Abstract
Dominant bacterial communities in the bulk soil of the plow layer in a Japanese paddy field were investigated over 1 year using denaturing gradient gel electrophoresis (DGGE) of 16S rRNA genes, and the succession and phylogenetic composition of the bacterial communities were estimated. “Existing” bacterial communities in the plow layer soil estimated from DNA were stable over 1 year irrespective of the soil conditions, although multivariate analysis of the DGGE patterns showed a difference in the communities between flooded and drained/upland conditions. Metabolically “active” bacterial communities based on the RNA analysis were also stable and all the bands detected in the DNA analysis existed, indicating that most of the bacterial members in the “existing” communities could be metabolically “active” in the plow layer soil. The DGGE bands that were commonly present and characteristic of certain periods were closely related to Chloroflexi, Actionobacteria, Alphaproteobacteria, Verrucomicrobia, Acidobacteria, Nitrospira, Chlorobi, Cyanobacteria and candidate division OP10, and most of their closest relatives were uncultured bacteria. Comparisons of these bacterial members in the bulk soil with those in other habitats in the plow layer showed that the bacterial members in the bulk soil comprised more diverse phylogenetic groups at phylum or class levels than those in other habitats. Heterogeneous and oligotrophic features of the soil may have enabled diverse and versatile bacterial members to grow and survive in the bulk soil.
INTRODUCTION
Soil environments consist of various habitats for soil microorganisms and these microorganisms grow on different substrates in the various habitats: for example, rhizodepositions in rhizosphere, plant residues and humic substances in bulk soil. In agricultural lands, the application of organic fertilizers brings in additional substrates. In addition, seasonal cycling of oxic and anoxic soil conditions in paddy field ecosystems significantly affects the microbial communities in respective habitats in the plow layer of paddy fields (anoxic during rice cultivation and oxic after harvest).
Microbial communities in paddy fields have been studied using culture methods, terminal restriction fragment length polymorphism (T-RFLP) analysis (CitationLüdemann et al. 2000; CitationNoll et al. 2005) and phospholipid fatty acid (PLFA) analysis (CitationBai et al. 2000; CitationReichard et al. 1997; CitationOkabe et al. 2000). And CitationKimura and Asakawa (2006) elucidated the establishment of different microbial communities in the abovementioned habitats in paddy fields by comparing PLFA compositions in microbial communities among the habitats. These authors found that the communities were distinct among the habitats, and that flooding did not exert a significant effect on the communities.
Phylogenetic studies have been conducted to identify bacteria inhabiting floodwater (CitationShibagaki-Shimizu et al. 2006), percolating water (CitationMurase et al. 2005), microcrustaceans in the floodwater (CitationNiswati et al. 2005), rice straw (CitationSugano et al. 2005), rice straw compost (CitationTanahashi et al. 2005), plant residues (CitationMatsuyama et al. 2007) and rice roots (CitationIkenaga et al. 2003) in Japanese paddy fields, and also in microcosm experiments where air-dried paddy soil was incubated (CitationHengstmann et al. 1999; CitationLüdemann et al. 2000; CitationNoll et al. 2005). However, there has been no phylogenetic study on the bacterial communities in the bulk soil of the plow layer under field conditions. Irrigated paddy fields in the temperate region of Asia are flooded during the rice cultivation period and drained under fallow conditions or cropped with wheat or barley under upland conditions (double-cropping) in winter. Thus, the moisture and redox conditions of the soils change drastically seasonally in the paddy fields and could influence bacterial communities in the bulk soil.
This study aimed first to elucidate the succession and phylogenetic composition of the dominant bacterial communities in the bulk soil under field conditions using denaturing gradient gel electrophoresis (DGGE) analyses targeting 16S rRNA genes followed by sequencing in the paddy field at the same Research Center that had conducted the phylogenetic studies of the various habitats described above. As ribosome and rRNA contents are correlated with bacterial cell activity and growth (CitationPoulsen et al. 1993; CitationWagner 1994), analyses of DNA and RNA that were retrieved from a soil sample could reveal the diversity of the existing members and potentially active members in the communities, respectively (CitationDuarte et al. 1998). The second objective of the present study was, therefore, to obtain information on potentially active members of the bacterial communities in the bulk soil and their seasonal behavior under field conditions by analyzing 16S rRNA genes amplified from RNA as well as DNA. We also examined the phylogenetic specificities of bulk soil bacterial communities from a comparison with those at various habitats in the plow layer.
MATERIALS AND METHODS
Experimental field
The experiment was carried out in a paddy field (E2 field) located at Aichi-ken Anjo Research and Extension Center, central Japan (latitude 34°8′Ν, longitude 137°5′Ε). The soil had the following properties: total C, 12.8 g kg−1; total N, 1.1 g kg−1; pH (H2O), 6.3; amorphous Fe content, 3.76 g kg−1; clay content, 230 g kg−1 (Oxyaquic Dystrudepts).
