Abstract
The effects of methionine and glutathione on the promoter activities of the β subunit gene of β-conglycinin, a seed storage protein of soybean, were studied in a transient assay system using protoplasts derived from Arabidopsis thaliana liquid callus cultures. The promoter activity of the β subunit gene was repressed by methionine and glutathione in a manner similar to that observed in transgenic plants. This is the first report of a transient assay system to study the regulation of gene expression by methionine and glutathione.
INTRODUCTION
Transient expression systems are rapid, independent of so-called position effects and suitable for analyzing a large number of different promoter constructs. To effectively use transient assay systems, the patterns of gene expression observed in intact plants and/or stable transformants have to be reproduced. A number of successful examples have been reported, including responses to abscisic acid (CitationMarocotte et al. 1988), responses to gibberellic acid (CitationGopalakrishnan et al. 1991) and responses to nitrogen nutrition of a seed storage protein gene (CitationMüller et al. 1997). However, to our knowledge, there is no successful report on the establishment of a transient assay system to study transcriptional regulation by sulfur nutrition.
An effect of sulfur nutrition on seed storage protein accumulation was first reported as early as 1970. The composition of seed storage proteins changes in response to sulfur nutrition in a number of plant species, including soybean (CitationGayler and Sykes 1985), pea (CitationRandall et al. 1979), lupine (CitationBlagrove et al. 1976), wheat (CitationWrigley et al. 1984), maize (CitationBaudet et al. 1986), cowpea (CitationEvans et al. 1985), rape and sunflower (CitationSpencer et al. 1990). In these plant species, the accumulation of sulfur-rich proteins decreases while the accumulation of sulfur-poor proteins increases under sulfur-deficient conditions, so that the total amount of the storage proteins remains relatively constant (CitationHiggins 1984).
The response to sulfur nutrition was shown to be regulated at the level of transcription in the case of the β subunit gene of soybean β-conglycinin (CitationHirai et al. 1995), and at the level of mRNA stability in the case of PA1, an 11S albumin of pea (CitationChandler et al. 1983; CitationHiggins et al. 1986). In the soybean β subunit gene, a 235-bp region responsible for the sulfur nutrition response was identified with the use of transgenic Arabidopsis (CitationAwazuhara et al. 2002a). In the case of PA1, 3′ untranslated regions of the mRNA are shown to be responsible for the stability of the mRNA in response to sulfur nutrition in transgenic Nicotiana tabacum (CitationMorton et al. 1998). Other than genes expressed in seeds, a cis-acting element conferring sulfur deficiency response was identified in a sulfate transporter gene in Arabidopsis (CitationMaruyama-Nakashita et al. 2005). Although these studies were successful in identifying sulfur-responsive regions in the corresponding genes, the production of transgenic plants is time-consuming and laborious. Development of a transient assay system would facilitate analysis of the regulation mechanisms.
Accumulation of the β subunit of β-conglycinin increases in response to sulfur deprivation (CitationGayler and Sykes 1985) and decreases with the application of methionine (Met) or glutathione (GSH) (CitationAwazuhara et al. 2002b; CitationHolowach et al. 1984). These patterns of regulation have been previously reported in transgenic plants (CitationFujiwara et al. 1992; CitationHirai et al. 1994; CitationNaito et al. 1994). Furthermore, it has been shown that the DNA fragment corresponding to the –307 to –72 region of the β subunit gene promoter was capable of conferring Met and GSH regulation to cauliflower mosaic virus (CaMV) 35S RNA promoter in transgenic Arabidopsis (CitationOhkama et al. 2002; CitationSogawa et al. 2005). In the present study, we established a transient assay system using Arabidopsis thaliana protoplasts that regulates promoter activity of the β subunit of the β-conglycinin gene in a manner similar to that observed in transgenic plants.
