Abstract
The use of heptachlor, a cyclodiene-type insecticide, has been banned since the 1970s because of its carcinogenic potential. However, its metabolite, heptachlor exo-epoxide (HEPX), has still been detected in the fruit of cucurbits produced in some areas. It is important to reduce the daily intake of HEPX contained in food. To address this issue, the effects of carbonaceous adsorbents on the uptake of HEPX from the soil by Cucurbita maxima Dutch. (winter squash) were investigated. Amorphous organic carbons, such as peat moss and wood chips, did not affect the concentration of HEPX in the soil solution or the amount present in the shoots. In contrast, relatively condensed carbon, such as activated carbons, decreased the concentration of HEPX in the soil solution and in the shoots. The uptake amount in the shoot was closely correlated with the concentration of HEPX in the soil solution, which suggests that its uptake by C. maxima depends on its concentration in the soil solution. Activated carbons did not affect the growth of C. maxima. Therefore, the application of activated carbon to soil contaminated with HEPX appears to be an effective method of reducing the uptake of HEPX by C. maxima.
INTRODUCTION
Heptachlor, a persistent organic pollutant (POP) (CitationUnited Nations Environment Programme 2001), is a cyclodiene-type insecticide for crops. Although heptachlor was used worldwide from 1953, its use has been banned since the 1970s because animal data suggests that it is carcinogenic in humans (CitationWorld Health Organization 1984). Heptachlor was not manufactured in Japan, but 1500 t was imported between 1958 and 1972 (CitationJapan Plant Protection Association 1959–1973). Although the actual amounts of heptachlor used in agriculture are not known, it appears to have been heavily used on cultivated land in Japan as an insecticide. In 1972, the Japanese Government banned the use of heptachlor for food crops.
In soil, the major metabolic pathways of heptachlor are epoxidation, hydrolysis and dechlorination (CitationMiles et al. 1969). Heptachlor exo-epoxide (HEPX), the most predominant metabolite of heptachlor, is more stable than heptachlor itself and the other metabolites (CitationLu et al. 1975). HEPX is also a POP (CitationUnited Nations Environment Programme 2001), and is a relatively hydrophobic compound. The octanol-water partition coefficient (log K OW) of HEPX is 5.10 (CitationMeador et al. 1997). Hydrophobic organic chemicals (HOCs) extensively adsorb onto soil particles, which gives these compounds low bioavailability and mobility in soil (CitationOgram et al. 1985; CitationWeissenfels et al. 1992). Therefore, HEPX in soil and sediment is more persistent than in other media, and can still be detected in soil (CitationAigner et al. 1998; CitationBidleman et al. 2006; CitationLeone et al. 2001; CitationShegunova et al. 2007) and sediment (CitationBirch and Taylor 2000) in countries where the use of heptachlor has been restricted. The amounts of readily extractable or bioavailable organic chemicals in soil decrease with time (CitationGevao et al. 2000). Although the soil bioavailability of HEPX is very low, it has been detected in some commercial winter squash in the US (CitationSchafer and Kegley 2002) and was detected in Japan in 2007 in Cucurbitaceae that take up large amounts of HOCs from soil (CitationHülster et al. 1994; CitationLichtenstein and Schulz 1965; CitationMattina et al. 2000; CitationOtani et al. 2007; CitationWhite 2001). Therefore, to reduce the daily intake of HEPX through food, it is important to decrease the amount of HEPX taken up from the soil by cucurbits.
Soil organic carbons, such as amorphous carbons (e.g. fulvic acid, humic acid, humin, lignin and unstructured kerogens) and relatively condensed carbons (e.g. char, soot, kerogens and hard coal), play an important role in the adsorption of organic chemicals on soil (CitationCornelissen et al. 2005; CitationPignatello and Xing 1996). It has been reported that the pesticides-adsorption capacity of soil is enhanced by amorphous carbons (CitationGuo et al. 1991; CitationMartinez-iñigo and Almendros 1992; CitationSluszny et al. 1999) and condensed carbons (CitationGuo et al. 1991). Amorphous carbons and condensed carbons have also been used to reduce the uptake by plants of cyclodiene-type insecticides from the soil. CitationNakamura (1990) reported that the uptake of aldrin and dieldrin by Brassica rapa L. var. rapifera (turnip) decreased in soil treated with compost. Activated carbon reduced the concentration of dieldrin in the fruit of Cucumis sativus L. (cucumber) grown in a dieldrin-contaminated field (CitationHashimoto 2007). These results suggest that both amorphous and condensed carbonaceous adsorbents might affect the uptake of heptachlors (heptachlor and its metabolites) from soil by cucurbits.