The experiment was conducted from 11 April 2003 to 10 March 2004. The field was cropped with rice (Oryza sativa L.) and wheat (Triticum aestivam L.) in summer and winter, respectively, and field management in 2003 was as follows: harvesting wheat (cv. Norin 61) in early June, flooding, puddling and basal fertilization on 16 June, transplanting rice plants (cv. Matsuribare) on 19 June, midseason drainage from 1 to 11 August, drainage on 17 October, harvesting rice on 24 October, and seeding wheat (cv. Norin 61) on 10 December. Topdressing for rice plants was carried out on 22 August.
The amounts of fertilizers applied to the rice plants were 62 kg N, 57 kg P2O5 and 17 kg K2O per hectare as ammonium sulfate, ammonium dihydrogen phosphate and diammonium monohydrogen phosphate, and potassium chloride, respectively, for basal fertilization, 22 kg N, 21 kg P2O5 and 6.4 kg K2O per hectare for the top dressing, while 56 kg N, 56 kg P2O5 and 52 kg K2O per hectare as ammonium sulfate, ammonium dihydrogen phosphate and diammonium monohydrogen phosphate, and potassium chloride were applied as basal fertilization for wheat. Rice straw residues were removed from the field after harvest, while wheat straw residues were incorporated into the field after harvest.
Soil sampling
The plow layer soils (10–15-cm depth; the soil sample mainly consisted of reduced layer soils during flooded periods) were collected from the center of four rice hills and between wheat rows at three randomly chosen points in the field and mixed well to make the composite sample in a polyethylene bag. The soil samples for DNA extraction were transported to the laboratory on ice, passed through a 2-mm mesh sieve and kept at 4°C until use (DNA was usually extracted within 2 days of sampling). A portion of the composite sample (approximately 15 g) was placed into a 15-mL polypropylene tube for RNA experiments, frozen with dry ice in a container in the field and then kept at –80°C until analysis.
The dates of soil sampling (9 occasions) were 11 April, 26 June, 28 July, 11 August, 4 September, 14 October and 11 December in 2003, and 13 January and 10 March in 2004. Thus, the soil sampling was carried out four times during the flooded period, four times during the drained (upland) period and on the last day of midseason drainage.
DNA and RNA extraction
The DNA was extracted from 0.5 g of soil (fresh weight) as described by CitationWatanabe et al. (2006) based on the method of CitationZhou et al. (1996) using a Mini-Beadbeater (Biospec Product, Bartlesville, OK, USA). Extracted DNA was further purified using a Sephadex-G200 column according to CitationCahyani et al. (2003) based on the method of CitationJackson et al. (1997).
The RNA extraction was conducted from 1 g of soil (fresh weight) according to T. Watanabe et al. (unpubl. data, 2007) based on the method of CitationLüdemann et al. (2000) using a Mini-Beadbeater. The RNA samples were treated with DNase to remove the remaining DNA by treating the extracted RNA with DNase I (5 U µL−1; TaKaRa, Otsu, Japan) at 37°C for 1 h. The RNA was then recovered from the mixture after phenol–chloroform treatment and ethanol precipitation, dissolved in 100 µL of 1% diethyl pyrocarbonate (DEPC; Sigma, St Louis, MO, USA) treated water supplemented with RNA secure (Ambion, Austin, TX, USA) at a final concentration of 1%, and finally stored at –80°C. No amplification of DNA from RNA samples was confirmed using polymerase chain reaction (PCR).
Polymerase chain reaction amplification and DGGE analysis
Bacterial 16S rRNA genes were first amplified with the primer combination of 357f-GC clamp (Escherichia coli position: 341–357, 5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGGG CTT ACG GGA GGC AGC AG-3′, the underlined sequence corresponds to the GC clamp) and 517 r (Escherichia coli position: 517–534, 5′-ATT ACC GCG GCT GCT GG-3′) (CitationMuyzer et al. 1993). The PCR was conducted as described previously (CitationCahyani et al. 2003). For the RNA samples, reverse transcriptase (RT)–PCR reaction was conducted using an AccessQuick RT–PCR System (Promega, Madison, WI, USA). The RT reaction was carried out at 45°C for 45 min in a 0.2-mL tube with a mixture containing 25 µL AccessQuick Master MIX (two strength solution), 2.5 µL of each primer (10 µmol L−1 solutions), 19 µL of DEPC-treated water, RNA solution and 1 µL of avian myeloblastosis virus (AMV) Reverse Transcriptase. The conditions for the PCR reaction were the same as those for the DNA samples. The PCR products (approximately 300 ng) were applied to a polyacrylamide gel (8% w/v) with a denaturing gradient ranging from 35 to 65% (100% denaturant, 7 mol L−1 urea and 40% (v/v) formamide), and the DGGE was conducted at 60°C and 100 V for 14 h. Visualization of the bands was achieved by staining with SYBR Green I nucleic acid gel stain (BMA, Rockland, ME, USA) for 20 min and photographing under ultraviolet (UV) light.
