Abstract
Root hydraulic conductivity (Lpr) and aquaporins were investigated in barley seedlings under salt stress of 100 mmol L−1 NaCl in hydroponic culture for 24 and 48 h. No reduction in root elongation was observed in stressed seedlings, and more that 75% of root and shoot growth was maintained under salt stress of 100 mmol L−1 NaCl. A slight increase in HvPIP2;1 transcript, which was the most abundant of the three aquaporins investigated, was observed. No significant changes in Lpr and HvPIP2;1 protein were detected. The possible reason for these complicated results and the function of aquaporins in Lpr under salt stress are discussed.
INTRODUCTION
Aquaporins are membrane proteins that mediate membrane transport of water and/or low molecular weight molecules (CitationTyerman et al. 2002). The first aquaporin gene (AQP1) was reported from erythrocytes (CitationPreston et al. 1992) and soon after this discovery plant aquaporins were identified (CitationMaurel et al. 1993). Today, aquaporins have been recognized among various animal, plant and bacterial cells. Researchers have isolated three genes (HvPIP2;1 accession number AB009307, HvPIP1;3 accession number AB009308 and HvPIP1;5 accession number AB009309) of plasma-membrane type aquaporins (PIPs) from barley (CitationKatsuhara et al. 2002). In roots, HvPIP2;1 was the most abundant among the three identified genes at the RNA level, and its expression was downregulated under severe salt stress of 200 mmol L−1 NaCl, where root growth was completely inhibited. Water potential of 200 mmol L−1 NaCl solution (approximately –0.9 MPa) was lower than that of barley root tissue (approximately –0.7 MPa), indicating that the direction of the water potential gradient between external and internal root tissue was reversed and resulted in water loss from tissue. Downregulation of aquaporins may be one way to prevent water loss from cells. This hypothesis is consistent with the result that HvPIP2;1 overexpression increased the salt sensitivity of transgenic rice plants (CitationKatsuhara et al. 2003b).
In 100 mmol L−1 NaCl salt stress, the water potential difference inside and outside the root is quite small, but the direction of the water potential gradient is not reversed. That is, cells can continue to uptake water at a reduced rate. Two strategies are theoretically possible to compensate for the reduced water influx in this situation. One is the re-establishment of a water potential difference inside and outside the roots by the accumulation of cytosolic solutes (inorganic ions and/or organic compatible solutes). This strategy is being well investigated (CitationTester and Davenport 2003). Another possibility is an increase in water permeability (root hydraulic conductivity [Lpr]). This hypothetical possibility is rarely investigated in detail and was examined in the present study. As PIPs provide molecular mechanisms for the regulation of cellular water permeability (CitationTyerman et al. 1999), the expression and regulation of barley PIPs in roots was analyzed simultaneously with Lpr.
MATERIALS AND METHODS
Plant materials and salt stress treatment
Seeds of barley, variety Akashinriki, were sterilized with 10% H2O2 for 10 min. After a 1-day immersion in distilled water with aeration, germinated seeds were transplanted and hydroponically cultured with aeration in 3.5-L pots filled with 0.25 mmol L−1 CaSO4 for 2 days and for one more day after replacing the medium with a hydroponic solution containing KNO3 (4 mmol L−1 as final concentration), NaNO3 (1 mmol L−1), NaH2PO4·2H2O (1 mmol L−1), CaCl2·2H2O (1 mmol L−1), MgSO4·7H2O (1 mmol L−1), Fe as Fe-citrate (1 mg L−1), Mn as MnCl2·4H2O (0.5 mg L−1), B as H3BO3 (0.5 mg L−1), Zn as ZnSO4·7H2O (0.05 mg L−1), Cu as CuSO4·5H2O (0.02 mg L−1) and Mo as Na2MoO4 (0.02 mg L−1). Solution pH was adjusted to 5.5 using NaOH.
