Abstract
Both selenium (Se) and antimony (Sb) are major soil and water pollutants. Their sorption behavior in a soil–plant system was studied. Soil–soil solution distribution coefficients (K ds) for Se and Sb were measured, using a radiotracer, as an indicator of their sorption levels. Both Se and Sb behave as oxoanions (SeO2− 4, H2PO− 4 and SO2− 4) in soil; thus, the effects of concentrations of two major oxoanions (SeO2− 4 and SeO2− 3) on Se and Sb sorption were also examined. The K d values for Se for Japanese soils significantly correlated with the K d values for Sb (n = 141). The K ds of both Se and Sb similarly decreased with increasing SbO− 3 concentration. These results indicated that the sorption of Se and Sb was similarly controlled by a ligand-exchange mechanism such as phosphate sorption in soil. However, an increase in the concentration of SeO2− 3 did not decrease the K ds of Se and Sb. Furthermore, the ligand-exchangeable fractions of stable Se and Sb in major Japanese soils were determined by extraction with 0.1 mol L−1 Na2HPO4 solution. For both Se and Sb, the phosphate-extractable fractions were 10-fold higher for Se and fivefold higher for Sb than their water-soluble fractions. Although the total Se and Sb amounts in soils were the same, their ligand-exchangeable fractions were different. Approximately 0.9–12% of total Se and 0.2–1.3% of total Sb were extracted by the phosphate solution. These findings suggested that Se was more likely to be mobilized by the addition of phosphate than Sb. The effect of plant-available phosphate in the soil and the phosphate sorption capacity of soil on Se and Sb availabilities for plants were also examined using a pot experiment with soybean plants. The experimental results suggested that a high content of available phosphate and/or low phosphate sorption capacity of soil increased both Se and Sb availabilities to the plant. However, the results also suggested that the soil Se availability to the plant was higher than that of Sb even though the soil total Se and Sb amounts were the same.
INTRODUCTION
Selenium (Se) and antimony (Sb) are naturally occurring trace elements, and both are major pollutants released from metal mining and smelting sites (CitationCrecelius et al. 1974; CitationLindsay and Spiers 2005; CitationRagaini et al. 1977). Both Se and Sb have been listed as priority pollutants by the US Environment Protection Agency (EPA) (CitationKeith and Telliard 1979). Selenium is an essential element for animals because it is required for normal enzyme function, however, it can cause blind staggers in animals that drink water or eat plants in areas with high Se contamination, such as that found in Kesterson Reservoir (CitationOhlendorf 1989). Antimony has toxic properties similar to arsenic. Antimony contamination has been reported near mining and smelting areas in Japan; for example, CitationAsami and Kubota (1993) found that the concentration of Sb reached more than 100 mg Sb kg−1 soil. In this paper, we have studied the sorption behavior of both Se and Sb in soil with respect to plant uptake of these elements. There were two objectives of the present study. One was to understand the environmental mobility of Se and Sb for risk assessment as a result of their contamination and the second was to evaluate the factors affecting Se availability for plants as an important nutrient for animals.
Previously we reported that both Se and Sb behave as oxoanions (SO2− 4 and SeO2− 4) in the Eh–pH range of Japanese soils, and that their sorption to soil was inhibited under high phosphate concentrations (CitationNakamaru et al. 2005a, Citation2006a,Citationb). In a soil environment, adsorption of some oxoanions is highly controlled by the ligand-exchange mechanism of Al/Fe-(hydr-)oxides. Many studies have reported the ligand-exchange of oxoanions such as H2PO− 4, SO2− 4, SeO2− 4 (CitationParfitt 1978; CitationYamaguchi et al. 1999) and SO2− 4 (CitationBalistrieri and Chao 1987; CitationHington et al. 1974; CitationParfitt 1978; CitationParfitt and Russell 1977; CitationRajan 1979; CitationRajan and Watkinson 1976; CitationSu and Suarez 2000) as a result of the presence of Al/Fe-(hydr-)oxides. In addition, the specific sorption of Sb as a result of the Al/Fe-(hydr-) oxides has been reported (CitationLintschinger et al. 1998; CitationMeima and Comans 1998). Therefore, it is probable that oxioanions such as H2PO− 4 behave as competing ions for the ligand exchange of Se and Sb. As H2PO− 4 and SO2− 4 are major components of fertilizer, their concentrations should affect the sorption of Se and Sb in agricultural fields. Thus, in this study, we used a radiotracer experiment to study the sorption behavior of Se and Sb in the presence of H2PO− 4 and SO2− 4.