Statistical analysis
To estimate the seasonal change in the bacterial communities in the soil, data obtained from the DGGE band patterns, based on the position and the intensity (0, no band; 1, weak; 2, moderate; 3, strong) of bands observed, were analyzed using cluster analysis and principal component analysis (PCA). The PCA was carried out using EXCEL STASTISTICS 1997 for Windows (SRI, Tokyo, Japan). A correlation matrix was used in the analysis. Cluster analysis was carried out according to the Blackbox program (CitationAoki 1996). The Ward method was used in the analysis. The Dice similarity coefficient (SD) between the DGGE patterns was calculated using the PAST data analysis package (CitationHammer et al. 2001).
Sequencing of the selected DGGE bands
The characterized DGGE bands with the same mobilities were excised from two different lanes and subjected to sequencing. DNA extraction from the DGGE bands and verification of the mobility of the band and sequencing of the DNA fragments were carried out as described previously by CitationIkenaga et al. (2003) and CitationCahyani et al. (2003).
Sequence analysis
Close relatives and phylogenetic affiliations of sequences of the DGGE bands were determined using the BLAST search program at the DNA Data Bank of Japan (DDBJ; http://www.ddbj.nig.ac.jp/E-mail/homology.html). The sequences of 16S rDNA obtained in this study are available in the DDBJ database. The accession numbers are AB255308 to AB255338.
RESULTS
Soil moisture content
The moisture contents of soil samples ranged from 0.5 to 0.6 g g−1 (dry soil basis) during the flooded period and were approximately 0.2 g g−1 during the drained (upland) period. Moisture content decreased to 0.35 g g−1 at the end of the midseason drainage on 11 August, indicating that drainage supplied sufficient air to the plow layer soil (data not shown).
DGGE band patterns of bacterial communities
Analysis based on DNA
The DGGE patterns of bacterial communities in the plow layer based on the DNA analysis are shown in . The patterns of bacterial communities in the plow layer based on the DNA analysis did not differ greatly and consisted of 40–47 bands. Total different DGGE bands amounted to 52. Twenty-three bands were constantly present in every soil sample and 47 different bands were frequently observed (five times or more in nine samplings).
Although the DGGE band patterns seemed to be similar to each other, cluster analysis of the DGGE band patterns revealed differences in the patterns. The analysis separated firstly the soil samples from 11 April to 11 August (samples before and at midseason drainage) from those after midseason drainage, and it further
Figure 1 Denaturing gradient gel electrophoresis pattern of bacterial communities in the plow layer soil based on DNA analysis. Bands with arrows were excised and subjected to sequencing (see Table 1).
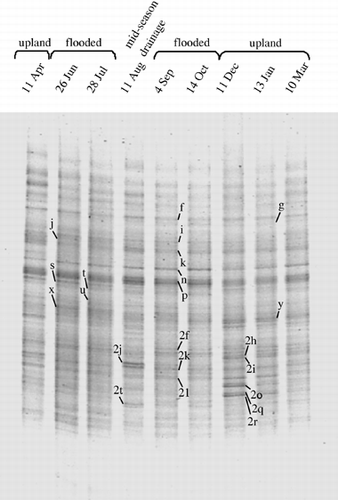
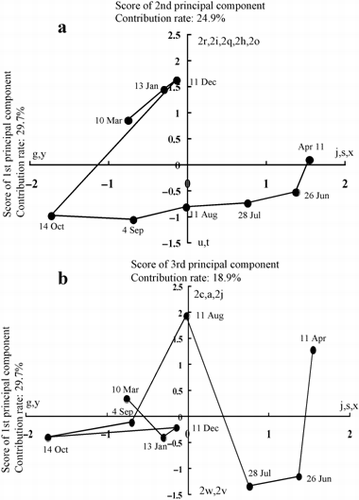
Figure 2 Principal component analysis of the denaturing gradient gel electrophoresis patterns of the bacterial communities in the plow layer soil based on DNA analysis. (a) Plots of first and second principal components and (b) plots of first and third principal components. Contribution rates of first, second and third principal components were 29.7%, 24.9% and 18.9%, respectively.