The purpose of the present study was to ascertain whether amorphous and condensed carbonaceous adsorbents reduce the uptake of heptachlors by Cucurbita maxima (winter squash) in contaminated soil. Some of these adsorbents were applied to soils contaminated with heptachlors. We observed the uptake amount of heptachlors by C. maxima and the behavior of the heptachlors in the soil.
MATERIALS AND METHODS
Soil and carbonaceous adsorbents
Surface soil (andosol, clay loam) contaminated with heptachlors was collected in 2007 from the upper layer (0–15 cm depth) of agricultural land in Japan. Precise records were not available, but this soil had received regular applications of heptachlor for insect control from the late 1950s until the early 1970s. The soil was air-dried, ground and passed through a 2-mm sieve before being used in the soil culture experiments.
Peat moss (Keiyo, Chiba, Japan) and wood chips (Forestry and Forest Products Research Institute, Tsukuba, Japan) were used as amorphous carbon adsorbents, and activated carbons (SS1 [Ajinomoto Fine-Techno Company, Kanagawa, Japan] and 4DX [Kuraray Chemical Company, Osaka, Japan]) were used as condensed carbon adsorbents. The adsorbents were dried at 50°C for 2 days, thoroughly ground in an agate mortar and passed through a 212-µm sieve. The effects of these adsorbents on the amount of heptachlors taken up by the plant and the concentration of heptachlors in the soil solution were observed.
Soil pH (H2O) and pH (KCl) were measured in 1:2.5 (w/w) soil and water or 1 mol L−1 potassium chloride (KCl) solution, respectively, using a pH meter (F-21; Horiba, Kyoto, Japan) with a glass electrode (6378-10D; Horiba). Carbonaceous adsorbent pH (H2O) was also measured in 1:100 (w/w) adsorbent and water. The carbon content in the soil or the adsorbent was determined using the dry combustion method (Sumigraph NC Analyzer NC-900; Sumika Chemical Analysis Service, Osaka, Japan). The cation exchange capacity (CEC) was measured according to the method of CitationWada and Harada (1969). The specific surface areas (SSAs) of the adsorbents were determined by N2 adsorption at –196°C using a surface area analyzer (Autosorb-1; Quantachrome Instruments, Boynton Beach, FL, USA).
The pH (H2O) and pH (KCl) values of the soil were 5.5 and 4.7, respectively. The CEC and the total carbon contents of the soil were 7.8 cmol(+) kg−1 and 30 mg g−1 dry soil, respectively. The physical and chemical properties of the adsorbents are listed in . The pH of the peat moss and wood chips was acidic, whereas the pH of SS1 and 4DX was alkaline. The total carbon contents of the peat moss, wood chips, SS1 and 4DX were 465, 472, 858 and 870 mg g−1, respectively. More than 85% (w/w) of SS1 and 4DX was carbon, and the carbon contents of SS1 and 4DX were approximately twofold higher than the carbon contents of the peat moss and wood chips. The SSAs of SS1 (803 m2 g−1) and 4DX (925 m2 g−1) were an order of magnitude higher than those of peat moss (16.5 m2 g−1) and wood chips (92.9 m2 g−1).
Plants
Cucurbita maxima Dutch. cv. Ebisu (winter squash; Takii Seed Company, Kyoto, Japan) was used as a test plant because C. maxima takes up dieldrin (CitationOtani et al. 2007), which has a similar structure to HEPX. Pumpkin seeds were individually placed in 150-mL plastic pots filled with granular pearlite (approximately 2 mm). The seeds were germinated and cultivated in a greenhouse at 20–25°C under natural light for 14 days. Tap water was used for the germination and cultivation. The seedlings were then transplanted into the soil.