As both Se and Sb sorption is affected by ligand exchange, a portion of the native Se and Sb in soil should exist as ligand-exchangeable forms. The ligand-exchangeable Se and Sb may become mobile with the addition of other oxoanions such as phosphate. To evaluate the potentially mobile Se and Sb amounts in different soils, we carried out an extraction experiment for 24 soil samples using 0.1 mol L−1 Na2HPO4 solution.
Furthermore, the effects of Se and Sb sorption behavior on the uptake of Se and Sb by plants were evaluated as a function of phosphate ions. A pot cultivation experiment of soybean plants was carried out to determine the effects of phosphate ions on the Se and Sb uptake by the plants.
MATERIALS AND METHODS
Soil samples
Soil samples were collected throughout Japan. One hundred and forty-one samples (69 upland soil samples and 72 paddy soil samples) were used for the soil–soil solution distribution coefficient (K d) analysis in Experiment 1. Soil samples were taken from the surface layer (0–20-cm depth). Samples were air-dried and passed through a 2-mm mesh sieve before experiments and analyses were carried out. In the present study, samples were classified into four soil groups using the soil classifications of CitationFood and Agriculture Organization United Nations Educational Scientific and Cultural Organization (1990). lists the numbers of samples for each soil group, their K d ranges for Se and Sb, and their corresponding (FAO-UNESCO) FAO-UNESCO classification and local classification in Japan (CitationCultivated Soil Classification Committee 1995). The Andosol group made up approximately half of the Japanese upland soil samples and the Fluvisol group contained most of the rice paddy soil samples. From the collected soils, 24 sample soils were chosen to include all major soil types in Japan, and they were used for experiment 2 and experiment 3. The chemical characteristics of these 141 soil samples are summarized in . Acid oxalate extractable Al and Fe contents were measured using the method of CitationBlakemore et al. (1981). Acid oxalate extractable Al and Fe contents refer to the amount of Al/Fe-(hydr)oxides and to the Al/Fe–humus complex. They are regarded as reactive components for anion adsorption (CitationParfitt 1978). The cation exchange capacity (CEC) was determined using the semi-micro Schollenberger method (CitationSchollenberger and Simon 1945). Total carbon (C) content in the soils was measured with a CHNS-analyzer (Euro EA3000; EuroVector S.p.A., Milan, Italy).
Table 1 Soil samples used in Experiment 1
Table 2 Characteristics of the soil samples used in this study†
Experiment 1: Measurement of the distribution coefficient (K d) using the batch process
We measured the K ds of Se and Sb (K d-Se and K d-Sb) for all soil samples by the batch process using 75Se (half life: 119.8 days) or 124Sb (half life: 60.2 days) as a tracer. Three-gram amounts of a prepared soil sample were placed into a 50-mL plastic bottle and 30 mL of deionized water was added. Prior to the addition of the radiotracer, the suspension was shaken at 120 r.p.m. for 24 h at 23°C using an end-over-end shaker. Approximately 10 kBq of 75Se or 30 kBq of 124Sb were then added to the suspension. The suspension was shaken for 7 days. The 7-day shaking time was sufficient to achieve equilibrium of Se and Sb adsorption for the soils used in this experiment (CitationNakamaru et al. 2005a, Citation2006b). The tracer, 75Se or 124Sb, was added as H2SeO3 or SbCl3, respectively, with 21 ng of stable Se or 0.3 µg of stable Sb as the carrier. In this experiment, selenite was selected as the Se source because it was considered to be the major Se form in Japanese soil (CitationAsagawa et al. 1977; CitationMaeta and Mizuno 1993; CitationYamada et al. 1998).
The sample suspension was separated by centrifugation at 1,000 G, and the supernatant was removed and filtered though a 0.45-µm membrane filter. Gamma rays of 75Se or 124Sb in the filtrate were measured with a NaI scintillation counter (ARC-300; Aloka, Tokyo, Japan) to determine their equilibrium concentration. For the 141 tested soil samples, the pH range of the batch solution was 4–8, and the Eh range was 300–450 mV from 1 day to 7 days after shaking. In this pH–Eh condition, the stable Sb chemical form is SeO2− 4 (CitationPourbaix 1974). Thus, the added SbCl3 changed to SeO2− 4 during the shaking period because most of the Sb tracer in the batch solution was the anion after 7 days (CitationNakamaru et al. 2006b).
K d (L kg−1) was calculated using the following equation:
where Ci is the initial concentration of radiotracer in the solution (Bq L−1), Cf is the concentration of the tracer in the supernatant solution after shaking with soil (Bq L−1), V is the solution volume (L) and M is the soil mass (kg).
Experiment 2: Determination of the effects of phosphate and sulfate concentrations on Se and Sb sorption onto soils
To evaluate the effects of H2PO− 4 and SO2− 4 on the sorption of Se and Sb we measured the K d values for Se and Sb under elevated concentrations of (NH4)2SO4 and KH2PO4.
