Abstract
Potassium chlorate is widely used as an active substance for flower induction in longan plantation fields for the off-season production of fruits in northern Thailand. The effects of the application of chlorate on the mineralization of organic matter in soil, nitrification in soil and the number of culturable soil bacteria were examined. No effect or a very small effect of chlorate on the mineralization of organic matter and soil bacterial number was observed, whereas nitrification was significantly suppressed. Suppression of nitrification should be taken into consideration as a possible side-effect of potassium chlorate in longan plantation soil.
INTRODUCTION
Chlorate (ClO− 3) has been used as a non-selective contact herbicide, defoliant and disinfectant in agriculture (CitationAgaev et al. 1986). Chlorate may cause hemolytic anaemia in humans (CitationCondie 1986; CitationDaniel et al. 1990) and is highly toxic to certain brown macroalgal species (Citationvan Wijk and Hutchinson 1995). As chlorate can appear in drinking water as a by-product of disinfectants such as chlorine dioxide, a provisional guideline value of 0.7 mg L−1 is proposed in the third edition of the World Health Organization Guidelines for Drinking-Water Quality (http://www.who.int/water_sanitation_health/dwq/gdwq0506.pdf).
In the longan plantation fields of northern Thailand, potassium chlorate (KClO3) has been applied to the soil as an active substance for flower induction, which enables the off-season production of longan fruits (CitationManochai et al. 2001). Our field survey (CitationOngprasert et al. 2002) revealed that the concentration of chlorate in the soil just after application was as high as 140–340 mg kg−1 soil. The survey also indicated the presence of residual chlorate in the field soils and contamination by chlorate of groundwater in the fields, suggesting its adverse effect not only on the soil and water ecosystems, but also on human health.
We previously reported the persistence of chlorate in soils collected from several longan plantation fields in Northern Thailand (CitationSutigoolabud et al. 2004a,Citationb). Our results indicated that chlorate disappeared from the soil because of microbial reduction to chloride ions, and that under upland soil conditions the disappearance of chlorate was relatively fast when it was added at a concentration of 34 mg kg−1 soil and very slow when the compound was added at a concentration of 341 mg kg−1 soil.
As a result of its oxidizing activity and disinfecting properties, the application of chlorate to soil may affect soil microorganisms and microbial activity. However, only limited information is available on the effect of chlorate on soil microorganisms in soil (CitationKarki et al. 1973). Therefore, we investigated how the above-mentioned levels of chlorate application affect soil respiration and soil bacterial number in longan field soils.
Chlorate shows an inhibitory effect on the nitrification process, especially nitrite oxidation to nitrate (CitationBelser and Mays 1980; CitationHynes and Knowles 1983; CitationLees and Quastel 1945). As a result, chlorate at a concentration of 2.5 g kg−1 soil is used as an inhibitor of nitrification in estimating the nitrification potential in soil (CitationKandeler 1996). Thus, we also examined how a lower concentration of chlorate affects soil nitrification activity in longan plantation fields.
MATERIALS AND METHODS
Effect of the application of chlorate on the mineralization of organic matter in soil
Three types of soil samples, Pak Chong (PC) soil, Tha Muang/Sanphaya (TMS) soil and Num Pong (NP) soil, were collected from longan plantation fields in the Chiang Mai and Lumphun Provinces of northern Thailand. These soils were selected as representative soils with high (PC soil), medium (TMS soil) and low (NP soil) clay and organic matter contents. The soil samples were taken from the surface layer (0–150 mm), air-dried and passed through a 2-mm mesh sieve. The chemical and physical properties of the soil samples are shown in CitationSutigoolabud et al. (2004b). Soil samples were pre-incubated for 1 week at 30°C and under 50% maximum water-holding capacity (MWHC) just before use in the incubation study. After that, powdered alfalfa meal (5 g kg−1soil) was added to the soils, mixed well, and then potassium chlorate solution (2,000 mg L−1) was added at two concentration levels, 50 and 500 mg KClO3 kg−1 soil (34 and 341 mg ClO− 3 kg−1 soil) (CitationSutigoolabud et al. 2004b). Soil with only alfalfa meal added was prepared as a control. The soil moisture content was adjusted to 50% MWHC. Each 10 g soil sample was placed into an hermetically sealed polyethylene bottle together with a vial containing 10 mL of 0.5 mol L−1 NaOH and incubated for 4 weeks at 30°C. The amount of carbon dioxide emitted from the soil was determined every week, as an indicator of alfalfa meal decomposition, by neutralizing NaOH solution containing trapped CO2 with 0.5 mol L−1 HCl solution (CitationZibilske 1994). Three replicate experiments were carried out. Statistical analysis of the data was carried out using Statistix ver. 3.5 software (Analytical Software, St Paul, MN, USA).