Table 1 Physical and chemical properties of the adsorbent and of the soil treated with the adsorbent
Chemicals
Special grade reagents (Wako Pure Chemical Industries, Osaka, Japan) were used for all experiments.
Heptachlors in the soil and soil solution
To determine the concentration of heptachlors in the soil, 8 g of soil was placed in an extraction thimble and subjected to Soxhlet extraction for 3 h (50 cycles) with 100 mL of acetone.
Soil solution was obtained by centrifugation of the soil (CitationDavis and Davis 1963), after which the amount of heptachlor in the solution was measured. After the adsorbent and fertilizer were applied to the soil, tap water was added to adjust the soil moisture condition to the equivalent of 60% water-holding capacity. The soil water content was 36.5% (w/w). The soil was maintained for 1 week under dark conditions at 25°C; after this time approximately 70 g of the soil was centrifuged at 16,000 g for 60 min at 25°C to obtain the soil solution using a double-bottomed container consisting of an upper soil-holding cup with a perforated base and a lower solution-holding cup. A glass filter (0.8 µm; Kiriyama Glass, Tokyo, Japan) was placed at the bottom of the upper cup to separate the soil solids from the soil solution. The centrifugal acceleration is equivalent to the water capacity at pF4.2, corresponding to the permanent wilting point (CitationSchofield 1935). All of the soil solution was used to measure the heptachlor contents using a gas chromatograph mass spectrometer (GC/MS).
Uptake of heptachlors by the plant
The amount of heptachlors taken up by C. maxima grown in soil culture was measured. Three hundred grams of air-dried soil was mixed with chemical fertilizer (0.34 g of nitrogen as (NH4)2SO4, 0.15 g of phosphorus as Ca(H2PO4)2·H2O and 0.29 g of potassium as KCl). Adsorbent was applied to the soil at a rate of 0, 0.3, 0.7, 1.0 or 3.0 g and then the soil was vigorously shaken by hand in a polyethylene bag to ensure thorough mixing. The soil was then placed into individual 400-mL plastic pots with a hole in the bottom. Polyester muslin was placed in the bottom of the pot to prevent the soil from leaking out. The pot was covered with a gray plastic sheet to prevent water evaporation from the soil surface. One seedling of C. maxima was transplanted into each pot and grown in the greenhouse described above. The pot was placed into a plastic dish and tap water was supplied to the dish. Fourteen days after transplanting, 20-g aliquots of the shoots of C. maxima were homogenized for 5 min in 200 mL of acetone and then passed through an 0.8-µm glass fiber filter. The amounts of heptachlors in the shoots of C. maxima were measured using a GC/MS. The remaining shoots and roots were dried at 70°C for 48 h and the dry weights were recorded.
Extraction and clean-up procedure
For both the plant and the soil extracts, 10-mL aliquots were taken and spiked with 50 ng of 13C10-labeled heptachlor and HEPX (Cambridge Isotope Laboratories, Andover, MA, USA) as internal standards. These solutions were concentrated to 1–2 mL, transferred to a separating funnel filled with 30 mL of n-hexane and washed twice with 5% (w/v) NaCl solution. In the case of the soil solution, the full amount of the solution (10–15 mL), 90 mL of distilled water, 5 g of NaCl, 13C10-labeled heptachlor and HEPX (Cambridge Isotope Laboratories) were placed into a separating funnel, twice extracted with 20 mL of dichloromethane and then with 20 mL of hexane by shaking. Each solution of plant, soil or soil solution was passed through Na2SO4 for dehydration and then concentrated to 1–2 mL in a rotary evaporator. The heptachlors in the extracts were purified using an ENVI-CARB/PSA column (Supelco, Bellefonte, PA, USA) by elution with 10 mL of n-hexane. The eluted solutions were concentrated to 1–2 mL in a rotary evaporator and then spiked with 5 ng of 13C12-labeled 2,2′,4,4′,5,5′-HxCB (Wellington Laboratories, Guelph, Ontario, Canada) as a syringe spike, and concentrated to 50 µL under a gentle stream of nitrogen.