Table 3 Chemical properties of the soils used for the pot cultivation experiment
For two samples, A1 and F1, the K d-Se and K d-Sb were measured under elevated concentrations of phosphate or sulfate. The phosphate and sulfate concentrations were adjusted with 0.1 mol L−1 KH2PO4 buffer solution (pH 4.5) and 0.1 mol L−1 (NH4)2SO4 solution (pH 5.3), respectively. Three grams of each soil sample was placed into a plastic bottle with 30 mL of KH2PO4 or (NH4)2SO4 solution (0, 0.1, 1, 5 or 10 mmol L−1 PO4 or SO4). With the KH2PO4 and (NH4)2SO4 solutions, the pH condition of the batch solution was not changed from that of the K d measurement of Experiment 1. As described above, K ds were measured by adding 75Se or 124Sb to this suspension. Each sample suspension was separated by centrifugation and filtration though a 0.45-µm membrane filter and the activity concentration of added tracer in the filtrate was measured.
Experiment 3: Determination of the ligand-exchangeable Se and Sb in soils
To evaluate the potentially mobile Se and Sb amounts in soils, we carried out an extraction experiment for the 24 soil samples listed in .
Native Se and Sb in the samples were extracted using a procedure modified from the method of CitationZawislanski et al. (2003). Three grams of air-dried and sieved soil sample was placed into a 50-mL plastic bottle. Then, 30 mL of 0.1 mol L−1 Na2HPO4 was added to the soil, and the bottle was shaken for 24 h to extract the ligand-exchangeable Se and Sb fractions. The suspension was centrifuged at 1,000 G and the supernatant was removed and filtered though a 0.45-µm membrane filter before measuring the extracted Se and Sb using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7500c; Yokogawa Electric Corporation, Tokyo, Japan). For comparison, another extraction with H2O was also carried out under the same extraction conditions to evaluate the water-soluble Se and Sb in the soils.
Experiment 4: Pot cultivation experiment of soybean plants
To evaluate the effects of soil-available phosphate and phosphate sorption capacity of soil on Se and Sb availabilities to plants, we carried out a pot cultivation experiment.
For the pot experiment, Andosol samples were used because of their high reactivity toward phosphate ions. Two different types of Andosol samples were collected from the Abashiri area. For comparison, we also used the results of a similar experiment from our previous study (CitationNakamaru et al. 2005b) with a third type of Andosol sample (Kawatabi-C). The properties of the soils used in experiment 4 are shown in . The plant-available phosphate amount was measured using the Truog method (CitationBlakemore et al. 1981), and the phosphate sorption capacity was measured using the method of CitationNanzyo et al. (1998). All three soils were categorized as Andosol, but their available phosphate contents and phosphate retention capacities were different. Abashiri-C soil had a high available phosphate level and low phosphate sorption capacity. Kawatabi-C soil was a non-allophanic Andosol and had a higher phosphate retention capacity than the other soils (CitationShoji et al. 1993).
The soybean plant (Glycin max (L.) Merrill) was selected as the test plant because some legumes, such as Astragalus sp., are known to accumulate Se to an extremely high level (CitationEhlig et al. 1968).
Two kilograms of soil was placed into each of 12 5-L plastic pots. The soil water content was adjusted daily with deionized water to field capacity (345 g H2O kg−1 dry soil).
At 1-day before sowing, all pots were fertilized with 200 mL of nutrient solution (25 mmol L−1 KNO3, 25 mmol L−1 Ca (NO3)2, 10 mmol L−1 MgSO4, 5 mmol L−1 KH2PO4) according to CitationHoagland and Arnon (1950). Four soybean seeds were sown into each of the 12 pots. The pots were placed in a greenhouse and the plants were grown for 99 days. Plants were grown with and without Se (+Se and –Se plots) added to each soil. For the +Se application, 20 mL of selenite solution (1 mg Se L−1) was added to the pots.
Soybean seeds were collected when the maturing stage was reached (99 days). Plant material and soil samples were ground in an agate mill. Then, the samples were digested with a mixture of concentrated HNO3 and HF using a microwave digestion system (CEM Mars 5; CEM Corporation, Matthews, NC, USA). The concentrations of Se and Sb in the soil samples and in the soybean seeds were measured using ICP-MS (Agilent 7500c; Yokogawa).
