Abstract
The origin of soil protease in field soil was estimated using culture-dependent and independent approaches. Overall soil protease activity was much higher in field soils with an annual application of liquid livestock feces (120 t ha−1 year−1 and 600 t ha−1 year−1) compared with the activity recorded in other field soils, and the character of the soil proteases became highly homogeneous (approximately 70% metalloprotease in a 600 t field). Selective incubation studies suggested that bacteria were the most important source of soil protease. There were significantly higher correlations between serratial metalloprotease and the overall soil protease in both feces-applied fields in terms of the effect of inhibitors, and the bacteria, which produced serratial metalloprotease, were suggested to proliferate in both the 120 t and 600 t fields. The gene homologous to serratial metalloprotease gene was amplified in directly extracted DNA from field soils using selective DNA primer and proteolytic Serratia marcescens was certified to be one source of soil protease in these field soils. Proteolytic S. marcescens and its metalloprotease gene have occasionally been isolated and detected in field soils applied with raw feces, and have rarely been isolated or detected from other field soils. Proteolytic S. marcescens is believed to be introduced in the raw feces and subsequently colonizes the field soil and replaces the indigenous bacteria in the soil.
INTRODUCTION
Proteolysis is an initial step in organic nitrogen cycles in field soils and information on the microorganisms involved in this step is indispensable for clarifying biological nitrogen turnover in field soils. In terms of the other steps involved in the inorganic nitrogen cycle, such as nitrification, denitrification or nitrogen fixation, in which typical bacterial groups, ammonia-oxidizing bacteria, denitrifying bacteria or nitrogen-fixing bacteria, specifically contribute to the process in the field, a culture-independent approach based on the molecular base has been used to identify these bacteria (CitationAvrahami et al. 2002; CitationBraker et al. 2000). However, this molecular-based approach is not adaptable unless the specific gene sequence or particular bacterial group concerned with proteolysis in field soil are specified because all microorganisms and plants produce various types of proteases to maintain their own life cycle. Therefore, specification of the bacterial group involved in proteolysis in field soil is a prerequisite for use of this molecular technique.
To obtain culture-independent proof showing the expression of specific protease genes in field soil, the similarity between the soil protease character and the extracellular protease characters of proteolytic soil isolates have been compared (CitationWatanabe and Hayano 1993b; CitationWatanabe et al. 2003). To obtain culture-independent proof showing the existence of a specific bacteria in field soil, the similarity between the protease gene in the soil DNA and the genes in the proteolytic soil isolates was compared. Based on these results, the origin of soil proteases was estimated in experimental field soils under a 20-year monoculture of corn supplied with liquid livestock feces (600 t ha−1 year−1 and 120 t ha−1 year−1) (CitationWatanabe and Niimi 2005), where the higher activity and the higher homogeneous character of the soil protease implied proliferation of certain types of soil microorganisms (44% were metalloproteases in the 600 t ha−1 slurry application field; CitationWatanabe et al. 2003). In the present study, we speculated on the function and origin of these metalloproteases.
Table 1 Soil protease activities (pKat leucine equivalent liberated g−1 dry soil) and culturable microbial counts (CFU g−1 dry soil ± 95% confidence interval)† of upland field soil treated with liquid livestock feces (600 t and 120 t ha−1 year−1)
MATERIALS AND METHODS
Soils
The experimental fields used in the present study were established at Miyakonojyo Miyazaki, Japan (Udand; Department of Upland Farming Research, National Agricultural Research Center for Kyushu-Okinawa Regions), to determine a control method for the soil nitrogen cycle, particularly to reduce NO- 3 leaching from applied livestock feces. Surface soils (depth 0–15 cm) were obtained from two upland fields where forage crops, such as corn and Italian ryegrass, have been cultivated with a heavy application of liquid livestock feces, one with 60 t ha−1 (120 t ha−1 year−1; 120 t field) and the other with 300 t ha−1 (600 t ha−1 year−1; 600 t field), twice a year from 1985. Sampling was done in duplicate on 6 June 1994. Soil samples were also collected from a neighboring area that had been left without cultivation for over 10 years and was not affected by feces application (0 t area).
Immediately after sampling, the soils were sieved (< 2 mm) and analyzed. The soil water contents and chemical properties at the time of sampling are shown in .
Soil protease activities
Benzyloxycarbonyl-l-phenylalanyl-l-leucine hydrolyzing (z-FLase) and caseinase activities were measured using the ninhydrin reaction (CitationIchishima 1972), and the activities were expressed as pKat (pmole sec−1) g−1 dry soil of leucine equivalent. The overall soil protease activities were measured as described previously (CitationWatanabe and Hayano 1993b, Citation1994) using soil suspension (CitationLadd and Butler 1972). The extracted soil protease activity was measured by the same methods using the enzyme solution extracted from soils with 0.1 mol L−1 phosphate buffer (pH 7) (CitationWatanabe and Hayano 1993b).