Measurement of heptachlors
The heptachlors in the plant and soil and in the soil solution were quantified using a GC/MS and a gas chromatograph high-resolution mass spectrometer (GC/HRMS), respectively. The GC/MS and GC/HRMS were operated on selective ion monitoring (SIM) mode, and their conditions are shown in . Unless otherwise noted, all heptachlor concentrations are expressed on a dry weight basis of the particular matrix. The limits of quantitation (LOQs) were calculated according to the CitationJapan Industrial Standard (JIS) K0312 (1999). The LOQs for heptachlor in soil, soil solution and plants were 1 ng g−1, 0.0008 ng mL−1 and 5 ng g−1, respectively; those for HEPX in soil, soil solution and plants were 0.4 ng g−1, 0.0003 ng mL−1 and 2 ng g−1, respectively, and those for heptachlor end-epoxide in soil, soil solution and plants were 0.00006 ng mL−1, 0.4 ng g−1 and 0.08 ng g−1, respectively.
Statistical methods
The source of variability in the experimental data was determined by anova, followed by Williams’ and Dunnett's multiple comparison tests (CitationHochberg and Tamhane 1987). A linear regression analysis was used to determine the correlation between the uptake amount of heptachlors by C. maxima and the concentration of heptachlors in the soil solution (CitationWonnacott and Wonnacott 1981). The statistical analyses were carried out using Base SAS ver. 9.1.3 (SAS Institute Japan, Tokyo, Japan) (CitationSAS Institute 2004).
Table 2 Measurement conditions of the gas chromatography
RESULTS
Physical and chemical properties of the soil and the adsorbents
The amounts of heptachlor, HEPX and heptachlor end-epoxide in the soil were 3.4, 57.5 and < 0.4 ng g−1 dry soil, respectively. The residual amounts of heptachlor and heptachlor end-epoxide in the soil were quite low.
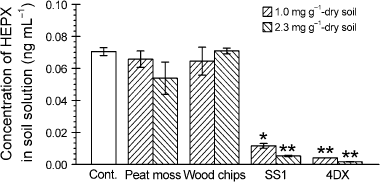
Effects of carbonaceous adsorbent on the concentration of HEPX in the soil solution
The concentrations of heptachlor and heptachlor end-epoxide in the soil solution were under the LOQs or threshold (data not shown). The concentration of HEPX in the untreated soil solution was 0.0705 ng mL−1 (). One gram of dry soil contained 0.365 mL of water. Accordingly, the HEPX amount in the soil water was equivalent to 0.0257 ng per 1 g of dry soil, and was only 0.0448% (w/w) of the total amount of HEPX in the soil (57.5 ng g−1 dry soil). SS1 and 4DX reduced the concentrations of HEPX in the soil solution, whereas peat moss and wood chips did not. At the rate of 1.0 mg of SS1 and 4DX to 1 g of dry soil, the concentration of HEPX decreased by 83% (0.0116 ng mL−1) and 94% (0.0041 ng mL−1), respectively, compared with the control. At the rate of 2.3 mg of SS1 and 4DX to 1 g of dry soil, the concentration of HEPX decreased by 92% (0.0053 ng mL−1) and 97% (0.0017 ng mL−1), respectively, compared with the control. Thus, the amount of HEPX in the soil solution in the soil treated with activated carbon was a very low 0.00108–0.00736% (w/w) of the total amount of HEPX in the soil.
Figure 1 Concentration of heptachlor exo-epoxide (HEPX) in the soil solutions. The soil was treated with peat moss, wood chips, SS1 and 4DX. The control group soil (Cont.) was not treated with any adsorbents. Error bars represent the standard error (n = 3). Statistical analyses were carried out using a Williams’ multiple comparison test only if the outcome of a one-way anova was significant. Asterisks indicate significant differences from the control group (*P ≤ 0.05; **P ≤ 0.01).