Effect of the application of chlorate on nitrification in soils
The PC soil and the NP soil were used in this experiment. Soils were pre-incubated and potassium chlorate was added at two concentration levels, 34 and 341 mg ClO− 3 kg−1 soil. Each 10 g soil sample was placed into a 100 mL Erlenmeyer flask, covered with aluminum foil and incubated at 30°C and 50% MWHC under dark conditions. At 0, 1, 2 and 4 weeks after incubation, ammonium sulfate solution was added to a different part of the flasks at a concentration of 50 mg N kg−1 soil, and the samples were subsequently incubated for 4 and 7 days. The amount of existing nitrate at the time of the ammonium sulfate addition, and the amount of nitrate formed by nitrification during the subsequent incubation after the addition of ammonium sulfate were determined as follows: at 0, 4 and 7 days after the ammonium sulfate addition, 100 mL of distilled water was added to each flask, tightly capped with a rubber plug and shaken for 30 min to extract the nitrate. The supernatant solution was taken, filtrated through filter paper and subjected to a determination of nitrate concentration using ion chromatography (HIC-6A; Shimadzu, Kyoto, Japan) equipped with a Shimpack IC/A1 analytical column and conductivity detector (CDD-6A). Analytical conditions were as follows: mobile phase, 2.5 mmol L−1 phthalic acid and 2.4 mmol L−1 tris (hydroxymethyl) aminomethane; flow rate, 1.2 mL min−1; column temperature, 40°C. Determination of the concentration of residual chlorate in the soils at the time of ammonium sulfate addition was also carried out using the filtrates and ion chromatography (CitationSutigoolabud et al. 2004b). Three replicate experiments were conducted. Statistical analysis of the data was carried out using Statistix ver. 3.5.
Effect of the application of chlorate on soil bacterial number
Incubation of soil samples
Ten-gram portions of pre-incubated PC and NP soils were placed in 30-mL beakers, potassium chlorate solution (341 mg ClO− 3 kg−1 soil) was added and the soil was incubated at 30°C under 50% MWHC for 2 weeks. Soil without chlorate was prepared as a control.
Enumeration of the number of bacteria in the soil
The number of bacteria in the soil was counted by the spread plating method (CitationReasoner 2004) using YG agar (yeast extract, 1.0 g; glucose, 1.0 g; K2HPO4, 0.3 g; KH2PO4, 0.2 g; MgSO4·7H2O, 0.2 g; agar, 15 g; water, 1 L, pH 6.8) at 0, 1, 3, 7 and 14 days after the addition of chlorate. Each soil sample was serially diluted with sterilized distilled water and spread onto an agar plate. The agar plates were incubated at 30°C for 24 h, and then the number of colonies appearing on each plate was counted and calculated as c.f.u. g−1 soil. Three replicates of this experiment were conducted. Statistical analysis of the data was carried out using Statistix ver. 3.5.
RESULTS AND DISCUSSION
Effect of the application of chlorate on the mineralization of organic matter in the soil
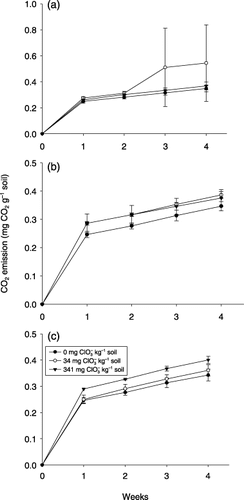
The amount of carbon dioxide emitted from the PC, TMS and NP soils is shown in . In the PC and TMS soils, there were no statistically significant differences in carbon dioxide emission between the chlorate added and non-added conditions, even when 341 mg kg−1 soil of chlorate was added. In the NP soil, the addition of 34 mg kg−1 soil of chlorate did not significantly affect carbon dioxide emission compared with the control soil, whereas 341 mg kg−1 soil of chlorate had a significant effect throughout the incubation period (P < 0.01 at 1, 2 and 3 weeks of incubation and P < 0.05 at 4 weeks).
Figure 1 Effect of the addition of chlorate on the mineralization of alfalfa meal in soil as indicated by CO2 emission from the soil. (a) Pak Chong soil, (b) Tha Muang/Sanpaya soil and (c) Num Pong soil. Chlorate was added to the soil at three concentration levels, 0, 34 or 314 mg kg−1 soil. Bars indicated standard deviation.