MATERIALS AND METHODS
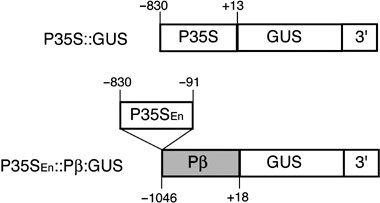
Protoplasts were prepared from liquid callus cultures of A. thaliana wild-type (ecotype Col-0) plants (CitationGuzman and Ecker 1998; CitationIshikawa et al. 1993). P35SEn:Pβ::β-glucuronidase (GUS) or P35S::GUS plasmids (; CitationAwazuhara et al. 2002a) were transfected by electoroporation as described in CitationChiba et al. (1999). P35SEn:Pβ::GUS carries the β-glucuronidase (GUS) gene driven by the β subunit promoter (Pβ; the –1046∼+18 region relative to the transcription start site) fused downstream of the –830∼–91 region (P35SEn) of the CaMV 35S RNA promoter. P35SEn includes the enhancer region of the promoter (CitationKay et al. 1987). The plasmid P35S::GUS that carries the GUS gene fused to the –830∼+13 region of the CaMV 35S RNA promoter (CitationAwazuhara et al. 2002a) was used as a control. The 221-LUC + plasmid carrying a modified firefly luciferase (LUC) gene fused directly to the CaMV 35S RNA promoter (CitationMatsuo et al. 2001) was co-transfected with a test plasmid to serve as an internal control for normalization of transfection. After transfection, half of the protoplasts were cultured in medium supplemented with 0.1 mmol L−1 Met (Wako Chemicals, Osaka, Japan) or 1 mmol L−1 GSH and the other half were cultured in medium without Met or GSH supplementation. The GUS and LUC activities were determined 48 h after transfection as described by CitationChiba et al. (1999), and GUS activity relative to LUC activity was calculated for each sample.
Figure 1 Schematic diagram of the plasmid constructs used in the transient assay (redrawn from Awazuhara et al. 2002a). P35S, the –830∼+13 region CaMV 35S RNA promoter; P35SEn, the –830∼–91 region of the CaMV 35S RNA promoter; Pβ, the –1046∼+18 region of the β subunit gene; GUS, β-glucuronidase Open Reading Frame (ORF); 3′, 3′ end of the α′ subunit gene of β-conglycinin (Adiputra and Anderson 1992).
RESULTS AND DISCUSSION
We used Arabidopsis protoplasts derived from calli and tested the ability of the chimeric gene construct carrying the β subunit gene promoter () to respond to Met in a transient assay system. P35SEn:Pβ::GUS or P35S::GUS plasmids were transfected by electoroporation. We have previously shown that construct P35SEn:Pβ::GUS responds to sulfur nutrition in non-seed tissues in transgenic A. thaliana (CitationAwazuhara et al. 2002a). The plasmid P35S::GUS was used as a control. In all the experiments described here, the plasmid carrying LUC gene fused directly to the CaMV 35S RNA promoter was co-transfected to serve as an internal control for normalization of transfection efficiency. After transfection, half of the protoplasts were cultured in medium supplemented with 0.1 mmol L−1 Met and the other half were cultured in medium without Met supplementation. The GUS and LUC activities were determined 48 h after transfection and GUS activity relative to LUC activity was calculated for each sample.
As shown in , relative GUS activity from P35SEn:Pβ::GUS was decreased by Met application to 48% of that without Met. Relative GUS activity from P35S::GUS with Met was not statistically different from that without Met. These results suggest that expression from the β subunit gene promoter is repressed by Met application in the present transient assay system using the protoplasts from the wild-type plants.
We also carried out transient assays using an A. thaliana mto1-1 mutant (Col-0 background) that overaccumulates soluble Met (CitationChiba et al. 1999; CitationOminato et al. 2002). Protoplasts were prepared from calli of wild-type Col-0 plants and mto1-1 plants cultured in standard medium. P35SEn:Pβ::GUS or P35S::GUS was transfected and relative GUS activity was determined at 48 h after transfection. As shown in , the relative GUS activities of P35SEn:Pβ::GUS were not significantly different between the wild type and mto1-1. GUS activities of P35S::GUS for wild type and mto1-1 were also not significantly different from each other. These results suggest that the mto1-1 mutation does not have a significant effect on transient expression of P35SEn:Pβ::GUS under our assay conditions.
Figure 2 Effect of methionine (Met) application on the expression of the β subunit gene in the transient assay. Plasmids carrying P35S::GUS or P35SEn:Pβ::GUS were transfected to protoplasts prepared from wild-type Col-0 (WT,□) and mto1-1 mutant (□) plants. The 221-LUC + plasmid was co-transfected as an internal control. Protoplasts were cultured in media with or without 0.1 mmol L−1 of Met for 48 h after transfection. The GUS activity relative to the LUC activity was calculated and the values were recalculated relative to the value from wild-type protoplasts transfected with P35S::GUS cultured without Met. (a) Effects of Met application in the wild-type protoplasts. (b) Effects of the mto1-1 mutation. (c) Effects of Met application in the mto1-1 mutant protoplasts. Means and standard deviations of three replicates are shown. Asterisks in panels (a, c) indicate significant differences between treatments with and without Met (Student's t-test, P < 0.05).