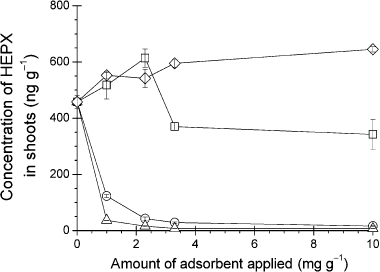
Effects of carbonaceous adsorbents on the uptake of HEPX by C. maxima
The amounts of heptachlor and heptachlor end-epoxide in the shoots of C. maxima were under the LOQs or threshold (data not shown). The HEPX amounts in the shoots of C. maxima grown in the soils treated with peat moss (541–645 ng g−1) and wood chips (342–613 ng g−1) were not significantly different from those of the control group (457 ng g−1) (). In contrast, the uptake amounts fell as the amount of activated carbon increased. This tendency was similar to the effect of the adsorbent on the concentration of HEPX in the soil solution. Compared with the control group, at the lowest application rates of SS1 and 4DX (1.0 mg g−1 dry soil), the uptake amounts decreased by 73% (123 ng g−1) and 92% (36 ng g−1), respectively, and at the highest application of the activated carbons (10 mg g−1 dry soil), the amounts decreased by 96% (16 ng g−1) and 98% (7 ng g−1), respectively.
Table 3 pH and cation exchange capacity of the soil treated with adsorbent at the highest rate (10 mg g−1 dry soil)
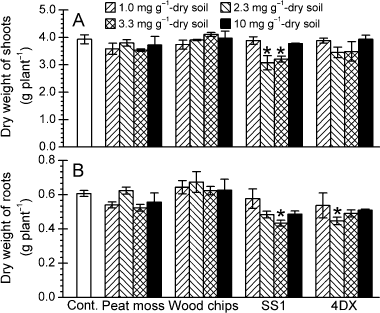
The pH and CEC of the soil treated with carbonaceous adsorbent ranged from 5.1 to 5.8 and from 8.4 to 8.7 cmol(+) kg−1 (), respectively; these values were similar to the values recorded in the soil without adsorbent. The weights of the dried shoots and roots of C. maxima were 3.07–4.11 g and 0.433–0.673 g, respectively. With some exceptions, no differences were observed between the control group and the treatment groups (), suggesting that the adsorbents did not affect the growth of C. maxima.
DISCUSSION
The collected soil was treated with heptachlor four decades ago. The content of heptachlor in the soil was very low and C. maxima took up little or no heptachlor. In the soil, heptachlor is metabolized to HEPX, which is more stable than heptachlor (CitationLu et al. 1975). Almost all the heptachlor applied to the collected soil appears to have been transformed to HEPX, which has remained in the soil. Only condensed carbon, such as activated carbon, and not amorphous carbon, such as peat moss or wood chips, had an effect on the concentration of HEPX in the soil solution (). Similarly, the effect of carbonaceous adsorbents on the uptake of HEPX by C. maxima was affected by activated carbons and not by peat moss and wood chips (). However, it has been reported that the adsorption amount of pesticides increases in soil treated with amorphous carbon (CitationGuo et al. 1991; CitationMartinez-iñigo and Almendros 1992; CitationSluszny et al. 1999). CitationNakamura (1990) also reported that compost (amorphous carbon) decreased the amount of dieldrin taken up by Brassica rapa L. var. rapifera (turnip). The reason for the different results between these previous studies and the present study must lie in the contact time of the chemicals with the soil. In previous studies, the chemicals and the adsorbents were applied to the test soil at the same time, and the contact time was shorter, several months at the longest. In contrast, the HEPX in the present study was applied to the soil four decades previously. Weak adsorbed or dissolved organic chemicals decrease with time (CitationGevao et al. 2000). In aged soil, most HOCs strongly adsorb on condensed carbon rather than on amorphous carbon, and these adsorbed HOCs are only slowly released into the water (CitationCornelissen et al. 2005; CitationPignatello and Xing 1996). In the test soil, we believe that most of the HEPX adsorbed onto the condensed carbon. Therefore, the sorption equilibrium must have been affected by activated carbons, which are condensed and have high SSAs, but not by peat moss and wood chips, which are amorphous carbons with low SSAs. Furthermore, the effects of activated carbon in our experiment support the previously reported finding of the effects of activated carbon on the concentration of dieldrin in the fruit of Cucumis sativus L. (cucumber) (CitationHashimoto 2007). Likewise, the same effect of activated carbon, that is, a reduction in HEPX in the fruit of cucurbits, can be anticipated.