The effects of etylenediaminetetraacetic acid (EDTA), 1,10-phenanthroline (1,10P), iodoacetic acid (IAA), p-chloromercuribenzoic acid (pCMB), diisopropyl fluorophosphate (DFP) and phenylmethylsulfonylfluoride (PMSF) on the overall soil protease activities were measured as described previously (CitationWatanabe and Hayano 1993b, Citation1994) using soil suspension and casein as the substrate (CitationLadd and Butler 1972).
The source of the soil protease was roughly estimated by monitoring the decrease or increase in protease activity after selective inhibition of specific microorganisms as described by CitationHayano and Tubaki (1985). The antibiotics, cycloheximide or chloramphenicol, were added in aqueous solution to 30 g of dry soil in a 100 mL beaker to give a concentration of 500 µg g−1 dry soil. The experimental conditions used were the same as those used in a former study (CitationWatanabe and Hayano 1994).
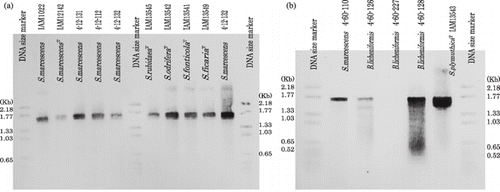
Figure 1 Amplified serratial metalloprotease gene from reference strains of (a) Serratia spp. and (b) isolated proteolytic Serratia marcescens and proteolytic Bacillus licheniformis. The DIG labeled 1.68 Kb amplified fragment of S. marcescens (4-12-132) was used as a probe and the DIG labeled size standard VI (pBR 328 digested by HinfI) was used as a size standard.
Viable counts of microorganisms and isolation of proteolytic bacteria
Microorganisms were counted as described in the paper by CitationWatanabe and Hayano (1994). Colony-forming units of fungi, proteolytic bacteria (azocoll degrader [AD]) and total bacteria in the soil were estimated using the dilution plate method, with a rose Bengal medium (CitationMartin 1950), an azocoll nutrient agar plate (CitationCaplan and Fahey 1982) and an albumin agar medium (CitationWaksman and Fred 1922). The numbers of total Bacillus spp. were estimated using the dilution plate method with a peptone–polymyxin medium (PP medium; CitationAkiba and Katoh 1986). The numbers of Bacillus spp. spores were estimated using the same method with an inoculation medium that had been pre-incubated at 80°C for 20 min. The numbers of vegetative cells were estimated using a selective medium for vegetative cells of Bacillus thuringiensis (BTV medium; CitationAkiba and Katoh 1986). As the selectivity of these media for Bacillus spp. was mainly attributed to the addition of polymyxin B and there were some polymyxin B resistant species in the feces-applied field soil (CitationWatanabe and Niimi 2005; CitationWatanabe 2008), the microorganisms counted included some portion of non-bacillus polymyxin B resistant bacteria (CitationWatanabe and Niimi 2005; CitationWatanabe 2008).
A total of 120 proteolytic bacteria (AD) were isolated from freshly collected soils and selected as described in former papers (CitationWatanabe and Hayano 1993a, Citation1994; CitationWatanabe et al. 1994) and some of these bacteria were identified (CitationWatanabe et al. 2008). Most reference strains used in the present study were kindly provided by the Institute of Applied Microbiology, University Tokyo, Japan.
Polymerase chain reaction amplification and Southern hybridization of bacterial isolates
The genomic DNAs of the isolates and reference strains were prepared as described by CitationSaito and Miura (1963). The serratial metalloprotease gene was amplified by polymerase chain reaction (PCR) using fpp5/rpp10 primers (CitationWatanabe et al. 2003) with the following parameters (CitationWatanabe et al. 2003) after a 5 min denaturation step at 95°C: 30 cycles of denaturation at 94°C for 40 s, primer annealing at 55°C for 40 s, and elongation at 72°C for 40 s. After the amplification cycles, the final elongation step at 72°C lasted for 10 min.
The PCR product (10 µL) was digested by each of 10 units of the restriction enzymes PstI and BamHI (Takara Bio, Shiga, Japan) in an overnight incubation at 37°C. The PCR product (5 µL; ) or the digested fragments (5 µL; ) was electrophoretically separated on 2% (w/v) low melting point agarose gel (Agarose X; Nippongene, Tokyo, Japan) in Tris–borate buffer.
The fragment DNA (1.68 Kb), which contained the whole serratial metalloprotease gene and the pro-peptide sequence of 4-12-132, was used as a DNA probe for Southern hybridization, which was prepared after isolation by agarose gel electrophoresis, purification with JETSORB (Genomed, Oeynhausen, Germany), and labeling by random priming with DIG-11-dUTP (DIG labelling kit; Boehringer-Mannheim, Mannheim, Germany).