RESULTS AND DISCUSSION
K d ranges of Se and Sb for the tested soils
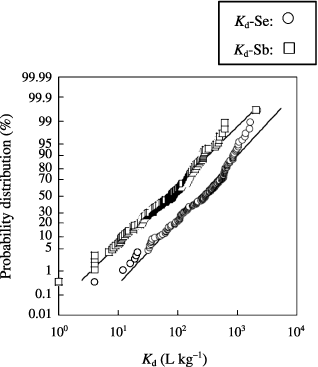
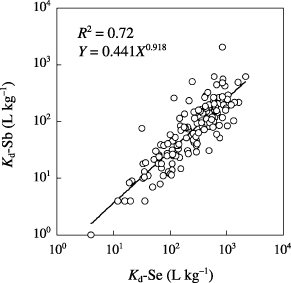
The K d ranges for the 141 soil samples are shown in . A K d range of 4–2,130 L kg−1 was observed for Se (80–99.95% of added Se was sorbed) and the geometric mean was 223 L kg−1 (99.55% of added Se was sorbed). For Sb, the K d range was 1–2,065 L kg−1 (50–99.95% of added Sb was sorbed) and the geometric mean was 65 L kg−1 (98.48% of added Sb was sorbed). The K d ranges determined in the present study were similar to those reported in the CitationInternational Atomic Energy Agency Report (1994). Although a high K d-Sb value of 2,065 was observed for one sample, K d-Sb values tended to be smaller than those for Se. The probability distributions of the K ds are shown in . From the Kolmogrov–Smirnov–Lilliefors test, the K d distributions for both Se and Sb were judged to be log-normal types. The logarithms of the K d values for Se and Sb were correlated significantly (). This indicated that the K ds of Se and Sb had a similar trend, although the K d level of Se was generally higher than that of Sb. In our previous study, we showed that the major chemical forms of Se and Sb present during the batch process were oxoanions (SO2− 4 and SeO2− 4) (CitationNakamaru et al. 2005a, Citation2006b). The primary adsorption mechanism of selenite (SO2− 4) has been regarded as ligand exchange (CitationParfitt 1978). Selenite has been shown to behave analogously to phosphate (CitationRajan 1979; CitationRajan and Watkinson 1976; CitationNakamaru et al. 2006a; CitationNeal et al. 1987a,Citationb). Although very little is known about Sb, sorption of Sb (III) and Sb (V) onto a goethite surface to form an inner-sphere complex (CitationLeuz et al. 2006) and high extractability of Sb by 0.1 mol L−1 Na2HPO4 solution (CitationEttler et al. 2007) have been reported. Therefore, from the results of this experiment, we hypothesized that both Se and Sb behaved as ligand-exchangeable oxoanions similar to phosphate. However, the results also suggested that the sorption level of Sb was lower than that of Se even with the same number of ligand-exchange sites.
Effect of phosphate concentration on the K ds of Se and Sb
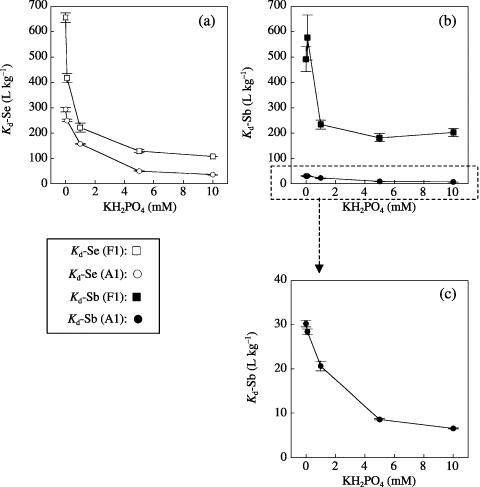
For both Se and Sb sorption, we determined the effect of phosphate concentration from the K d-Se and K d-Sb measurements for elevated concentrations of KH2PO4. shows the changes in K d-Se and K d-Sb with different concentrations of KH2PO4. The addition of phosphate solution decreased the K d-Se and K d-Sb values significantly. This indicated that the H2PO− 4 ion inhibited SO2− 4 and SeO2− 4 sorption as a competitor for the ligand-exchange reaction. However, the sharpness of the response when increasing the PO4 concentration was stronger for Se than for Sb. For Se, a significant K d decrease was observed with only 0.1 mmol L−1 PO4 and no significant decrease was observed for K d-Sb at that phosphate level. The phosphate level sufficient for inhibition of Se and Sb sorption was 1 mmol L−1 PO4. The added phosphate level for 30 mL of 1 mmol L−1 PO4 solution corresponded to 0.3 g P kg−1 dry soil. This value was the same level as that in phosphate fertilizer commonly applied to paddy fields in Japan (0.2–0.5 g P kg−1 dry soil). Our results suggested that mobilization of Se and Sb by phosphate addition could occur in agricultural fields. Thus, phosphate fertilization could make the uptake of Se and Sb by crops easier and could increase the risk from Se and Sb in contaminated soil. However, Se is an essential nutrient for animals. Our results also suggested that we could raise the amount of Se nutrient in agricultural products by phosphate fertilization of the soil.