All cultures were carried out in the dark in an air-conditioned room (25 ± 0.5°C) and seedlings were covered with wet sheets to maintain saturated humidity around the seedlings. Five-day old seedlings were subjected to salt stress by the application of 100 mmol L−1 NaCl to the hydroponic solution. Total root length (sum of the total roots of a seedling with three main roots) and shoot height were measured before (0 h) and after (24 and 48 h) 100 mmol L−1 NaCl of salt stress application in one batch of control seedlings and in another batch of stressed seedlings. Over the same period, root samples were harvested from other batches for the analysis of Lpr, RNA and protein. For RNA and protein extraction, samples were weighed separately, frozen with liquid N2 and stored at –80°C until analysis.
Measurement of Lpr and estimation of osmotic pressure
A double-chamber volumeter (CitationKatsuhara et al. 2003a) was used to measure Lpr. After removal of the root apoplastic gas, one part of an individual root (4 cm in length) was set in one room and separated from another part hydraulically with a mixture of vaseline and lanolin. Initially, the control solution was filled in both rooms. Transroot osmosis was induced by replacing the solution with a second solution supplemented with 100 mmol L−1 sorbitol. The volume of the water flow was microscopically monitored and root water permeability was calculated from the water flow per unit time, the surface area of the root, and the osmotic pressure difference between the solutions (CitationKatsuhara et al. 2003a).
Root tissue was mechanically extracted and the osmotic potential of the extraction solution was estimated using a 5520 vapor pressure osmometer (Wescor, Logan, UT, USA).
Isolation and quantification of RNA and protein
Total RNA was isolated according to the method of CitationShirzadegan et al. (1991) with a slight modification (ice cold extraction buffer was used in the present study). Transcript was quantified using a competitive polymerase chain reaction (PCR) method (CitationKatsuhara et al. 2002). Internal fragments of the cDNAs were deleted at the restriction sites (NarI–NarI fragment for HvPIP1;3, the AvrII–AvrII fragment for HvPIP1;5 and the HpaI–BstEII fragment for HvPIP2;1) to make shortened synthetic RNAs as competitors. The PCR primers were CTC TTC CTC TAC ATC TCG GTG CTC (upper) and GTC GTC CCA CGC CTG CTT CTT (down) for HvPIP1;3, GAA TCT CAG GAG GAC ACA TCA AC (upper) and AAT GGA AAC TAC AGC AAC CAT AAA (down) for HvPIP1;5, and GCT AGC TTA GCA ATG GCC AAG GAC (upper) and GTC GGA CTG GTG CTT GTA CC (down) for HvPIP2;1. Two independent experiments were carried out and 2–4 samples were quantified in each experiment.
Total protein was extracted, solubilized and submitted to sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western analysis as described previously (CitationKatsuhara et al. 2002). Anti-PIP2;1 antibody was used, and the detected bands were scanned and their density was normalized against cytochrome c as a control.
Functional expression in Xenopus oocytes
For the assay of water transport activities, capped RNAs of HvPIP1;3 or HvPIP2;1 were synthesized using a mMESSAGE mMACHINE kit (Ambion, Austin, TX, USA) and were injected into Xenopus oocytes. Swelling experiments were carried out and data were analyzed as previously described (CitationKatsuhara et al. 2002).
RESULTS
Total root length was measured in 5-day old seedlings (time 0 h in ). A second measurement was carried out 24 h after and a third measurement was taken 48 h after in treatments with or without salt stress. Root elongation () over 24 h (or 48 h) was calculated as the difference between total root lengths at time 0 and 24 h (or 48 h). No significant difference was observed between control and stressed seedlings in root elongation over the experimental period (). Shoot heights () and shoot elongations () were measured and calculated as per roots. A slight reduction in shoot elongation was detected during the 24 h treatment with 100 mmol L−1 NaCl, but not over 48 h.