Analysis based on RNA
The DGGE band pattern of bacterial communities based on RNA analysis did not differ greatly from those based on DNA analysis (). In total, 52 DGGE bands were obtained from bacterial RNA and all the DGGE bands of bacterial RNA except for the band R-1 were also observed in the DGGE pattern of bacterial DNA with the same mobilities. In the RNA analysis, the number of DGGE bands ranged from 29 to 46, with 22 bands commonly present in every soil sample and 40 different bands were observed frequently (five or more times in nine samplings). The patterns of both bacterial DNA and RNA showed no conspicuous changes throughout the year.
Figure 3 Denaturing gradient gel electrophoresis pattern of the bacterial communities in the plow layer soil based on DNA and RNA analyses. Bands with arrows were excised and subjected to sequencing (see Table 1). D, DNA; R, RNA.
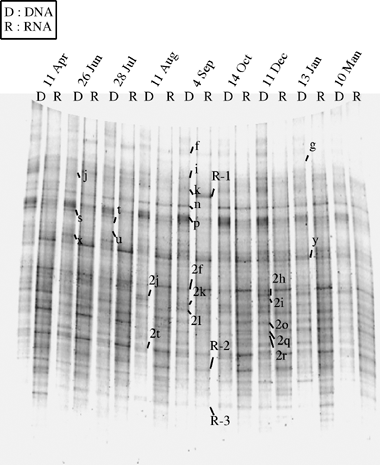
Similarities in the DGGE patterns of the bacterial communities between DNA and RNA at the same sampling period were compared using the Dice coefficient (SD), in which band intensity was evaluated using 1 (present) or 0 (absent). The SD values were more than 0.85, indicating that the patterns were similar between bacterial DNA and RNA, with one exception, that is, the patterns on 4 September had a slightly lower SD value (0.77). The differences between the patterns of bacterial DNA and RNA were mainly attributed to an increase or decrease in the band intensity because the two patterns shared almost all bands.
Phylogenetic positions of characteristic DGGE bands
In addition to the characteristic DGGE bands that were recognized from the PCA, eight bands (f, i, k, n, p, 2f, 2k and 2l) that appeared constantly in every soil sample with relatively strong intensity and one band 2t with strong intensity only at the time of midseason drainage (11 August) on the DGGE gel of bacterial DNA were subjected to sequencing (). From the DGGE gel of bacterial RNA (), bands that exhibited stronger intensity than in the gel of bacterial DNA were selected because the band patterns showed no conspicuous changes throughout the year like those of bacterial DNA. The band R-1, which was not detected in the gel of bacterial DNA, and the bands R-2 and R-3, which showed markedly stronger intensity than in the DGGE pattern of bacterial DNA, were subjected to sequencing. Sequences of bands n, y, 2f, 2o 2q and 2t were confirmed between the gels of bacterial DNA and RNA by excising the fragments from the gel of bacterial RNA because of strong intensity in the gel of bacterial RNA. The total number of sequenced DGGE bands was 25 ().
Most of their closest relatives (top BLAST hits) were uncultured bacteria during the flooded period, while the closest relatives of the bands 2h, 2i, 2o and 2q that characterized the bacterial communities during the upland period were cultured strains of Actinobacteria (). The closest relatives of the constantly present DGGE bands belonged to the phyla, Verrucomicrobia, Chloroflexi, Nitrospira, Acidobacteria and Chlorobi. Members of Chloroflexi were also obtained as the characteristic bands (x and 2j), which were constantly present throughout the period with weak intensities, for the periods before the midseason drainage and during the midseason drainage.
Sequences of the DGGE bands y, 2o, 2q and 2t in bacterial RNA were identical to or similar to those determined from the corresponding bands in DGGE gel of bacterial DNA (). Band n showed slightly different sequences between the fragments of DNA and RNA, but they were closely related with each other and belonged to the Nitrospira group. In contrast, the sequence of band 2f in the gel of bacterial RNA was phylogenetically different from that of band 2f in the gel of bacterial DNA: the former was affiliated to Methylomonas (Gammaproteobacteria) and the latter to Chloroflexi. Bands R-2 and R-3, which had stronger intensities in RNA samples than in DNA samples, showed close relationships to Anaeromyxobacter, anaerobic myxobacteria in Deltaproteobacteria. The band R-1, specific to bacterial RNA, was related to a plant chloroplast.