Figure 3 Weight of dried (A) shoots and (B) roots of Cucurbita maxima grown in soil treated with adsorbent. The control group soil (Cont.) was not treated with any adsorbent. Error bars represent the standard error (n = 3). Statistical analyses were carried out using a Dunnett's multiple comparison test only if the outcome of a one-way anova was significant. Asterisks indicate significant differences from the control group (*P ≤ 0.05).
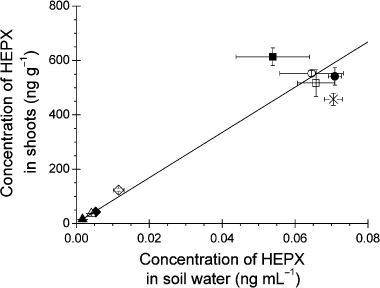
The growth of C. maxima was not inhibited by the adsorbents (). Therefore, the uptake of HEPX by C. maxima does not appear to depend on the plant activity (e.g. transpiration amount), but rather on the bioavailability of HEPX in the soil. There appears to be a close relationship between the uptake amount () and the concentration in the soil solution (). Thus, the amount of HEPX in the shoots of C. maxima was compared with its concentration in the soil solution (). A linear regression analysis was carried out to examine the relationship between the concentration in the shoot and in the soil solution. As a result of this calculation, there was a linear relationship between these values. The coefficient of determination was 0.966 (r 2; P ≤ 0.001). Although the abundance ratio of the HEPX in the soil solution is somewhat lower than the net HEPX in the soil, the amount of HEPX taken up by C. maxima closely depended on the HEPX level in the soil solution. The uptake of organic chemicals in soil by plants has been studied by many researchers. CitationBriggs et al. (1982) proposed the first model for the uptake of organic chemicals from soil solution by plants. In addition, it has been reported that the concentration of a relatively hydrophilic herbicide in soil solution collected by centrifugation is related to its activity in plants (CitationLee et al. 2004; CitationMurano et al. 2007). These studies and our results suggest that the concentration of HEPX in the soil solution is an important factor in the bioavailability of HEPX in the soil to C. maxima.
Activated carbons reduced the uptake of HEPX by C. maxima. Moreover, the chemical properties of the soil, such as the pH and CEC, were not affected by the application of activated carbons. Growth of C. maxima was similarly unaffected. These results suggest that activated carbon is effective in reducing the amount of HEPX in the early stages of cucurbits. Further studies are needed to confirm the effect of activated carbon in decreasing the concentration of heptachlor in winter squash fruits.
Figure 4 Effects of the application of adsorbent to soil on the heptachlor exo-epoxide (HEPX) concentration in Cucurbita maxima shoots and in the soil solution. The soil was treated with 1.0 ng mg−1 dry soil (□) and 2.3 ng mg−1 dry soil (▪) of peat moss, 1.0 ng mg−1 dry soil (⋄) and 2.3 ng mg−1 dry soil (♦) of wood chips, 1.0 ng mg−1 dry soil (○) and 2.3 ng mg−1 dry soil (•) of SS1.0, 1.0 ng mg−1 dry soil (▵) and 2.3 ng mg−1 dry soil (▴) of 4DX, or no adsorbent (×). Error bars represent the standard error (n = 3). The solid line shows the fitting of the linear regression analysis.
ACKNOWLEDGMENT
This work was supported in part by Grants-in-Aid for the Research Project for Ensuring Food Safety from Farm to Table (PO-2221) and the Research Project for Utilizing Advanced Technologies in Agriculture, Forestry and Fisheries (1907) from the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- Aigner , EJ , Leone , AD and Falconer , RL . 1998 . Concentrations and enantiomeric ratios of organochlorine pesticides in soil from the US Corn Belt . Environ. Sci. Technol , 32 : 1162 – 1168 .