Decreased Lpr (from 10.6 × 10−14 m s−1 Pa−1 to 5.8 × 10−14 m s−1 Pa−1) was observed () after 24 h in 100 mmol L−1 NaCl salt stress; however, statistical analyses indicated that this decrease was not significant as a result of large deviations in the data. In both control and stressed seedlings, a similar Lpr was observed 48 h after treatment.
Osmotic pressure of the hydroponic solution supplemented with 100 mmol L−1 NaCl was 183 mosm kg−1. Osmotic pressure of the root extraction before the NaCl application was 284 ± 14 mosm kg−1, indicating that the water potential deference outside and inside the roots was estimated (inside base) as 0.71 MPa (equivalent to 284 mosm kg−1) and 0.25 MPa (101 mosm kg−1) before
Figure 1 (A) Total root length and (B) shoot height of 15 seedlings without (□) and with salt stress of 100 mmol L−1 NaCl (░). Data are means ± standard deviation.
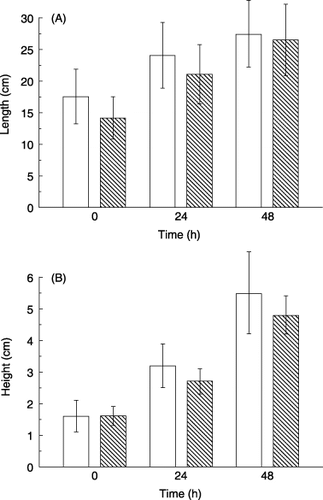
Table 1 Elongations of roots and shoots during the salt treatments (cm)
Transcripts of three PIPs were quantitatively analyzed during salt stress of 100 mmol L−1 NaCl.
Figure 2 Changes in root hydraulic conductivity without (•) or with salt stress of 100 mmol L−1 NaCl (º). Data are means ± standard deviation.
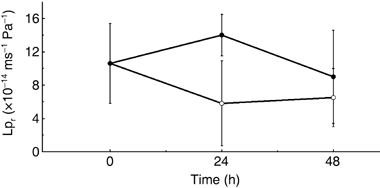
Figure 3 Amounts of HvPIP2;1 (□). HvPIP1;3 (░) or HvPIP1;5 (▒) transcripts in barley roots after the application of 100 mmol L−1 NaCl to the hydroponic solution. Data are means of two independent experiments.
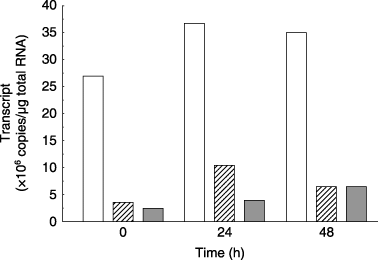
Table 2 Osmotic water permeability (Pf) of oocytes injected with cRNAs
In oocytes expressing HvPIP2;1, enhanced water transport activity was observed () as consistent with our previous study (CitationKatsuhara et al. 2002). An
Figure 4 Relative amounts of HvPIP2;1 protein in barley roots after the application of 100 mmol L−1 NaCl to the hydroponic solution. Data are means ± standard deviation.
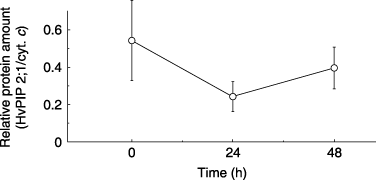
It seems that HvPIP2;1 protein slightly decreased in roots 24 h after salt stress in 100 mmol L−1 NaCl from the data with three repliactes (). However, no significant difference was found using a t-test because of large deviations in the data.
DISCUSSION
In the present study, root hydraulic conductivity and the expression of PIPs were focused in barley seedlings subjected to salt stress of 100 mmol L−1 NaCl. Root elongation was not reduced in stressed seedlings. And although a reduction in shoot growth was detected, the data indicated that 75% of shoot elongation was maintained over 24 h in salt stress. Because of the moist environment around the seedlings and because there was no light in the experimental condition, we assumed that almost no transpiration and no photosynthesis occurred and, therefore, the amount of water uptake was assumed to be almost proportional to the net growth (i.e. root and shoot elongations) of the seedlings. The water flow from outside to inside the root (J) is defined as the water uptake per unit time. The results indicated that J of stressed seedlings remained at approximately 75% of that in the unstressed seedlings during 24 h of salt stress.