DISCUSSION
Succession of dominant bacterial communities in the bulk soil
As the DGGE patterns based on both DNA and RNA analyses showed no conspicuous changes during the study periods, bacterial communities in the plow layer soil were estimated to be stable throughout the year irrespective of the soil conditions, which was in accordance with the findings obtained from an PLFA analysis (CitationOkabe et al. 2000). Effects of field managements, such as flooding, mid-season drainage, wheat cultivation and wheat straw application, on the bacterial communities in the plow layer soil seemed to be small, although slight changes in intensities of the respective DGGE
Table 1 Close relatives of excised denaturing gradient gel electrophoresis bands (see Figs 1,3) that were commonly present or characterized the bacterial communities in the plow layer soil at the respective periods based on DNA and RNA analyses
“Existing” and “active” bacterial communities in the bulk soil
All the DGGE bands of bacterial DNA were retrieved in the DGGE pattern of bacterial RNA with the same mobilities except for one band (R-1) (). This indicates that almost all the bacterial members detected by the DNA analysis maintained rRNA to some extent and had more or less potential activities for growth. The DGGE patterns of the bacterial communities were similar, but not identical mainly because of differences in band intensities between bacterial DNA and RNA (), which indicates that the community structure of the “active” bacteria (RNA) was slightly distinct from that of the “existing” bacteria (DNA). These findings suggest that the composition of bacterial members was similar between the “existing” (DNA) and the “active” (RNA) bacterial communities, but that the relative abundance of respective members and their activities were different. CitationTanahashi et al. (2005) investigated bacterial communities in rice straw compost incorporated into a paddy field at the same Research Center during the flooded period using DGGE analysis of 16S rRNA genes amplified from DNA and RNA and reported that the bacterial communities were influenced by the mid-season drainage and that 62–81% of the bacteria present in rice straw compost were metabolically “active” from the comparison of DGGE bands of DNA and RNA. Ecophysiological conditions for bacterial growth, such as substrate availability, were different between the plow layer soil and rice straw compost in soil and contributed to those differences in bacterial DNA and RNA patterns between the bulk soil and rice straw compost in soil. Plow layer soil, which is less eutrophic, but a more stable environment, might enable many kinds of versatile bacterial members to grow and survive, while rice straw compost could support the prominent growth of certain bacterial members because of greater substrate availability.
Composition of the dominant bacterial communities in the bulk soil
Most of the top BLAST hits of the DGGE bands were uncultured bacteria (). This result was unique to the soil samples in comparison with the results of rice straw (CitationSugano et al. 2005), rice straw compost (CitationTanahashi et al. 2005) and plant residues (CitationMatsuyama et al. 2007) recovered from the paddy field at the same Research Center, and rice roots grown in pots with the same paddy soil (CitationIkenaga et al. 2003), where many of the sequenced DGGE bands were affiliated to cultured bacterial isolates. In contrast, most of the closest relatives of the DGGE bands were also uncultured bacterial clones in the DGGE analysis of the bacterial communities in the floodwater of a paddy field at the same Research Center (CitationShibagaki-Shimizu et al. 2006). The oligotrophic feature of the environments, plow layer soil and floodwater, may have been partly related to the scarce detection of cultured members as closest relatives. On the contrary, the closest relatives of the bands 2h, 2i, 2o and 2q that characterized the bacterial communities during the upland period were cultured strains of Actinobacteria, indicating that plow layer soil under upland conditions might have slightly different features for bacterial inhabitants from soil under flooded conditions, although the reason for this is not known.
As members of Chloroflexi were constantly present throughout the period with weak intensities and were also the characteristic bands (x and 2j) for the periods before the midseason drainage and during the midseason drainage (), they seemed to be one of the dominant bacterial inhabitants in the paddy field soil. The members may have adapted to various soil conditions in the paddy field because the Chloroflexi group comprises diverse species that have versatile metabolic abilities (CitationPierson and Castenholz 1992). Gram-negative anaerobic Verrucomicrobia have been reported to be numerically dominant in a rice paddy microcosm (CitationChin et al. 1999; CitationHengstmann et al. 1999). Members of Verrucomicrobia have also been detected in rice straw (CitationSugano et al. 2005) and rice straw compost (CitationTanahashi et al. 2005) incorporated into soil, plant residues (CitationMatsuyama et al. 2007), percolating water (CitationMurase et al. 2005) and rice roots (CitationIkenaga et al. 2003). These findings indicate that Verrucomicrobia is a common genus in paddy field ecosystems. Sequences affiliated to Chlorobi (obligate anaerobic phototrophs [green sulfur bacteria]) and Nitrospira (nitrite oxidizing bacteria) were also obtained from rice straw (CitationSugano et al. 2005) and rice straw compost (CitationTanahashi et al. 2005) buried into soil, and plant residues (CitationMatsuyama et al. 2007), respectively, and those members may have contributed to photosynthesis and sulfur (CitationOvermann 2001) or nitrogen cycles (CitationSpieck and Bock 2001) in the soil. These findings indicate that some members of the bacterial communities in the plow layer soil were similar to members of the communities in the other habitats in the paddy field ecosystem. All of the above bacterial groups are Gram-negative bacteria and there was no band belonging to Gram-positive bacteria that was constantly present in the plow layer soil, although CitationOkabe et al. (2000) indicated the predominance of Gram-positive bacteria in that soil layer from a PLFA analysis. This apparent discrepancy between DGGE and sequence analyses and PLFA analysis indicates that there were no specific, numerous members among abundant Gram-positive bacteria. This difference may also have arisen as a result of methodological differences in PLFA and PCR-based DGGE.