- Bidleman , TF , Leone , AD , Wong , F , van Vliet , L , Szeto , S and Ripley , BD . 2006 . Emission of legacy chlorinated pesticides from agricultural and orchard soils in British Columbia, Canada . Environ. Toxicol. Chem , 25 : 1448 – 1457 .
- Birch , GF and Taylor , SE . 2000 . Distribution and possible sources of organochlorine residues in sediments of a large urban estuary, Port Jackson, Sydney . Aust. J. Earth Sci , 47 : 749 – 756 .
- Briggs , GG , Bromilow , RH and Evans , AA . 1982 . Relationships between lipophilicity and root uptake and translocation of non-ionised chemicals by barley . Pestic. Sci , 13 : 495 – 504 .
- Cornelissen , G , Gustafsson , Ö , Bucheli , TD , Jonker , MTO , Koelmans , AA and Van Noort , PCM . 2005 . Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: Mechanisms and consequences for distribution, bioaccumulation, and biodegradation . Environ. Sci. Technol , 39 : 6881 – 6895 .
- Davis , EB and Davis , IR . 1963 . A simple centrifugation method for obtaining small samples of soil solution . Nature , 198 : 216 – 217 .
- Gevao , B , Semple , K and Jones , K . 2000 . Bound pesticide residues in soils: a review . Environ. Pollut , 108 : 3 – 14 .
- Guo , L , Bicki , TJ , Hinesly , TD and Felsot , AS . 1991 . Effect of carbon-rich waste materials on movement and sorption of atrazine in a sandy, coarse-textured soil . Environ. Toxicol. Chem , 10 : 1273 – 1282 .
- Hashimoto , Y . 2007 . Reduction of dieldrin concentration in cucumber fruits using Cucurbita rootstocks and activated carbon . J. Pestic. Sci , 32 : 229 – 234 .
- Hochberg , Y and Tamhane , AT . 1987 . Multiple Comparison Procedures , New York : John Wiley & Sons .
- Hülster , A , Müller , JF and Marschner , H . 1994 . Soil–plant transfer of polychlorinated dibenzo-p-dioxins and dibenzofurans to vegetables of the cucumber family (Cucurbitaceae) . Environ. Sci. Technol , 28 : 1110 – 1115 .
- Japan Industrial Standard . 1999 . Method for Determination of Tetra- Through Octa-chlorodibenzo-p-dioxins, Tetra- Through Octa-chlorodibenzofrans, and Coplanar Polychlorinated Biphenyls in Industrial Water and Waste Water (K0312) , Tokyo : Lewis Publishers .
- Japan Plant Protection Association . 1959–1973 . “Nôyaku Yôran” [Agrochemicals Handbook] , Tokyo : Japan Plant Protection Association . (in Japanese)
- Lee , DJ , Senseman , SA , O’Barr , JH , Chandler , JM , Krutz , LJ , McCauley , GN and Kuk , YI . 2004 . Soil characteristics and water potential effects on plant-available clomazone in rice . Weed Sci , 52 : 310 – 318 .
- Leone , AD , Amato , S and Falconer , RL . 2001 . Emission of chiral organochlorine pesticides from agricultural soils in the cornbelt region of the US . Environ. Sci. Technol , 35 : 4592 – 4596 .
- Lichtenstein , EP and Schulz , KR . 1965 . Residues of aldrin and heptachlor in soils and their translocation into various crops . J. Agric. Food Chem , 13 : 57 – 63 .
- Lu , PY , Metcalf , RL , Hirwe , AS and Williams , JW . 1975 . Evaluation of environmental distribution and fate of hexachlorocyclopentadiene, chlordene, heptachlor, and heptachlor epoxide in a laboratory model ecosystem . J. Agric. Food Chem , 23 : 967 – 973 .