Figure 3 Changes in the soil–soil solution distribution coefficients of (a) selenium (Kd-Se) and (b,c) antimony (Kd-Sd) of Fluvisol (F1: , ) and Andosol (A1: , ) plotted against the phosphate concentration in the soil solution under elevated levels of PO4 concentration. Error bars indicate the standard deviation of three replicates.
Effect of sulfate concentration on the K ds of Se and Sb
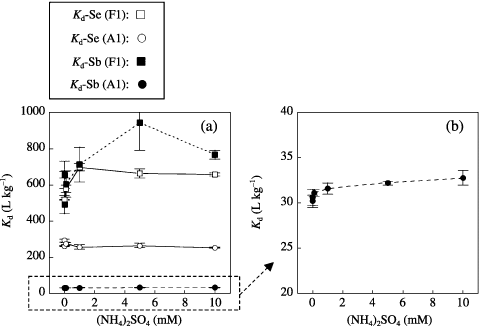
We also studied the effect of sulfate concentration on the sorption behavior of Se and Sb. The changes in K d-Se and K d-Sb with different concentrations of (NH4)2SO4 are shown in . No decrease of K d values was observed for either Se or Sb when sulfate was added. For K d-Sb of the Fluvisol sample (F1), the K ds slightly increased with increasing sulfate concentration. This increase should be caused by the decrease in pH associated with the addition of (NH4)2SO4. For F1, the final pH value decreased from 6.3 to 5.9 when SO4 was increased from 0 to 5 mmol L−1. From these results, it seemed that sulfate concentration did not affect Se and Sb sorption in soil.
While sorption behavior of selenate to oxide surfaces has been reported to be similar to that of sulfate (CitationNeal and Sposito 1989; CitationWijnja and Schulthess 2000), the chemical form of Se in our experimental system was regarded as selenite (CitationNakamaru et al. 2005a). The relative retainment of sulfate on aluminum oxides has been reported to be smaller than that of selenite and phosphate (CitationRajan and Watkinson 1976; CitationWu et al. 2000). Although less information is available for Sb, it is known that Sb (III) and Sb (V) can form an inner-sphere complex with goethite surface similar to selenite (CitationLeuz et al. 2006). However, in the pH condition of this experiment, SO2− 4 predominantly forms an outer-sphere surface complex with Al and Fe oxides (CitationWijnja and Schulthess 2000). Therefore, we considered that the adsorption strength of SO2− 4 onto ligand-exchange sites was much smaller than that of SO2− 4 and SeO2− 4.
Ligand-exchangeable fractions of native Se and Sb in soil
The ligand-exchangeable Se and Sb fractions in Japanese agricultural soils were determined using an extraction experiment with 0.1 mol L−1 Na2HPO4 solution. The results are listed in . For the tested soils, the total contents and the soluble fractions of native Se and Sb were the same. Total-Se and total-Sb contents were 0.2–1.0 mg kg−1 and 0.3–1.4 mg kg−1, respectively. In addition, both the soluble-Se and soluble-Sb contents were 0.2–3.2 µg kg−1. These values were within the ranges measured for world soils, that is, 0.02–1.9 mg kg−1 and 0.05–4.0 mg kg−1 for total-Se and total-Sb, respectively (CitationKabata-Pendias 2001). For both elements, the phosphate-extractable fractions were significantly higher than the soluble fractions. However, the phosphate-extractable Se and Sb fractions were different. For Se, 0.9–12% of total Se was phosphate extractable. In contrast, the value for Sb was approximately 10-fold lower than that of Se; 0.2–1.3% of total Sb was extracted by the phosphate solution.
Table 4 Soil–soil solution distribution coefficients (Kd) and the stable selenium and antimony amounts in each extracted fraction of the tested soils
Figure 4 Changes in the soil–soil solution distribution coefficients of (a) selenium (Kd-Se) and (a,b) antimony (Kd-Sd) of Fluvisol (F1: , ) and Andosol (A1: , ) plotted against the sulfate concentration in the soil solution under elevated levels of SO4 concentration Error bars indicate the standard deviation of three replicates.