Members related to Alphaproteobacteria characterized the bacterial communities before and after the midseason drainage (). Members of Alphaproteobacteria were obtained from soils of rice paddy microcosms using a culture method (CitationChin et al. 1999), 16S rDNA clone analysis (CitationHengstmann et al. 1999) and T-RFLP method (CitationLüdemann et al. 2000). These findings indicate that the alphaproteobacterial group was one of the common members in paddy field soil, especially under flooded conditions. The band y, which characterized the bacterial communities after the midseason drainage, was closely related to “BANA domain”, including the genera Bradyrhizobium, Agromyces, Nitrobacter and Afipia in Alphaproteobacteria (CitationSaito et al. 1998). As oligotrophy and slow growth seem to be common features for bacterial members in the BANA domain group and the members are autochtonous inhabitants in soil (CitationMinanisawa and Mitsui 2000), the bacterium corresponding to band y may have been an autochtonous member in the paddy field soil.
All of the DGGE bands that characterized bacterial communities during the drained (upland) period in the plow layer soil belonged to a high G + C Gram-positive bacterial group, Actinobacteria (). In addition, one band (2t), which was characteristic of the midseason drainage, was also affiliated to Actinobacteria. Sufficient supply of oxygen to the field under drained conditions presumably stimulated the growth of those actinobacterial members, most of which are strictly aerobic.
Most of the DGGE bands in bacterial RNA showed identical or similar sequences to those determined from the corresponding bands in the DGGE gel of bacterial DNA in the present study (). The exception was band 2f and it may have contained two kinds of fragments as shown in , but the fragments showed similar mobilities on the gels () because they had very similar G + C contents of DNA (54% and 55%). Methylomonas is an obligate aerobic methanotroph and it is not clear that the bacterium retained the activity throughout the year under both anoxic and oxic conditions. These results suggest that most of the bacterial members present in plow layer soil (obtained in the DGGE gel of bacterial DNA) could be metabolically “active” at certain periods over the year, although an exception was observed for bands such as band 2f.
The RNA specific bands (R-1, R-2 and R-3) were related to myxobacteria and chloroplast (). Myxobacterial members were obtained from rice straw (CitationSugano et al. 2005) and rice straw compost buried into soil (CitationTanahashi et al. 2005) and plant residues (CitationMatsuyama et al. 2007), and they seemed to be involved in the decomposition of organic matter in soils. As for band R-1, cells of some plants, such as weeds, might have been contaminated in the soil sample on 4 September, but the reason why it was detected specifically on that date is not known.
Table 2 Comparison of the distribution of characteristic bacteria at the phylum and class levels among bulk soil (this study) and other habitats in the plow layer
Phylogenetic comparison of characteristic bacteria among various habitats in the plow layer
compares the distribution of characteristic bacteria at the phylum and class levels among the bulk soil and other habitats in the plow layer, such as rice straw (CitationSugano et al. 2005), plant residues (CitationMatsuyama et al. 2007), rice straw compost (CitationTanahashi et al. 2005), rice roots (CitationIkenaga et al. 2003) and percolating water (CitationMurase et al. 2005). These studies were conducted on the same or adjacent paddy fields, or the soil samples collected from the same paddy field inside the same Research Center, and the DGGE analyses were carried out using the same primer pair. Therefore, compares the phylogenetic characteristics of bacterial communities among various habitats in the plow layer of “a Japanese paddy field”.
Characteristic bacteria were mostly restricted to specific groups for the percolating water and rice roots (five phyla), while those in the plow layer soil were most diverse (nine phyla). In addition, Actinobacteria and Chlorofrexi, to which the largest number of bands (six bands) belonged, accounted for only 26% of the total in the plow layer soil. In contrast, Proteobacteria occupied 65% of the total in rice roots. These comparisons indicate that the plow layer soil is a highly heterogeneous habitat for versatile bacterial members to grow and survive.
Members of Verrucomicrobia and Proteobacteria were commonly present at various habitats, including the plow layer soil, while members of Bacteroidetes, Spirochaetes and Firmicutes were not detected as characteristic bacteria from the plow layer soil, although they characterized bacterial communities in some other habitats (e.g. rice straw, rice straw compost and plant residues) in the plow layer. In contrast, bacteria belonging to Chlorofrexi, candidate division OP10 and Cyanobacteria were specific to the bulk soil. The DGGE bands belonging to Proteobacteria were most abundantly identified as the characteristic bands at the respective habitats in the plow layer except for the plow layer soil, probably because substrates for bacterial growth in the plow layer soil are mainly humic substances and differ from the other habitats where substrates deriving from rice plants are predominant. The predominance of Proteobacteria in the percolating water probably resulted from the aquatic feature of the habitat. Gram-negative bacteria are well known to be predominant in aquatic environments (CitationOkabe et al. 2000; CitationShibagaki-Shimizu et al. 2006; CitationShimizu et al. 2002a, Citation2002b).