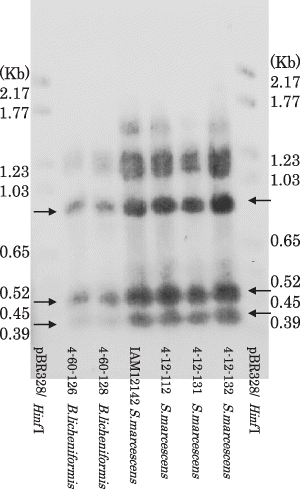
Figure 2 PstI and BamHI digested fragments (0.89, 0.45 and 0.37 Kb) of the amplified metalloprotease gene of isolated proteolytic Serratia marcescens, proteolytic Bacillus licheniformis and a reference strain of S. marcescens (indicated by arrows). The DIG labeled 1.68 Kb amplified fragment of S. marcescens (4-12-132) was used as a probe. The DIG labeled size standard VI (pBR 328 digested by HinfI) was used as a size standard.
After agarose gel electrophoresis separation, the DNA fragments were transferred onto Hybond N nylon membrane (Amersham, Piscataway, NJ, USA) by a capillary technique using 20× sodium chloride–citrate buffer. The DNA was linked to a filter by ultraviolet exposure for 5 min. Prehybridization, hybridization and washes were done at 68°C. The DIG-labeled DNA was detected by enzyme immunoassay and enzyme-catalyzed color detection in accordance with the manufacturer's protocol (DIG detection kit; Boehringer-Mannheim).
The detected DNA fragment on the nylon membrane was digitized using a Bioimage analyzer (Vilber Lourmat /M&S Instrument Trading, Tokyo, Japan) through a charge–coupled device camera and saved using an image documentation system (BIO-PRINT V96, M&S). The sizes of the fragments were calculated using RFLPscan (Vilber Lourmat/M&S Instrument Trading). To estimate the fragment size, DIG labeled size standard VI (pBR 328 digested by Hinf I; Boehringer-Mannheim) was used.
Selectivity of the DNA primers was not only checked by the above PCR experiments, but also by simulation using the method of CitationWatanabe and Okuda (2003) and CitationWatanabe et al. (2008) as follows: among 418 DNA sequences of metalloprotease genes and serine protease genes, X04127 (extracellular metalloprotease gene of Serratia sp. E-15) and X55521 (extracellular metalloprotease gene of Serratia marcescens SM6) afforded the post-amplification sequence (1684 bp and 1689 bp) files for fpp5/rpp10 primers by permitting a five-base mismatch in both primer recognition sites.
Direct extraction of soil DNA and detection by polymerase chain reaction
The DNA was extracted using the method of CitationTsai and Olson (1991). After washing twice with 120 mmol L−1 sodium phosphate buffer (2 mL, pH 8.0), soil samples (1 g, wet weight) were treated with lysozyme (15 mg mL−1) in the lysis solution (2 mL, 0.15 mol L−1 NaCl, 0.1 mol L−1 Na2EDTA, pH 8.0) at 37°C for 2 h with agitation at 15-min intervals. After the addition of 10% (w/v) sodium dodecyl sulfate (2 mL) in the lysis solution (0.1 mol L−1 NaCl, 0.5 mol L−1 Tris–HCl, pH 8.0), the cells were lysed by rapid freezing and thawing (–70°C to 65°C three times). After the washing using Tris–HCl (0.1 mol L−1, pH 8.0) saturated phenol (2 mL), phenol/chloroform/isoamyl alcohol (25:24:1, 3.0 mL) and a chloroform mixture (chloroform : isoamyl alcohol = 24:1), the DNA in the extracted aqueous phase (2 mL) was precipitated with isopropanol (2 mL) at –20°C for 1 h, and any impurities were removed by Qiagen tip in accordance with the manufacturer's protocol (Qiagen Science, Maryland, MD, USA). After electrophoresis separation (1.5% agarose gel) and extraction with Qiaex II Gel Extraction Kit (Qiagen Science), a 10-fold dilution series of DNA was used as the temperate DNA for PCR amplification (CitationTebbe and Vahjen 1993).
In the PCR amplification, T4 gene 32 protein (0.4 µL, 2.5 µg 100 µL−1; Boehringer-Mannheim) was added. After denaturation at 94°C for 2 min, amplification was carried out with the following parameters; 30 cycles of denaturation at 94°C for 2 min, primer annealing at 50°C for 60 s, and elongation at 72°C for 60 s; the last elongation cycle was at 72°C for 3 min. Using the 10-fold dilution of purified soil DNA, the serratial metalloprotease gene was amplified, which was certified by Southern hybridization (data not shown).
To estimate the number of bacteria with this gene in the soil, the amount of the PCR products (1.68 Kb) was compared to the amount of the PCR products (1.68 Kb) of pure temperate DNA (4-12-132) (). By comparing the amount of the PCR products (1.68 Kb) of pure temperate DNA (4-12-132) with that of pure temperate DNA added with purified soil DNA, it was certified that humic substances in soil DNA did not cause a serious effect on the estimated bacterial number (data not shown).