CitationEttler et al. (2007) tested five reagents (H2O, 0.01 mol L−1 CaCl2, 1 mol L−1 NH4NO3, 0.005 mol L−1 diethylene-triamine-pentaacetic acid and 0.1 mol L−1 Na2HPO4) for Sb extraction from Sb-contaminated soils, and reported that the highest extractability was observed for Na2HPO4 (1–9% of total Sb was extracted). They also reported that Sb (V) was the predominant Sb species in the extracted Sb. CitationFuentes et al. (2003) also reported that most of the soil-extracted Sb (H2O, 0.05 mol L−1 ethylenediaminetetraacetic acid and 0.25 mol L−1 H2SO4 extraction) was Sb (V). These reports suggested that Sb (III) species tended to be less extractable than Sb (V) and tended to be in the residual fraction. Therefore, the lower extractability of Sb compared with Se could result from the Sb chemical forms in soil. For Japanese soils, 14–60% and 3–18% of total Se were reported as selenite and selenate, respectively (CitationYamada et al. 1998). As selenite and selenate may be present in the ligand-exchangeable fraction in soil (CitationBalistrieri and Chao 1987; CitationHington et al. 1974; CitationParfitt 1978; CitationParfitt and Russell 1977; CitationRajan 1979; CitationRajan and Watkinson 1976; CitationSu and Suarez 2000), the proportion of selenite and selenate could be sufficient to explain the ligand-exchangeable Se amount.
These results indicated that significant mobilization could occur for both Se and Sb in soil with phosphate addition, and the potentially mobile Sb amount was smaller than that of Se, although the total amounts of Se and Sb were the same level. Although the sorption level of Se onto soil was generally higher than that of Sb, Se was more likely than Sb to become mobile with the addition of phosphate.
Se and Sb uptake by plants as a function of phosphate ions
Sorption of both Se and Sb was affected by phosphate ions in the soil. Therefore, the effects of Se and Sb sorption on their uptake by plants were evaluated as a function of the amount of phosphate ions.
The Se and Sb contents of soybean seeds are listed in . The contents of both Se and Sb in soybean seeds were affected by soil phosphate sorption capacity. Significantly higher amounts of Se and Sb were found in the seeds of soybean plants grown in Abashiri-C and Abashiri-F soil samples than in the seeds of soybean plants grown in the Kawatabi-C soil sample. Therefore, we thought that the Se and Sb availabilities for plants were high in soils with a high available amount of phosphate and/or low phosphate sorption capacity. The phosphate sorption capacity should also indicate the sorption capacity of Se, and the Se sorption capacity would be the dominant factor controlling Se availability to the plant. In contrast, no increase in Se uptake by soybean plants was observed with the addition of Se. Thus, we considered that the added Se was sorbed immediately to the soil and the amount (100 µg Se kg−1 soil) was not sufficient to increase Se uptake by soybean plants.
Table 5 Selenium and antimony contents of the soybean plants in Experiment 4
The amount of plant-available phosphate in the soil would also affect Se availability to the plant because Se concentration levels were highest for the Abashiri-C sample. However, such an effect was not significant for Sb. The Se content in soybean seeds was 10-fold higher than the Sb content (), although the soil Se and Sb contents were the same (). This indicated that soil Se availability to soybean plants was higher than that of Sb. According to the results of Experiment 2 and Experiment 3, the ligand-exchangeable Sb fraction was smaller than that for Se. This is one factor that may explain the lower availability of Sb than Se for soybean plants because the ligand-exchangeable Se and Sb fractions could be made available for the plants by phosphate fertilization.
Conclusions
The K ds of Se and Sb showed a similar trend, although the K d level of Se was generally higher than that of Sb. The addition of phosphate decreased the K d-Se and K d-Sb values significantly, although the addition of SO2− 4 had no effect. This indicated that H2PO− 4 ions specifically inhibited SO2− 4 and SeO2− 4 sorption as a competitor for the ligand-exchange reaction. Therefore, the sorption behavior of Se and Sb should be affected by the number of ligand-exchange sites and by the available phosphate in the soil. The extraction of ligand-exchangeable Se and Sb fractions in Japanese agricultural soils showed that significant mobilization could occur for both Se and Sb in soils with phosphate addition. However, the potentially mobile Sb amount was smaller than that of Se, although the total amounts of Se and Sb in the soil were the same. The difference between Se and Sb sorption behavior would result from the different chemical forms of Se and Sb in the soil.
Moreover, the results of the pot experiment suggested that both Se and Sb availabilities for soybean plants were high in soils with high available phosphate and/or low phosphate sorption capacity, although soil Se availability to soybean plants was higher than that of Sb. The ligand-exchangeable Se and Sb amounts in soil were considered to be an important factor influencing the difference between the Se and Sb availabilities to plants. However, many factors determining Se and Sb availabilities to plants are still unclear, further study is needed to clarify their bioavailabilities in different soils.
ACKNOWLEDGMENTS
This work has been partially supported by the Takano Life Science Research Foundation, Japan. The authors thank Dr S. Uchida and Dr K. Tagami (National Institute of Radiological Sciences, Japan) for their kind advice on the radiotracer experiment and for the analysis of the trace elements.