Notes
Present addresses: Aichi Steel Corporation, Arao, Tokai 476-8666, Japan
Max Planck Institut für terrestrische Mikrobiologie, Karl-von-Frisch-Straße, Marburg D-35043, Germany.
REFERENCES
- AokiS1996Black-box: Data analysis on the wwwAvailable from URL: http://aoki2.si.gunma-u.ac.jp/bb0/BlackBox0.html
- Bai , Q , Gattinger , A and Zelles , L . 2000 . Characterization of microbial consortia in paddy rice soil by phospholipid analysis . Microb. Ecol , 39 : 273 – 281 .
- Cahyani , VR , Matsuya , K , Asakawa , S and Kimura , M . 2003 . Succession and phylogenetic composition of bacterial communities responsible for the composting process of rice straw estimated by PCR-DGGE analysis . Soil Sci. Plant Nutr , 49 : 619 – 630 .
- Chin , K-J , Hahn , D , Hengstmann , U , Liesack , W and Janssen , PH . 1999 . Characterization and identification of numerically abundant culturable bacteria from the anoxic bulk soil of rice paddy microcosms . Appl. Environ. Microbiol , 65 : 5042 – 5049 .
- Duarte , GF , Rosado , AS , Seldin , L , Keijzer-Wolters , AC and van Elsas , JD . 1998 . Extraction of ribosomal RNA and genomic DNA from soil for studying the diversity of the indigenous bacterial community . J. Microbiol. Methods , 32 : 21 – 29 .
- Hammer , Ø , Harper , DAT and Ryan , PD . 2001 . PAST: paleontological statistics software package for education and data analysis . Palaeontol. Electron , 4 9pp
- Hengstmann , U , Chin , K-J , Janssen , PH and Liesack , W . 1999 . Comparative phylogenetic assignment of environmental sequences of genes encoding 16S rRNA and numerically abundant culturable bacteria from an anoxic rice paddy soil . Appl. Environ. Microbiol , 65 : 5050 – 5058 .
- Ikenaga , M , Asakawa , S , Muraoka , Y and Kimura , M . 2003 . Bacterial communities associated with nodal roots of rice plants along with the growth stages: estimation by PCR-DGGE and sequence analyses . Soil Sci. Plant Nutr , 49 : 591 – 602 .
- Jackson , CR , Harper , JP , Willoughby , D , Roden , EE and Churchill , PF . 1997 . A simple, efficient method for the separation of humic substances and DNA from environmental samples . Appl. Environ. Microbiol , 63 : 4993 – 4995 .
- Kimura , M and Asakawa , S . 2006 . Comparison of community structures of microbiota at main habitats in rice field ecosystems based on phospholipid fatty acid analysis . Biol. Fertil. Soils , 43 : 20 – 30 .
- Lüdemann , H , Arth , I and Liesack , W . 2000 . Spatial changes in the bacterial community structure along a vertical oxygen gradient in flooded paddy soil cores . Appl. Environ. Microbiol , 66 : 754 – 762 .
- Matsuyama , T , Nakajima , Y , Matsuya , K , Ikenaga , M , Asakawa , S and Kimura , M . 2007 . Bacterial community in plant residues in a Japanese paddy field estimated by RFLP and DGGE analyses . Soil Biol. Biochem , 39 : 463 – 472 .
- Minanisawa , K and Mitsui , H . 2000 . “ Genetic ecology of soybean bradyrhizobia ” . In Soil Biochemistry , Edited by: Bollag , J-M and Stotzky , G . vol. 10. , 349 – 377 . New York : Marcel Dekker .
- Murase , J , Shimizu , M , Hayashi , M , Matsuya , K and Kimura , M . 2005 . Vertical changes in bacterial communities in percolating water of a Japanese paddy field as revealed by PCR-DGGE . Soil Sci. Plant Nutr , 51 : 83 – 90 .
- Muyzer , G , de Wall , EC and Uitterlinden , AG . 1993 . Profiling of complex microbial populations by denaturing gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA . Appl. Environ. Microbiol , 59 : 695 – 700 .
- Niswati , A , Murase , J and Kimura , M . 2005 . Comparison of bacterial communities associated with microcrustaceans from the floodwater of a paddy field microcosm: estimation based on DGGE pattern and sequence analyses . Soil Sci. Plant Nutr , 51 : 281 – 290 .