Table 2 Effects of inhibitors on overall soil protease (% of blanks ± 95% confidence interval)
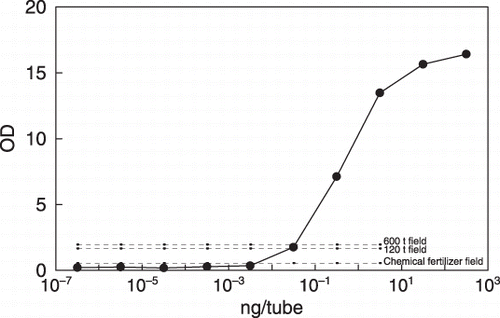
Figure 3 Estimation of the amount of bacterial DNA with serratial metalloprotease gene in the 600 t field, the 120 t field and the chemical fertilizer field by comparing the amount of polymerase chain reaction product (1.68 Kb in Fig. 5; dashed line) in the soil DNA with the amount of pure temperate DNA (4-12-132; solid line).
RESULTS
Soil protease activity and viable counts of microorganisms in the field soils
The overall soil z-FLase and caseinase activities were 652 and 1607 pKat g−1 dry soil in the 120 t field and 1764 and 3902 pKat g−1 dry soil in the 600 t field (), which were several times higher (120 t field, 3.2-fold for zFLase and 5.6-fold for caseinase; 600 t field, 8.6-fold for zFLase and 13.6-fold for caseinase) than those recorded in an upland field supplied with chemical fertilizer, an uncultivated field and a paddy field under upland conditions () (CitationWatanabe and Hayano 1994), and also higher than the values reported by CitationKandeler et al. (1994).
The ratios of the extracted soil protease activities to the overall soil protease activities in the 600 t field were 13.1% (z-FLase) and 7.4% (caseinase) and 27.2% and 7.0% of those in the 120 t field (). There was not a large difference in culturable microbial numbers of fungi, bacteria and AD after the application of liquid livestock feces, although the total numbers of Bacillus spp. counted on PP medium, vegetative cells of Bacillus spp. counted on BTV medium and the total spore number of Bacillus spp. counted on PP medium after 80°C pre-heating for 20 min were higher in both feces-applied fields (). However, the numbers of Bacillus spp. in these fields were overestimated because the bacterial numbers included a high ratio of non-bacillus polymyxin B resistant bacteria (CitationWatanabe and Niimi 2005; CitationWatanabe 2008).
Using 1,10P, the overall soil caseinase activity of both feces-applied fields was highly inhibited () and the inhibitory activities of 1,10P decreased in relation to the decrease in applied liquid livestock feces (10 mmol L−1 inhibitor, 600 t field 69.7%, 120 t field 51.3%, 0 t area 31.7%; 2.5 mmol L−1 inhibitor, 600 t field 46.4%, 120 t field 37.5%, chemical fertilizer 25.9%; uncultivated 8.2%; ). However, the inhibitory activities of pCMB, PMSF and DFP increased in relation to the decrease in applied liquid livestock feces () (pCMB; 10 mmol L−1 inhibitor, 600 t field 21.4%, 120 t field 29.5%, 0 t area 35.0%; 2.5 mmol L−1 inhibitor, 600 t field 14.2%, 120 t field 26.6%, chemical fertilizer field 31.4%, uncultivated field, 39.5%: PMSF; 10 mmol L−1 inhibitor, 600 t field 14.3%, 120 t field 24.4%, 0 t area 39.7%; 2.5 mmol L−1 inhibitor, 600 t field 3.0%, 120 t field 1.1%, chemical fertilizer field, 11.7%, uncultivated field, 24.4%: DFP; 10 mmol L−1 inhibitor, 600 t field 4.7%, 120 t field 11.4%, 0 t area 17.5%; 2.5 mmol L−1 inhibitor, 600 t field 5.8%, 120 t field 6.9%, chemical fertilizer field 11.7%, uncultivated field 24.4%). As the maximum inhibitory activities (approximately 70%) were attained with a single application of 1,10P and EDTA, and by combined application of the both metalloprotease inhibitors (), metalloprotease was suggested to occupy most of soil protease in the 600 t field (approximately 70%).
Table 3 Correlation coefficients between the percentage inhibition of bacterial caseinase by six inhibitors (EDTA, 1,10P, IAA, pCMB, PMSF, DFP) and the inhibition of overall soil caseinase
Table 4 Changes in the overall soil protease activities and microbial counts (ratio to control ± 95% confidence interval) after antibiotic treatment
Figure 4 Additive effect of different metalloprotease inhibitors (ethylenediaminetetraacetic acid [EDTA] and 1,10-phenanthroline) on overall soil protease activities in the 600 t field.
![Figure 4 Additive effect of different metalloprotease inhibitors (ethylenediaminetetraacetic acid [EDTA] and 1,10-phenanthroline) on overall soil protease activities in the 600 t field.](/cms/asset/7e3c0625-c863-4bcb-be23-fc4a11b9b288/tssp_a_10382672_o_f0004g.gif)
Correlation coefficients between the percentage of remaining activity of bacterial caseinase by six inhibitors (EDTA, 1,10P, IAA, pCMB, PMSF and DFP) and the overall soil caseinase are listed in . As there were significantly higher correlations between the effect of inhibitors on bacterial caseinase (4-12-131) and the overall soil caseinase in the 120 t field and the 600 t field, and between that of 4-12-132 and that of 600 t field, and between that of 4-60-110 and that of 120 t field (), bacteria, which produced serratial metalloprotease in vitro, were suggested to produce serratial metalloprotease in both the 120 t and 600 t fields.