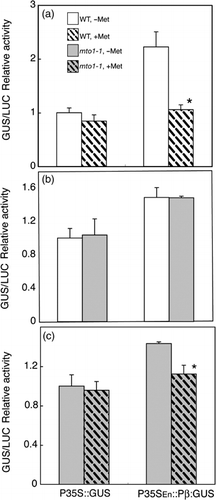
We examined the effects of exogenous application of Met in mto1-1. The transfection was carried out with protoplasts derived from mto1-1 calli and half of the protoplasts were cultured in medium with and half were cultured without 0.1 mmol L−1 of Met. Expression from the P35S::GUS did not significantly decrease with exogenous Met application, whereas GUS activity from P35SEn:Pβ::GUS in mto1-1 was significantly downregulated by exogenous Met (). These results suggest that P35SEn:Pβ::GUS responds to exogenous Met in the mto1-1 mutant () as well as in wild-type protoplasts ().
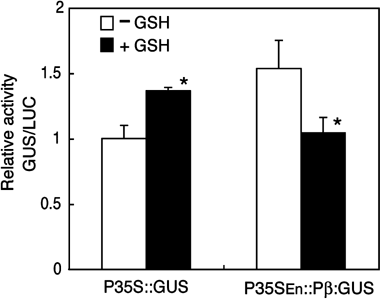
The β subunit gene has been also reported to be repressed by GSH application (CitationAwazuhara et al. 2002b; CitationSogawa et al. 2005). To determine if the β subunit gene also responds to GSH in our transient assay system, we tested the effects of GSH on P35SEn:Pβ::GUS expression in the protoplasts. After transfection, half of the protoplasts were cultured for 48 h in medium supplemented with 1 mmol L−1 GSH and the other half were cultured without GSH. Relative GUS activity from P35SEn:Pβ::GUS decreased with GSH application to 68% of that without GSH (). In contrast, expression of P35S::GUS was not repressed; rather it increased by 40% with GSH application (). These results suggested that the β subunit gene was downregulated by GSH application in our transient assay system.
It has been shown that P35SEn:Pβ::GUS expression is downregulated with mto1-1 mutation in transgenic plants (CitationOhkama et al. 2002). The results obtained in this study suggest that in the present transient assay system, the effect of the mto1-1 mutation on expression of the β subunit gene was different from the expression observed in transgenic plants, whereas the response to exogenously applied Met was similar to that in transgenic plants.
It is not clear why the response of P35SEn:Pβ::GUS to Met was evident when applied exogenously, but not when Met levels were internally elevated by the mto1-1 mutation. Met concentrations in calli of mto1-1 are approximately threefold those of wild-type calli (F. Kijima and S. Naito, unpubl. data, 1999). However, it was possible that the effect of the mto1-1 mutation on the free Met concentration in the cells was affected by protoplast isolation and subsequent cultures, and for proper repression by Met, it was necessary to supply Met in the media.
Figure 3 Effect of glutathione (GSH) application on the β subunit gene in the transient assay. Plasmids carrying P35S::GUS or P35SEn:Pβ::GUS were transfected to protoplasts prepared from wild-type (WT) plants. The 221-LUC + plasmid was co-transfected as an internal control. Protoplasts were cultured in medium with (▪) or without (□) 1 mmol L−1 GSH for 48 h after transfection. The GUS activity relative to the LUC activity was calculated for each sample. The values were recalculated relative to the value from protoplasts transfected with P35S::GUS and cultured without GSH. Means and standard deviations of three replicates are shown. Asterisks indicate significant difference between treatments with and without GSH (Student's t-test, P < 0.05).
In summary, we established a transient assay system for gene regulation by metabolites in the sulfur assimilation pathway. The present study provides a demonstration of the repression of the β subunit gene expression by Met or GSH application in a transient assay system. To our knowledge this is the first report of the regulation of seed storage protein genes by metabolites in the sulfur assimilation pathway in a transient assay system. With the use of the transient assay system established here, rapid identification of the cis-elements and trans-acting factors required for sulfur regulation of genes, including seed storage protein genes, becomes feasible.