Effect of the application of chlorate on nitrification in the soil
The concentration of residual chlorate in the soil gradually decreased as the incubation period proceeded (). At 4 weeks of incubation, 6–7 mg and 220–270 mg kg−1 soil of chlorate was present in soils that had 34 mg and 341 mg kg−1 soil of chlorate added, respectively. Nitrification in both the PC soil and the NP soil () was significantly suppressed by those concentration levels of chlorate. In soils to which 34 mg kg−1 soil of chlorate had been added, the amounts of nitrate formed during 4 and 7 days after the addition of ammonium were significantly smaller than the amounts in the control soil. Suppression of nitrification was observed even in soils at 4 weeks after the addition of chlorate, when the residual chlorate in the soils was as low as 6 mg kg−1 soil. Remarkable suppression of nitrification was observed in both soil types to which 341 mg kg−1 soil of chlorate had been added. In particular, in soils at 2 and 4 weeks after the addition of chlorate, only a trace amount of nitrate (0–4 mg ClO− 3-N kg−1 soil) was formed during the subsequent incubation after the addition of ammonium.
Chlorate is known to inhibit the nitrification process, especially nitrite oxidation to nitrate (CitationBelser and Mays 1980; CitationHynes and Knowles 1983; CitationLees and Quastel 1945). This property is used for the estimation of potential nitrification in soil (CitationBelser and Mays 1980; CitationKandeler 1996). In the standard method, the addition of 2.5 g kg−1 of chlorate is recommended for arable soil with a humus content of 1.5–3.5% (CitationKandeler 1996). The concentrations of chlorate tested in this experiment (34 and 341 mg kg−1 soil) were much lower than the levels used as a soil nitrification inhibitor. It should be noted that the low concentration of chlorate in the soil tested in this study affected nitrification activity, and that attention must be paid to nitrogen transformation in longan plantation soils.
Existing nitrate in the soils at the time of ammonium addition, indicated by parentheses in , corresponds to nitrification of ammonium originally present in the soil and/or derived from soil organic matter decomposition during incubation. Nitrification of such “potential” ammonium was also suppressed by chlorate; however, the degree of suppression was not as marked as that observed in nitrification of added ammonium. Thus, there might be different groups of nitrifiers in the soil: one that rapidly oxidizes relatively high (e.g. 50 mg N kg−1 soil) concentrations of ammonium and another that slowly oxidizes low concentrations of ammonium. It is assumed that the latter group of nitirifiers might be responsible for nitrification of “potential” ammonium and would be less susceptible to chlorate than the former group.
Table 1 Effect of chlorate on nitrification in soil
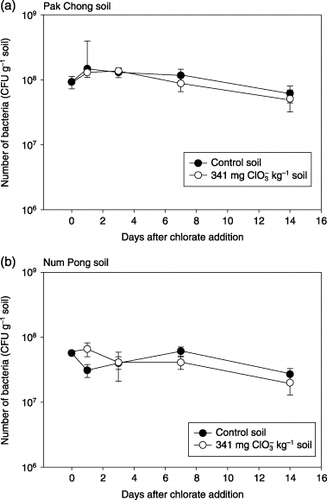
Effect of the application of chlorate on the number of bacteria in the soils
The effect of the addition of chlorate on the number of soil bacteria is shown in . During the incubation, the bacterial number was kept at a level of 107–108 c.f.u. g−1 soil. There was no statistically significant difference in the bacterial number between the control soil and the chlorate added soil. This result indicated that chlorate did not affect the number of culturable heterotrophic bacteria in the soil examined in this study using this method.
Our results indicated that mineralization of organic matter and the bacterial number are not largely affected by chlorate application, while nitrification was significantly suppressed. Other available information on the effect of chlorate on general soil microorganisms in agricultural soil is quite limited. CitationKarki et al. (1973) reported that the application of sodium chlorate as a herbicide at 150 kg ha−1 did not affect the number of total microflora, actinomycetes, cellulolytic, ammonifying, nitrifying or denitrifying bacteria, but slightly affected the evolution of CO2. By rough calculation, 150 kg ha−1 of sodium chlorate corresponds to approximately 60 mg kg−1 soil of chlorate, which is not far from our experimental conditions. Our results, in part, agree with this report. The effect of chlorate on the activity and community structure of fungi, which also play important and diverse roles in agricultural soils (CitationHoshino and Matsumoto 2007), is of interest and should be elucidated in future studies.