According to the following formula, J is described as the product of the motive force (V) and conductance (G):
where V is described as the water potential difference between two compartments (outside and inside roots in the present study) and G is Lpr in the present case. Salt stress (100 mmol L−1 NaCl) decreases V from 0.71 MPa to 0.25 MPa. From this change in V and using Eq. (Equation1), J is calculated to be reduced to 35% (0.25/0.71) if G is constant. A big change in Lpr (= G) was not observed in the present study; however, a large reduction of J was not justified. Therefore, it was considered that the most likely major cellular mechanism maintaining J was re-establishment of V under salt stress (CitationTester and Davenport 2003) by osmotic adjustment. In barley, proline (CitationPesci 1992; CitationSanada et al. 1995) and glycine betaine (CitationAraya et al. 1991; CitationSakamoto and Murata 2002) are recognized as osmolytes. Although a decrease in root hydraulic conductance has been reported in a few plant species (CitationMartínez-Ballesta et al. 2006; CitationRodriguez et al. 1997), a very early effect of salt stress on Lpr after a few days of salt application has rarely been investigated (CitationMartínez-Ballesta et al. 2003).
In the roots of barley seedlings, three PIPs have been established previously (CitationKatsuhara et al. 2002). Among them, HvPIP1;3 and HvPIP1;5 transcripts were always much less than HvPIP2;1. In general, aquaporins in the PIP1 subgroup have no or very low water transport activity (CitationChaumont et al. 2000; CitationTyerman et al. 2002), as confirmed by the present data (). Taking together these results, it is concluded that HvPIP2;1 is the most responsible for Lpr among the three genes investigated in the present study.
A slight, but not large, increase in HvPI2;1 transcript was observed 24 h after the application of 100 mmol L−1 NaCl. HvPIP2;1 protein and Lpr seemed to be almost constant. Other PIPs, in addition to PIP2;1, may also contribute to Lpr. It is likely that 10 PIPs are expressing in barley according to the HarvEST barley contig database (http://harvest.ucr.edu/).
The role of aquaporin in Lpr (CitationHenzler et al. 1999; CitationJavot et al. 2003) and the regulation of aquaporins during osmotic and salt stress (CitationJang et al. 2004; CitationMartínez-Ballesta 2006; CitationSuga et al. 2002; CitationVera-Estrella et al. 2004; CitationZhu et al. 2005) have been investigated in some plants. To understand the plant strategy to uptake water under salt stress at the molecular level, simultaneous analysis of root hydraulic properties and aquaporins under salt stress in further studies will complement the present results.
ACKNOWLEDGMENTS
The authors are thankful for the technical assistance provided by Mrs Akiyama, Mrs Akiyoshi, Mr Nishimura, Mrs Sasano and Mrs Takahashi. This work was supported by the Program for the Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
REFERENCES
- Araya , F , Abarco , O , Zuniga , GE and Corcuera , LJ . 1991 . Effects of sodium chloride on glycine betaine and on aphids in cereal seedlings . Phytochem , 30 : 1793 – 1796 .
- Chaumont , F , Barrieu , F , Jung , R and Chrispeels , MJ . 2000 . Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity . Plant Physiol , 122 : 1025 – 1034 .
- Henzler , T , Waterhouse , RN Smyth , AJ . 1999 . Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus . Planta , 210 : 50 – 60 .
- Jang , JY , Kim , DG , Kim , YO , Kim , JS and Kang , H . 2004 . An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana . Plant Mol. Biol , 54 : 713 – 725 .
- Javot , H , Lauvergeat , V Santoni , V . 2003 . Role of a single aquaporin isoform in root rater uptake . Plant Cell , 15 : 509 – 522 .