REFERENCES
- Asagawa , M , Kusizaki , M and Ishizuka , J . 1977 . Distribution and behavior of selenium in Japanese grasslands (II) . Nihon-Dojoh-Hiryougaku-Zasshi (Jpn. J. Soil Sci. Plant Nutr.) , 48 : 293 – 296 . (in Japanese)
- Asami , T and Kubota , M . 1993 . Antimony and bismuth pollution of soils in Japan . Intern. Conf., Heavy Metals Environ , 2 : 140 – 143 .
- Balistrieri , LS and Chao , TT . 1987 . Selenium adsorption by goethite . Soil Sci. Soc. Am. J , 51 : 1145 – 1151 .
- Blakemore , LC , Searle , PL and Daly , BK . 1981 . Methods for Chemical Analysis of Soils , New Zealand Soil Bureau Scientific Report 10A Lower Hutt City : Department of Scientific and Industrial Research .
- Crecelius , EA , Johnson , CJ and Hofer , GC . 1974 . Contamination of soils near a copper smelter by arsenic, antimony and lead . Water Air Soil Pollut , 3 : 337 – 342 .
- Cultivated Soil Classification Committee . 1995 . New Classification of Cultivated Soils in Japan , National Institute for Agro-Environmental Sciences Annual Report 17 69 – 76 . Tsukuba : National Institute for Agro-Environmental Sciences .
- Ehlig , CF , Allaway , WH , Cary , EE and Kubota , J . 1968 . Differences among plant species in selenium accumulation from soil low in available selenium . Agron. J , 60 : 43 – 47 .
- Ettler , V , Mihaljevic , M , Sebek , O and Nechutny , Z . 2007 . Antimony availability in highly polluted soils and sediments – A comparison of single extractions . Chemosphere , 68 : 455 – 463 .
- Food , Agriculture Organization–United Nations Educational Scientific and Cultural Organization . 1990 . Soil Map of the World Revised Legend , World Soil Resources Report 60 Rome : Food and Agriculture Organization .
- Fuentes , E , Pinochet , H , Gregori , ID and Potin-Gautier , M . 2003 . Redox speciation analysis of antimony in soil extracts by hydride generation atomic fluorescence spectrometry . Spectrochim. Acta Part B , 58 : 1279 – 1289 .
- Hington , FJ , Posner , AM and Quirk , JP . 1974 . Anion adsorption by goethite and gibbsite II. Desorption of anions from hydrous oxide surfaces . J. Soil Sci , 25 : 16 – 26 .
- Hoagland , DR and Arnon , DI . 1950 . The Water Culture Method for Growing Plants without Soil Circ , Vol. 347 , Barkley : California Agricultural Experiment Station .
- International Atomic Energy Agency . 1994 . Handbook of Parameter Values for the Prediction of Radionuclide Transfer in Temperate Environments , IAEA Technical Reports Series, No. 364 Vienna : IAEA .
- Kabata-Pendias , A . 2001 . Trace Elements in Soils and Plants , 3rd edn , Boca Raton : Chemical Rubber Company Press .
- Keith , LH and Telliard , WA . 1979 . Priority pollutants . Environ. Sci. Technol , 13 : 416 – 423 .
- Leuz , A , Monch , H and Johnson , A . 2006 . Sorption of Sb and Sb (V) to goethite: influence on Sb (III) oxidation and mobilization . Environ. Sci. Technol , 40 : 7277 – 7282 .
- Lindsay , AR and Spiers , G . Speciation and fractionation of selenium in smelter contaminated soils . Proc of 8th International Conference on the Biogeochemistry . April 3–7 2005 , Adelaide, Australia.
- Lintschinger , J , Michalke , B , Schulte-Hostede , S and Schramel , P . 1998 . Studies on speciation of antimony in soil contaminated by industrial activity . Int. J. Environ. Anal. Chem , 72 : 11 – 25 .
- Maeta , Y and Mizuno , N . 1993 . Selenium concentrations in soils and grasses of horse breeding farms in eastern Hidaka district, Hokkaido . J. Japan. Grassl. Sci , 39 : 147 – 154 .
- Meima , JA and Comans , RNJ . 1998 . Reducing Sb-leaching from municipal solid waste incinerator bottom ash by addition of sorbent minerals . J. Geochem. Explor , 62 : 299 – 304 .
- Nakamaru , Y , Tagami , K and Uchida , S . 2005a . Distribution coefficient of selenium in Japanese agricultural soils . Chemosphere , 58 : 1347 – 1354 .