- Noll , M , Matthies , D , Frenzel , P , Derakshani , M and Liesack , W . 2005 . Succession of bacterial community structure and diversity in a paddy soil oxygen gradient . Environ. Microbiol , 7 : 382 – 395 .
- Okabe , A , Oike , H , Toyota , K and Kimura , M . 2000 . Comparison of phospholipid fatty acid composition in floodwater and plow layer soil during the rice cultivation period in a Japanese paddy field . Soil Sci. Plant Nutr , 46 : 893 – 904 .
- Overmann , J . 2001 . “ Green sulfur bacteria ” . In Bergey's Manual of Systematic Bacteriology, Vol 1, The Archaea and the Deeply Branching and Phototrophic Bacteria , 2nd edn , Edited by: Boone , DR and Castenholz , RW . Vol 1 , 601 – 605 . New York : Springer-Verlag .
- Pierson , BK and Castenholz , RW . 1992 . “ The Family Chloroflexaceae ” . In The Prokaryotes , Edited by: Balows , A , Trüper , HG , Dworkin , M , Harder , W and Schleifer , K-H . 3754 – 3774 . New York : Springer-Verlag .
- Poulsen , LK , Ballard , G and Stahl , DA . 1993 . Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms . Appl. Environ. Microbiol , 59 : 1354 – 1360 .
- Reichard , W , Mascariña , G , Padre , B and Doll , J . 1997 . Microbial communities of continuously cropped, irrigated rice fields . Appl. Environ. Microbiol , 63 : 233 – 238 .
- Saito , A , Mitsui , H , Hattori , R , Minamisawa , K and Hattori , T . 1998 . Slow-growing and oligotrophic soil bacteria phylogenetically close to Bradyrhizobium japonicum . FEMS Microbiol. Ecol , 25 : 277 – 286 .
- Shibagaki-Shimizu , T , Nakayama , N , Nakajima , Y , Matsuya , K , Kimura , M and Asakawa , S . 2006 . Phylogenetic study on a bacterial community in the floodwater of a Japanese paddy field estimated by sequencing 16S rDNA fragments after denaturing gradient gel electrophoresis . Biol. Fertil. Soils , 42 : 362 – 365 .
- Shimizu , M , Hayashi , M , Matsuya , K , Murase , J and Kimura , M . 2002a . Comparison of microbial communities in percolating water from plow layer and subsoil layer estimated by phospholipid fatty acid (PLFA) analysis . Soil Sci. Plant Nutr , 48 : 877 – 881 .
- Shimizu , M , Nakajima , Y , Matsuya , K and Kimura , M . 2002b . Comparison of phospholipid fatty acid composition in percolating water, floodwater, and the plow layer soil during the rice cultivation period in a Japanese paddy field . Soil Sci. Plant Nutr , 48 : 595 – 600 .
- Spieck , E and Bock , E . 2001 . “ Genus I. Nitrospira. ” . In Bergey's Manual of Systematic Bacteriology, Vol 1, The Archaea and the Deeply Branching and Phototrophic Bacteria , 2nd edn , Edited by: Boone , DR and Castenholz , RW . Vol 1 , 451 – 453 . New York : Springer-Verlag .
- Sugano , A , Tsuchimoto , H , Tun , CC , Asakawa , S and Kimura , M . 2005 . Succession and phylogenetic profile of eubacterial communities in rice straw incorporated into a rice field: estimation by PCR-DGGE analysis . Soil Sci. Plant Nutr , 51 : 51 – 60 .
- Tanahashi , T , Murase , J , Matsuya , K , Hayashi , M , Kimura , M and Asakawa , S . 2005 . Bacterial communities responsible for the decomposition of rice straw compost in Japanese rice paddy field estimated by DGGE analysis of amplified 16S rDNA and 16S rRNA fragmants . Soil Sci. Plant Nutr , 51 : 351 – 360 .
- Wagner , R . 1994 . The regulation of ribosomal RNA synthesis and bacterial cell growth . Arch. Microbiol , 161 : 100 – 109 .
- Watanabe , T , Kimura , M and Asakawa , S . 2006 . Community structure of methanogenic archaea in paddy field soil under double cropping (rice–wheat) . Soil Biol. Biochem , 38 : 1264 – 1274 .
- Zhou , J , Bruns , MA and Tienje , JM . 1996 . DNA recovery from soils of diverse composition . Appl. Environ. Microbiol , 62 : 316 – 322 .
- Present addresses: Aichi Steel Corporation, Arao, Tokai 476-8666, Japan
- Max Planck Institut für terrestrische Mikrobiologie, Karl-von-Frisch-Straße, Marburg D-35043, Germany.