Selective inhibition of protease activities and growth of microorganisms by antibiotic treatment
The cycloheximide treatment, which highly suppressed fungal growth and had no serious effect on bacterial growth and promoted growth of Bacillus spp., increased soil caseinase activities in both feces-applied fields, but decreased z-FLase activity in the 120 t field (). This result indicated that fungus was not a major source of soil caseinase in either feces-applied field, but was a major source of z-FLase in the 120 t field.
Table 5 Characterization of the isolated azocoll degraders (AD) and gelatin liquefiers (GL)
The chloramphenicol treatment, which highly promoted fungal growth, suppressed bacterial growth and slightly suppressed the growth of Bacillus spp., had no serious effect on soil caseinase and soil z-FLase activities. Only z-FLase activity of the 120 t field was slightly suppressed ().
Compared to former studies on upland field soils (CitationWatanabe and Hayano 1994) where the soil caseinase activities were completely inhibited and the soil z-FLase activities were significantly inhibited by chloramphenicol treatment, chloramphenicol treatment did not completely decrease soil protease activities. Chloramphenicol treatment did not completely inhibit the growth of bacteria or Bacillus spp. in these fields, where remaining bacteria might contribute to hold the soil protease activities.
Isolation and characterization of proteolytic bacteria
Bacillus spp. comprised 58.1% of the AD isolated from the 600 t field and 81% of the AD isolated from the 120 t field (). Bacillus spp. comprised 68.4% of the gelatin liquefiers (GL) screened in AD isolated from the 600 t field and 44% of the GL from the 120 t field (). All of the Bacillus spp. in GL belonged to group I Bacillus spp., which were aerobic spore-forming rods with ellipsoideal spores that did not cause the sporangium to swell (data not shown). Ratios of Gram-negative bacteria in GL (120 t field, 55.6%; 600 t field 31.6%) were higher than those in the chemical fertilizer field (2.9%), uncultivated field (0%) and paddy field (16.0%) ().
Among the GL in both feces-applied fields, S. marcescens (4-12-112, 4-12-131, 4-12-132 and 4-60-110) and Xanthomonas sp. (4-12-214) had higher extracellular caseinase activities (). Among the GL in the chemical fertilizer and uncultivated fields, Bacillus spp. had the higher extracellular caseinase activities (), which was similar to a previous report on paddy field soils (CitationWatanabe and Hayano 1993a).
DNA structure of protease genes of the proteolytic isolates and reference strains
The DNA primers not only amplified the genomic genes of the proteolytic S. marcecens isolates (4-12-131, 4-12-112, 4-12-132 and 4-60-110), but also the genomic genes of various type strains of Serratia spp., such as S. marcescens, S. rubidaea, S. odorifera, S. fonticola and S. ficria () and proteolytic Bacillus licheniformis isolates (4-60-128 and 4-60-126) () to give a 1.68 Kb fragment that was highly homologous to the metalloprotease gene of S. marcescens (4-12-132).
The amplified DNA had one PstI and BamHI site to give 0.89, 0.45 and 0.37 Kb fragments (), which were identical to the predictions from the published serratial metalloprotease genes; 892, 437 and 354 bp in E15 (X04127; CitationNakahama et al. 1986) and 895, 437 and 356 bp in SM6 by PstI and BamHI digestions (X55521; CitationBraunagel and Benedik 1990).
Polymerase chain reaction amplification of the metalloprotease gene in directly extracted DNA from soil
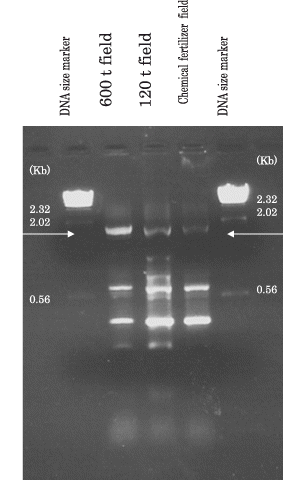
The total purified DNA recovered from the 600 t field, 120 t field and the chemical fertilizer field using freezing and thawing cycles was 1.38 µg g−1, 1.21 µg g−1 and 1.37 µg g−1 dry soil, respectively, and the numbers of bacteria were estimated to be 5.8 × 108 cells, 5.1 × 108 cells and 5.8 × 108 cells g−1 dry soils using one DNA weight of Escherichia coli. Although several amplified DNA were detected using these purified DNA, only DNA of 1.68 Kb () hybridized with the metalloprotease gene of 4-12-132.
By using a ball mill instead of freezing and thawing cycles (CitationTebbe and Vahjen 1993), the recovery ratios of the DNA from soils increased after two purification steps (600 t field, 5.08 µg g −1 dry soil; 120 t field, 3.07 µg g −1 dry soil; 0 t area, 1.97 µg g −1 dry soil) and metalloprotease genes homogeneous to the serratial metalloprotease genes were detected in the field soil (Phaeozem, Braunshweig, Germany; CitationWatanabe et al. 1995), whereas homogeneous metalloprotease genes were not amplified in either feces-applied field (Andosol field soil) because of PCR inhibition by residual humic substances.