ACKNOWLEDGMENTS
We are grateful to Kumi Fujiwara for general assistance and Dr Annita G. Peterson for critical reading of the manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan to S. Naito and T. Fujiwara.
Notes
Present addresses: †Department of Genetics, Development and Cell Biology, Iowa State University, Ames, IA 50011, USA
Tobacco Science Research Center, Japan Tobacco, Aoba-ku, Yokohama 227-8512, Japan.
Division of Life Science, Graduate School of Life Science, Hokkaido University, Sapporo 060-8589, Japan
Contributed equally.
REFERENCES
- Adiputra , IGK and Anderson , JW . 1992 . Distribution and redistribution of sulphur taken up from nutrient solution during vegetative growth in barley . Physiol Plant , 85 : 453 – 460 .
- Awazuhara , M , Kim , H Goto , DB . 2002a . A 235-bp region from a nutritionally regulated soybean seed-specific gene promoter can confer its sulfur and nitrogen response to a constitutive promoter in aerial tissues of Arabidopsis thaliana . Plant Sci , 163 : 75 – 82 .
- Awazuhara , M , Kim , H , Hayashi , H , Chino , M , Kim , S-G and Fujiwara , T . 2002b . Composition of seed storage proteins changed by glutathione treatment of soybeans . Biosci. Biotech. Biochem , 66 : 1751 – 1754 .
- Baudet , J , Huet , JC , Jolivet , E , Lesaint , C , Mosse , J and Pernollet , JC . 1986 . Changes in accumulation of seed nitrogen compounds in maize under condition in sulfur-deficiency . Physiol. Plant , 68 : 608 – 614 .
- Blagrove , RJ , Gillespie , JM and Randall , PJ . 1976 . Effect of sulphur supply on the seed globulin composition of Lupinus angustifolius . Aust. J. Plant Physiol , 3 : 173 – 184 .
- Chandler , PM , Higgins , TJV , Randall , PJ and Spencer , D . 1983 . Regulation of legumin levels in developing pea seeds under conditions of sulfur deficiency: rates of legumin synthesis and levels of mRNA . Plant Physiol , 71 : 47 – 54 .
- Chiba , Y , Ishikawa , M Kijima , F . 1999 . Evidence for autoregulation of cystathionine γ-synthase mRNA stability in Arabidopsis . Science , 286 : 1371 – 1374 .
- Evans , IM , Gatehouse , JA and Boulter , D . 1985 . Regulation of storage-protein synthesis in pea (Pisum sativum L.) cotyledons under conditions of sulphur deficiency . Biochem. J , 232 : 261 – 265 .
- Fujiwara , T , Hirai , MY , Chino , M , Komeda , Y and Naito , S . 1992 . Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia . Plant Physiol , 99 : 263 – 268 .
- Gayler , KR and Sykes , GE . 1985 . Effects of nutritional stress on the storage proteins of soybean . Plant Physiol , 78 : 582 – 585 .
- Gopalakrishnan , B , Sonthayanon , B , Rahmatullah , R and Muthukrishnan , S . 1991 . Barley aleurone layer cell protoplasts as a transient expression system . Plant Mol. Biol , 16 : 463 – 467 .
- Guzman , P and Ecker , JR . 1988 . Development of large DNA methods for plants: molecular cloning of large segments of Arabidopsis and carrot DNA in yeast . Nucl. Acids Res , 16 : 11091 – 11105 .
- Higgins , TJV . 1984 . Synthesis and regulation of major proteins in seeds . Annu. Rev. Plant Physiol , 35 : 191 – 221 .
- Higgins , TJV , Chandler , PM Randall , PJ . 1986 . Gene structure, protein structure and regulation of the synthesis of a sulfur-rich protein in pea seeds . J. Biol. Chem , 25 : 11124 – 11130 .
- Hirai , MY , Fujiwara , T , Chino , M and Naito , S . 1995 . Effects of sulfate concentration on the expression of a soybean seed storage protein gene and its reversibility in transgenic Arabidopsis thaliana . Plant Cell Physiol , 36 : 1331 – 1339 .