- Martinez-iñigo , MJ and Almendros , G . 1992 . Pesticide sorption on soils treated with evergreen oak biomass at different humification stages . Commun. Soil Sci. Plant Anal , 23 : 1717 – 1729 .
- Mattina , MI , Iannucci-Berger , W and Dykas , L . 2000 . Chlordane uptake and its translocation in food crops . J. Agric. Food Chem , 48 : 1909 – 1915 .
- Meador , JP , Adams , NG , Casillas , E and Bolton , JL . 1997 . Comparative bioaccumulation of chlorinated hydrocarbons from sediment by two infaunal invertebrates . Arch. Environ. Contam. Toxicol , 33 : 388 – 400 .
- Miles , JRW , Tu , CM and Harris , CR . 1969 . Metabolism of heptachlor and its degradation products by soil microorganisms . J. Econ. Entomol , 62 : 1334 – 1338 .
- Murano , H , Kobayashi , K and Fujihara , S . 2007 . Residual thenylchlor concentration in soil water and its phytotoxic activity in soil . Weed Biol. Manage , 7 : 158 – 163 .
- Nakamura , K . 1990 . [Behavior of pesticides in soil and other environment.] . J. Pestic. Sci , 15 : 271 – 281 . (in Japanese with English abstract)
- Ogram , AV , Jessup , RE , Ou , LT and Rao , PSC . 1985 . Effects of sorption on biological degradation rates of (2,4-dichlorophenoxy)acetic acid in soils . Appl. Environ. Microbiol , 49 : 582 – 587 .
- Otani , T , Seike , N and Sakata , Y . 2007 . Differential uptake of dieldrin and endrin from soil by several plant families and Cucurbita genera . Soil Sci. Plant Nutr , 53 : 86 – 94 .
- Pignatello , JJ and Xing , BS . 1996 . Mechanisms of slow sorption of organic chemicals to natural particles . Environ. Sci. Technol , 30 : 1 – 11 .
- SAS Institute . 2004 . SAS/STAT 9.1 User's Guide , Cary : SAS Institute .
- Schafer , KS and Kegley , SE . 2002 . Persistent toxic chemicals in the US food supply . J. Epidemiol. Comm. Health , 56 : 813 – 817 .
- Schofield , R . The pF of water in soil . Third International Congress on Soil Science . Vol. 2 , 30 July – 7 August . pp. 37 – 48 . Oxford, , UK : Plenary Session Papers . 1935
- Shegunova , P , Klánová , J and Holoubek , I . 2007 . Residues of organochlorinated pesticides in soils from the Czech Republic . Environ. Pollut , 146 : 257 – 261 .
- Sluszny , C , Graber , ER and Gerstl , Z . 1999 . Sorption of s-triazine herbicides in organic matter-amended soils: Fresh and incubated systems . Water Air Soil Poll , 115 : 395 – 410 .
- United Nations Environment Programme . Final Act of the Conference of Plenipotentiaries on the Stockholm Convention on Persistent Organic Pollutants . Conference of Plenipotentiaries on the Stockholm Convention on Persistent Organic Pollutants . May 22–23 2001 . Stockholm, United Nations, NY, USA, , Sweden
- Wada , K and Harada , Y . Effects of salt concentration and cation species on the measured cation exchange capacity of soils and clays . Proceedings of the International Clay Conference . September 5–10 1969 , Tokyo, Japan. vol. 1 , pp. 561 – 572 .
- Weissenfels , WD , Klewer , HJ and Langhoff , J . 1992 . Adsorption of polycyclic aromatic hydrocarbons (PAHs) by soil particles: Influence on biodegradability and biotoxicity . Appl. Microbiol. Biotechnol , 36 : 689 – 696 .
- White , JC . 2001 . Plant-facilitated mobilization and translocation of weathered 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene (p,p’-DDE) from an agricultural soil . Environ. Toxicol. Chem , 20 : 2047 – 2052 .
- World Health Organization . 1984 . Environmental Health Criteria 38; Heptachlor , Geneva : World Health Organization .
- Wonnacott , TH and Wonnacott , RJ . 1981 . Regression , New York : John Wiley & Sons .