Table 6 Extracellular protease activities (pKat leucine equivalent liberated per mL−1 incubation medium for z-FLase and tyrosine equivalent for caseinase) of proteolytic bacteria isolated from various upland field soils
DISCUSSION
As soil proteases have been categorized in terms of their attitude towards inhibitors and depend on fertilizer management (CitationWatanabe and Hayano 1993b, Citation1994), we had assumed that a difference in soil protease type was caused by a difference in the extracellular protease produced by the proteolytic bacteria that had proliferated in the field. Our former comparative studies examining the properties of soil proteases and extracellular proteases of culturable bacteria isolated from eight typical field soils, suggested that serine-type protease resulted from the subtilisin-type protease of Bacillus subtilis that existed in soils such as paddy field soil (CitationWatanabe and Hayano 1993b) and uncultivated field soil (; CitationWatanabe et al. 2003). As 1,10P inhibited the soil proteases of the 120 t and 600 t fields more than in the other fields and more than the other inhibitors (), a specific bacterial group that produced the other type of protease was more numerous in the feces-applied field soil than in the other field soils ().
The ratios of the proteolytic Bacillus spp. to total proteolytic bacteria (AD) in the feces-applied field soils were lower than those in the upland field soil (), which suggested that Gram-negative bacteria might become a more important source of soil protease than Bacillus spp. in the feces-applied field soils (). Among the protelytic bacteria isolated from the feces-applied field soils, S. marcescens (4-12-132, 4-12-131, 4-12-112 and 4-60-110) produced the highest amount of extracellular protease in vitro and showed similar properties to the soil protease in the feces-applied field soils (; CitationWatanabe et al. 2003).
As the metalloprotease genes homologous to these serratial metalloprotease genes were amplified using directly extracted DNA from the feces-applied field soils in relation to the amount of applied livestock feces (, ), these proteolytic S. marcescens, which might include B. licheniformis, were supposed to exist in relation to the amount of applied livestock feces. By comparing the amount of PCR products (1.68 Kb) of pure temperate DNA amounts (4-12-132), the DNA amounts of bacteria with serratial metalloprotease gene were equal to 0.0708 ng g−1 dry soil (chemical fertilizer), 0.256 ng g−1 soil (120 t field) and 0.975 ng g−1 soil (600 t field), respectively (, ), and were estimated to be equal to 4.2 × 104 cells, 15.0 × 104 cells and 60.0 × 104 cells, respectively.
Table 7 Proteolytic bacteria isolated from field soils with different fertilizer managements
Figure 5 Amplified serratial metalloprotease gene (1.68 Kb indicated by arrows) using soil DNA directly extracted from the soil in the 600 t field, the 120 t field and the upland field supplied with chemical fertilizer.
Real-time PCR could not be used for the quantification of this gene for the following reasons. As pro-peptide is indispensable for the excretion of serratial metalloprotease out of cells (CitationLee et al. 1984), DNA including both pro-peptide and mature protease sequences (1.68 Kb), which was much longer than the maximum length (0.5 Kb) found by quantification using real-time PCR, had to be quantified at the same time. As the amount of total amplified DNA was measured in real-time PCR, it was impossible to quantify the amount of target DNA when the PCR amplification product included non-selectively amplified DNA (). As the humic substance that remained in the purified DNA disturbed the efficiency of the PCR amplification, the same amount of humic substance must be added to compensate for the inhibition in quantification using Andosol field soil.
As the amplified gene included the whole serratial metalloprotease gene and the pro-peptide sequence, the difference in the estimated bacterial number reflected the activity of the serratial metalloprotease excreting into these fields. As the characters of the soil proteases of the feces-applied field soils resembled those of the serratial metalloprotease in relation to the amount of applied livestock feces (,), these proteolytic S. marcescens, which might include B. licheniformis, were suggested to produce soil protease in the feces-applied field soils.
A change in the ratio of bacterial numbers with these metalloprotease genes to the total bacterial number, 7.2 × 10−5 (chemical fertilizer), 29.4 × 10−5 (120 t field) and 103.4 × 10−5 (600 t field), clearly explained the results in that there was not a large difference in the culturable bacterial numbers among these field soils, although soil caseinase activity was higher in relation to the amount of applied liquid livestock feces.