The suppression of nitrification by chlorate should be taken into consideration as a possible side-effect of potassium chlorate in longan plantation soils, as well as groundwater contamination. It is desirable to use the minimum necessary amount of chlorate in longan plantation fields, and to clean up the residual chlorate in the soil, if any, after use (i.e. flower induction). Decontamination of chlorate in soil by bio-stimulation with molasses amendment (CitationSutigoolabud et al. 2005) is a candidate for such field management.
ACKNOWLEDGMENT
Pathipan Sutigoolabud was financially supported by the Ministry of Education, Sports, Culture, Science and Technology, Japan.
REFERENCES
- Agaev , R , Danilov , V , Khachaturov , V , Kasymov , B and Tishabaev , B . 1986 . The toxicity to warm-blooded animals and fish of new defoliants based on sodium and magnesium chlorates . Uzb. Biol. Zh , 1 : 40 – 43 .
- Belser , LW and Mays , EL . 1980 . Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments . Appl. Environ. Microbiol , 39 : 505 – 510 .
- Condie , L . 1986 . Toxicology problems associated with chlorine dioxide . J. Am. Water Wks. Assoc , 78 : 73 – 78 .
- Daniel , F , Condie , L Robinson , M . 1990 . Comparative subchronic toxicity study of three disinfectants . J. Am. Water Wks. Assoc , 82 : 61 – 69 .
- Hoshino , YT and Matsumoto , N . 2007 . Changes in fungal community structure in bulk soil and spinach rhizosphere soil after chemical fumigation as revealed by 18S rDNA PCR-DGGE . Soil Sci. Plant Nutr , 53 : 40 – 55 .
- Hynes , RK and Knowles , R . 1983 . Inhibition of chemoautotrophic nitrification by sodium chlorate and sodium chlorite: a reexamination . Appl. Environ. Microbiol , 45 : 1178 – 1182 .
- Kandeler , E . 1996 . “ Potential nitrification ” . In Methods in Soil Biology , Edited by: Schinner , F , Ohlinger , R , Kandeler , E and Margesin , R . 146 – 149 . Berlin : Springer-Verlag .
- Karki , AB , Coupin , L , Kaiser , P and Moussin , M . 1973 . Effets du chlorate de sodium sur les microorganisms du sol, leur respiration et leur activite enzymatique . Weed Res , 13 : 133 – 139 . (in French with English abstract
- Lees , H and Quastel , JH . 1945 . Bacteriostatic effects of potassium chlorate on soil nitrification . Nature , 155 : 276 – 278 .
- Manochai , P , Suthon , W , Wiriya-alomgkorn , W , Sruamsiri , P and Bangerth , F . Effects of application of KClO3on the flowering habit of longan Extended . Abstract of the 9th International Symposium on Plant Bioregulations in Fruit Production . August 19–22 2001 , Seoul, Korea. Vol. 29 ,
- Ongprasert , S , Sutigoolabud , P and Aumtong , S . The impact of application of chlorates in longan plantations on the environment and the remedy . Proceedings of the 17th World Congress of Soil Science, 1191-1–1191-10 . August 14–21 2002 , Bangkok, Thailand.
- Reasoner , DJ . 2004 . Heterotrophic plate count methodology in the United States . Int. J. Food. Microbiol , 92 : 307 – 315 .
- Sutigoolabud , P , Senoo , K Ongprasert , S . 2004b . Decontamination of chlorate in longan plantation soils by bio-stimulation with sugar amendment . Soil Sci. Plant Nutr , 50 : 249 – 256 .
- Sutigoolabud , P , Senoo , K , Ongprasert , S and Hisamatsu , M . 2004a . Suppression of chlorate degradation in longan plantation soil after multiple applications and accelerated degradation by sugar amendment . Soil Sci. Plant Nutr , 50 : 293 – 296 .
- Sutigoolabud , P , Senoo , K Ongprasert , S . 2005 . Decontamination of chlorate in longan plantation soils by bio-stimulation with molasses amendment . Soil Sci. Plant Nutr , 51 : 583 – 588 .
- van Wijk , DJ and Hutchinson , TH . 1995 . The ecotoxicity of chlorate to aquatic organism: a critical review . Ecotoxic. Environ. Safety , 32 : 244 – 253 .
- Zibilske , LM . 1994 . “ Carbon mineralization ” . In Methods of Soil Analysis. Part 2, Microbiological and Biochemical Properties , Edited by: Weaver , RW , Angel , S Bottomley , P . 835 – 863 . Wisconsin : Soil Science Society of America Book Series No. 5 .