- Hirai , MY , Fujiwara , T , Goto , K , Komeda , Y , Chino , M and Naito , S . 1994 . Differential expression of soybean storage protein gene promoter-GUS fusions by exogenously applied methionine in transgenic Arabidopsis thaliana . Plant Cell Physiol , 35 : 927 – 934 .
- Holowach , LP , Thompson , JF and Madison , JT . 1984 . Effects of exogenous methionine on storage protein composition of soybean cotyledons cultured in vitro . Plant Physiol , 74 : 576 – 583 .
- Ishikawa , M , Naito , S and Ohno , T . 1993 . Effects of tom1 mutation of Arabidopsis on the multiplication of tobacco mosaic virus RNA in protoplasts . J. Virol , 67 : 5328 – 5338 .
- Kay , R , Chan , A , Daly , M and McPherson , J . 1987 . Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes . Science , 236 : 1299 – 1302 .
- Marocotte , WR , Bayley , CC and Quantrano , RS . 1988 . Regulation of a wheat promoter by abscisic acid in rice protoplasts . Nature , 335 : 454 – 457 .
- Maruyama-Nakashita , A , Nakamura , Y , Watanabe-Takahashi , A , Inoue , E , Yamaya , T and Takahashi , H . 2005 . Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsisroots . Plant J , 42 : 305 – 314 .
- Matsuo , N , Minami , M , Maeda , T and Hiratsuka , K . 2001 . Dual luciferase assay for monitoring transient gene expression in higher plants . Plant Biotechnol , 18 : 71 – 75 .
- Morton , RJ , Ellery , AJ and Higgins , TJV . 1998 . Downstream elements from the pea albumin 1 gene confer sulfur responsiveness on a reporter gene . Mol. Gen. Genet , 259 : 309 – 316 .
- Müller , M , Dues , G , Balconi , C , Salamini , F and Thompson , RD . 1997 . Nitrogen and hormonal responsiveness of the 22 kDa α-zein and b-32 genes in maize endosperm is displayed in the absence of the transcriptional regulator Opaque-2 . Plant J , 12 : 281 – 291 .
- Naito , S , Inaba-Higano , K Kumagai , T . 1994 . Maternal effects of mto1 mutation, that causes overaccumulation of soluble methionine, on the expression of a soybean β-conglycinin gene promoter-GUS fusion in transgenic Arabidopsis thaliana . Plant Cell Physiol , 35 : 1057 – 1063 .
- Ohkama , N , Goto , DB , Fujiwara , T and Naito , S . 2002 . Differential tissue-specific response to sulfate and methionine of a soybean seed storage protein promoter region in transgenic Arabidopsis . Plant Cell Physiol , 43 : 1266 – 1275 .
- Ominato , K , Akita , H Suzuki , A . 2002 . Identification of a short highly conserved amino acid sequence as the functional region required for posttranscriptional autoregulation of the cystathionine γ-synthase gene in Arabidopsis . J Biol. Chem , 227 : 36380 – 36386 .
- Randall , PJ , Thomson , JA and Schroeder , HE . 1979 . Cotyledonary storage proteins in Pisum sativam, IV, Effects of sulfur, phosphorus, potassium, and magnesium deficiencies . Aust. J Plant Physiol , 6 : 11 – 24 .
- Sogawa , Y , Ohkama-Ohtsu , N , Hayashi , H , Yoneyama , T and Fujiwara , T . 2005 . Independent roles of glutathione and O-acetyl-L-serine in regulation of sulfur-responsive gene expression in Arabidopsis thaliana . Plant Biotechnol , 22 : 51 – 54 .
- Spencer , D , Rerie , WG , Randall , PJ and Higgins , TJV . 1990 . The regulation of pea storage protein genes by sulfur stress . Aust J. Plant Physiol , 17 : 355 – 363 .
- Wrigley , CW , Cros , DL , Fullington , JG and Kasarada , DD . 1984 . Changes in polypeptide composition and grain quality due to sulfur deficiency in wheat . J Cereal Sci , 2 : 15 – 24 .
- Present addresses: †Department of Genetics, Development and Cell Biology, Iowa State University, Ames, IA 50011, USA
- Tobacco Science Research Center, Japan Tobacco, Aoba-ku, Yokohama 227-8512, Japan.
- Division of Life Science, Graduate School of Life Science, Hokkaido University, Sapporo 060-8589, Japan
- Contributed equally.