Recently, various types of composted or non-composted organic waste, including various types of microorganisms, have been introduced into field soils instead of chemical fertilizer, and this makes it difficult to clarify the function of indigenous microorganisms in field soils. As only the number of Bacillus spp. counted on the peptone–polymyxin medium increased in relation to the amount of applied livestock feces and soil protease activities, Bacillus spp. were considered to be the source of soil proteases (). However, most bacteria counted on the peptone–polymyxin medium in the feces-applied field soils were found to be non-bacillus polymyxin B resistant bacteria in previous reports (CitationWatanabe and Niimi 2005; CitationWatanabe 2008). Serratia marcescens, is not only recognized as an opportunistic pathogen for insects and animals (CitationDecedue et al. 1979), but also shows multi-drug resistance (CitationMammeri et al. 2004). Although we did not test the antibiotic resistance of the proteolytic S. marcescens, it is possible that the proteolytic S. marcescens is one of the polymyxin B resistant bacteria (CitationWatanabe 2008). Lower growth suppression by chloramphenicol treatment in the 600 t field soils might also be attributed to the existence of chloramphenicol-resistant bacteria in these field soils (). The number of bacteria remaining after the chloramphenicol treatment was assumed to be the number of chloramphenicol-resistant bacteria (120 t field, 14.8 × 106 CFU g−1 dry soil; 600 t field 52.1 × 106 CFU), and the number of Bacillus was assumed to be the number of both polymyxin B and chloramphenicol-resistant bacteria (120 t field, 24.0 × 106 CFU g−1 dry soil; 600 t field 34.4 × 106 CFU).
The present results support our former suggestions that various types of non-indigenous bacteria, such as Gram-negative proteolytic bacteria (; CitationWatanabe et al. 2008), non-bacillus polymyxin B resistant bacteria (CitationWatanabe and Niimi 2005; CitationWatanabe 2008) and bacteria concerned with NH+ 4 reduction (CitationWatanabe et al. 2006) were introduced with liquid livestock feces and survived for several months. The present results suggested that proteolytic bacteria exogenously introduced from feces might play a role in the biotransformation of field soil and might replace soil indigenous bacteria. In the feces-applied field soils, the serratial metalloprotease gene was amplified in 1994 and 1996, but was not detected in 1998 or 2004 (data not shown), and the exotoxin A gene of another opportunistic pathogen (Pseudomonas aeruginosa; CitationKhan and Cerniglia 1994) was similarly detected in 1998, but was not detected in 2004 (data not shown). These results also suggest that opportunistic pathogens for insects and animals are occasionally introduced from liquid livestock feces. In contrast to these bacteria of feces origin, the genes of indigenous soil bacteria, such as the lecithinase gene of the Bacillus cereus group (CitationSchraft and Griffiths 1995) and the nitrous oxide reductase gene (CitationViebrock and Zumft 1988) of denitrifying bacteria, were detected in 1998 and in 2004 in the 0 t area and in both feces-applied fields (data not shown).
We propose that the homologous metalloprotease gene in B. licheniformis isolated from the 600 t field (,) might be transmitted from S. marcescens because the homologous sequence to the serratial metalloprotease gene was not found in the reported DNA sequence of B. licheniformis (data not shown). The livestock feces were found to include large numbers of multi-drug-resistant bacteria, which not only had resistance to poly-peptide antibiotics, such as polymyxin B, but also to various type of antibiotics, such as chloramphenicol, streptomycin, ampicillin and ciprofloxacin (data not shown). The results of the antibiotic resistance of the bacteria included in the livestock feces will be presented in a subsequent paper.
ACKNOWLEDGMENTS
The author thanks Mr Niimi, Department of Upland Farming Research, the National Agricultural Research Center for Kyushu–Okinawa Regions, for help with the soil sampling. The author also thanks Dr Christoph C. Tebbe, Institut für Agraökologie, Federal Agricultural Research Centre (FAL), Germany, for various suggestions with regard to this research. This research was supported by a grant from the Japanese Ministry of Agriculture, Forestry and Fisheries from April 1992 to March 1999 (“Development of new agricultural techniques compatible with the conservation of eco-systems by innovative method for the recycling of agricultural resources”) and by the OECD “Co-operative research programme biological resource management for sustainable agricultural systems”.
REFERENCES
- Akiba , Y and Katoh , K . 1986 . Microbial ecology of Bacillus thuringiensis; V Selective medium for Bacillus thuringiensis vegetative cells . Appl. Entomol. Zool , 21 : 210 – 215 .
- Avrahami , S , Conrad , R and Braker , G . 2002 . Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers . Appl. Environ. Microbiol , 68 : 5685 – 5692 .
- Braker , G , Zhou , J , Wu , L , Devol , AH and Tiedje , JM . 2000 . Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in pacific northwest marine sediment communities . Appl. Environ. Microbiol , 66 : 2096 – 2104 .
- Braunagel , SC and Benedik , MJ . 1990 . The metalloprotease gene of Serratia marcescens strain SM6 . Mol. Gen. Genet , 222 : 446 – 451 .
- Caplan , JA and Fahey , JM . 1982 . Plate-clearing technique to screen mixed microbial population for protein degraders . Soil Biol. Biochem , 14 : 373 – 375 .
- Decedue , CJ , Broussard , EA , Larson , AD and Braymer , HD . 1979 . Purification and characterization of the extracellular proteinase of Serratia marcescens . Biochim. Biophys. Acta , 569 : 293 – 301 .
- Hayano , K and Tubaki , K . 1985 . Origin and properties of β-glucosidase activity of tomato-field . Soil Biol. Biochem , 17 : 553 – 557 .
- Ichishima , E . 1972 . Purification and characterization a new type of acid carboxypeptidase . Biochim. Biophys. Acta , 258 : 274 – 288 .