- Nakamaru , Y , Tagami , K and Uchida , S . 2005b . Depletion of selenium in soil solution due to its enhanced sorption in the rhizosphere of soybean . Plant Soil , 278 : 293 – 301 .
- Nakamaru , Y , Tagami , K and Uchida , S . 2006b . Antimony mobility in Japanese agricultural soils and the factors affecting antimony sorption behavior . Environ. Pollut , 141 : 321 – 326 .
- Nakamaru , Y , Tagami , K and Uchida , S . 2006a . Effect of phosphate addition on the sorption–desorption reaction of selenium in Japanese agricultural soils . Chemosphere , 63 : 109 – 115 .
- Nanzyo , M , Nakamaru , Y , Ito , T and Yamasaki , S . 1998 . Relationship between forms of active aluminum and iron, and phosphate retention capacity free from precipitation of Ca and Mg phosphates . Jpn. J. Soil Sci. Plant Nutr , 69 : 144 – 149 .
- Neal , RH and Sposito , G . 1989 . Selenate adsorption on alluvial soils . Soil Sci. Soc. Am. J , 53 : 70 – 74 .
- Neal , RH , Sposito , G , Holtzclaw , KM and Traina , SJ . 1987a . Selenite adsorption on alluvial soils: I. Soil composition and pH effects . Soil Sci. Soc. Am. J , 51 : 1161 – 1165 .
- Neal , RH , Sposito , G , Holtzclaw , KM and Traina , SJ . 1987b . Selenite adsorption on alluvial soils: II. Solution composition effects . Soil Sci. Soc. Am. J , 51 : 1165 – 1169 .
- Ohlendorf , HM . 1989 . “ Bioaccumlation and effects of selenium in wildlife ” . In Selenium in Agriculture and Environment , SSSA Special Publication No. 23 Edited by: Jacobs , LW . 133 – 177 . Madison : American Society of Agronomy: Soil Science Society of America .
- Parfitt , RL . 1978 . Anion absorption by soils and soil materials . Adv. Agron , 30 : 1 – 50 .
- Parfitt , RL and Russell , JD . 1977 . Adsorption on hydrous oxides. IV. Mechanisms of adsorption of various ions on goethite . J. Soil Sci , 28 : 297 – 305 .
- Pourbaix , M . 1974 . “ Antimony ” . In Atlas of Electrochemical Equilibria , 524 – 532 . Houston : National Association of Corrosion Engineers .
- Ragaini , RC , Ralston , HR and Roberts , N . 1977 . Environmental trace metal contamination in Kellogg, Idaho, near a lead smelting complex . Environ. Sci. Technol , 11 : 773 – 781 .
- Rajan , SSS . 1979 . Adsorption of selenite, phosphate and sulphate on hydrous alumina . J. Soil Sci , 30 : 709 – 718 .
- Rajan , SSS and Watkinson , JH . 1976 . Adsorption of selenite and phosphate on allophane clay . Soil Sci. Soc. Am. J , 40 : 51 – 53 .
- Schollenberger , CJ and Simon , RH . 1945 . Determination of exchangeable capacity and exchangeable bases in soils . Soil Sci , 59 : 13 – 24 .
- Shoji , S , Nanzyo , M and Dahlgren , RA . 1993 . Volcanic Ash Soils – Genesis, Properties and Utilization , New York : Elsevier .
- Su , C and Suarez , DL . 2000 . Selenate and selenite sorption on iron oxides: an infrared and electrophoretic study . Soil Sci. Soc. Am. J , 64 : 101 – 111 .
- Wijnja , H and Schulthess , P . 2000 . Vibrational spectroscopy study of selenate and sulfate adsorption mechanisms on Fe and Al (hydr)oxide surfaces . J. Colloid Interface Sci , 229 : 286 – 297 .
- Wu , CH , Lo , SL and Lin , CF . 2000 . Competitive adsorption of molybdate, chromate, sulfate, selenate, and selenite on γ-Al2O3 . Colloids Surf. A , 166 : 251 – 259 .
- Yamada , H , Kang , Y , Aso , T , Uesugi , H , Fujimura , T and Yonebayashi , K . 1998 . Chemical forms and stability of selenium in soil . Soil Sci. Plant Nutr , 44 : 285 – 391 .
- Yamaguchi , NU , Okazaki , M and Hashitani , T . 1999 . Volume changes due to SO2- 4, SeO2- 4 and H2 PO- 4adsorption on amorphous iron(III) hydroxide in an aqueous suspension . J. Colloid Interface Sci , 209 : 386 – 391 .
- Zawislanski , PT , Benson , SM , Terberg , R and Borglin , SE . 2003 . Selenium speciation, solubility, and mobility in land-disposed dredged sediments . Environ. Sci. Technol , 37 : 2415 – 2424 .