- Katsuhara , M , Koshio , K , Akiyama , Y , Shibasaka , M and Kasamo , K . 2002 . Functional analysis of water channels in barley roots . Plant Cell Physiol , 43 : 885 – 893 .
- Katsuhara , M , Koshio , K , Shibasaka , M and Kasamo , K . 2003a . Expression of an aquaporin at night in relation to growth and root water permeability in barley seedlings . Soil Sci. Plant Nutr , 49 : 883 – 888 .
- Katsuhara , M , Koshio , K , Shibasaka , M , Hayashi , Y , Hayakawa , T and Kasamo , K . 2003b . Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants . Plant Cell Physiol , 44 : 1378 – 1383 .
- Martínez-Ballesta , MC , Aparicio , F , Pallás , V , Martínez , V and Carvajal , M . 2003 . Influence of saline stress on root hydraulic conductance and PIP expression in Arabidopsis . J. Plant Physiol , 160 : 689 – 697 .
- Martínez-Ballesta , MC , Silva , C , López-Berenguer , C , Cabañero , FJ and Carvajal , M . 2006 . Plant aquaporins: New perspectives on water and nutrient uptake in saline environment . Plant Biol , 8 : 535 – 546 .
- Maurel , C , Reizer , J , Schroeder , JI and Chrispeels , MJ . 1993 . The vacuolar membrane protein γ-TIP creates water specific channels in Xenopus oocytes . EMBO J , 12 : 2241 – 2247 .
- Pesci , P . 1992 . Effect of light on abscisic acid-induced proline accumulation in leaves: Comparison between barley and wheat . Physiol. Plant , 86 : 209 – 214 .
- Preston , GM , Carroll , TP , Guggino , WB and Agre , P . 1992 . Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein . Science , 256 : 385 – 387 .
- Rodriguez , P , Dell’amico , J , Morales , D , Sánchez Blanco , MJ and Alarco , JJ . 1997 . Effects of salinity on growth, shoot water relations and root hydraulic conductivity in tomato plants . J Agri. Sci , 128 : 439 – 444 .
- Sakamoto , A and Murata , N . 2002 . The role of glycine betaine in the protection of plants from stress: Clues from transgenic plants . Plant Cell Environ , 25 : 163 – 171 .
- Sanada , Y , Ueda , H Kuribayashi , K . 1995 . Novel light–dark change of proline levels in halophyte (Mesembryanthemum crystallinum L.) and glycophytes (Hordeum vulgare L. and Triticum aestivum L.) leaves and roots under salt stress . Plant Cell Physiol , 36 : 965 – 970 .
- Shirzadegan , M , Christie , P and Seeman , JR . 1991 . An efficient method for isolation of RNA from tissue cultured plant cells . Nucl. Acids Res , 19 : 6055
- Suga , S , Komatsu , S and Maeshima , M . 2002 . Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings . Plant Cell Physiol , 43 : 1229 – 1237 .
- Tester , M and Davenport , R . 2003 . Na+tolerance and Na+transport in higher plants . Ann. Botany , 91 : 503 – 527 .
- Tyerman , SD , Bohnert , H , Maurel , C , Steudle , E and Smith , JAC . 1999 . Plant aquaporins: their molecular biology, biophysics and significance for plant water relations . J. Exp. Bot , 50 : 1055 – 1071 .
- Tyerman , SD , Niemietz , CM and Bramly , H . 2002 . Plant aquaporins: multifunctional water and solute channels with expanding roles . Plant Cell Environ , 25 : 173 – 194 .
- Vera-Estrella , R , Brown , J , Bohnert , HJ and Pantoja , O . 2004 . Novel regulation of aquaporins during osmotic stress . Plant Physiol , 135 : 2318 – 2329 .
- Zhu , CS , Schraut , D , Hartung , W and Schäffner , AR . 2005 . Differential responses of maize MIP genes to salt stress and ABA . J. Exp. Bot , 56 : 2971 – 2981 .