- Kandeler , E , Eder , G and Sobotik , M . 1994 . Microbial biomass, N mineralization, and the activities of various enzymes in relation to nitrate leaching and root distribution in a slurry-amended grassland . Biol. Fertil. Soils , 18 : 7 – 12 .
- Khan , AA and Cerniglia , CE . 1994 . Detection of Pseudomonas aeruginosafrom clinical and environmental samples by amplification of exotoxin A gene using PCR . Appl. Environ. Microbiol , 60 : 3739 – 3745 .
- Ladd , JN and Butler , JHA . 1972 . Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates . Soil Biol. Biochem , 4 : 19 – 30 .
- Lee , IS , Wakabayashi , S Miyata , K . 1984 . Serratia protease. Amino acid sequences of both termini, the 53 residues in the middle region containing the sole methionine residue, and a probable zinc-binding region . J. Biochem , 96 : 1409 – 1418 .
- Mammeri , H , Poirel , L , Bemer , P , Drugeon , H and Nordmann , P . 2004 . Resistance to cefepime and cefpirome due to a 4-amino-acid deletion in the chromosome-encoded AmpC ß-lactamase of a Serratia marcescens clinical isolate . Antimicrob. Agents Chemother , 48 : 716 – 720 .
- Martin , JP . 1950 . Use of acid, rose Bengal and streptomycin in the plate method for estimating soil fungi . Soil Sci , 69 : 215 – 232 .
- Nakahama , K , Yoshimura , K Marumoto , R . 1986 . Cloning and sequencing of Serratia protease gene . Nucleic Acids Res , 14 : 5843 – 5855 .
- Saito , H and Miura , T . 1963 . Preparation of transforming DNA by phenol treatment . Biochim. Biophys. Acta , 72 : 619 – 629 .
- Schraft , H and Griffiths , MW . 1995 . Specific oligonucleotide primers for detection of lecithinase-positive Bacillusspp. by PCR . Appl. Environ. Microbiol , 61 : 98 – 102 .
- Tebbe , CC and Vahjen , W . 1993 . Interference of humic acids and DNA extracted directly from soil in detection and transformation of recombinant DNA from bacteria and a yeast . Appl. Environ. Microbiol , 59 : 2657 – 2665 .
- Tsai , YL and Olson , BH . 1991 . Rapid method for direct extraction of DNA from soil and sediments . Appl. Environ. Microbiol , 57 : 1070 – 1074 .
- Viebrock , A and Zumft , WG . 1988 . Molecular cloning, heterologous expression, and primary structure of the structural gene for the copper enzyme nitrous oxide reductase from denitrifying Pseudomonas stutzeri . J. Bacteriol , 170 : 4658 – 4668 .
- Waksman , SA and Fred , EB . 1922 . A tentative outline of the plate method for determining the number of microorganisms in soil . Soil Sci , 14 : 27 – 28 .
- Watanabe , K , Asakawa , S and Hayano , K . 1994 . Evaluation of extracellular protease activities of soil bacteria . Soil Biol. Biochem , 26 : 479 – 482 .
- Watanabe , K and Hayano , K . 1993a . Distribution and identification of proteolytic Bacillus spp. in paddy field . Can. J. Microbiol , 39 : 674 – 680 .
- Watanabe , K and Hayano , K . 1993b . Source of soil protease in paddy field . Can. J. Microbiol , 39 : 1035 – 1040 .
- Watanabe , K and Hayano , K . 1994 . Estimate of the source of soil protease in upland fields . Biol. Fertil. Soils , 18 : 341 – 346 .
- Watanabe , K , Koga , N and Niimi , H . 2006 . Bacterial community shift in field soil by annual application of liquid livestock feces . JARQ , 40 : 31 – 36 .
- Watanabe , K and Niimi , H . 2005 . Changes in soil protease activity and numbers of culturable bacteria in upland fields by the application of liquid livestock feces . Soil. Sci. Plant Nutr , 51 : 491 – 496 .
- Watanabe , K and Okuda , M . Method and system for searching for relationships between base sequences in genesJapanese . patent3,431,135; US patent, 7,006,924 . 2003 .
- Watanabe , K , Okuda , M and Koga , N . 2008 . A newly developed system based on multiple enzyme restriction fragment length polymorphism–An application to proteolytic bacterial flora analysis . Soil .Sci. Plant Nutr , 54 : 202 – 213 .
- Watanabe , K , Sakai , J and Hayano , K . 2003 . Bacterial extracellular protease activities in field soils under different fertilizer managements . Can. J. Microbiol , 49 : 305 – 312 .
- Watanabe , K , Sakai , J , Munch , JC and Tebbe , CC . Preparation of DNA primers and probes for the detection of metalloprotease genes in soil . Proc. of the International Symposium-Exploration of Microbial Diversity Divers . June , Goslar, Germany. pp. 61
- Watanabe , K . 2008 . Application of multiple enzyme restriction fragment length polymorphism analysis and microchip electrophoresis for estimation of antibiotic tolerant bacterial group . J. Pestic. Sci , 33 : 249 – 260 .