Abstract
5-Aminolevulinic acid (ALA), a key precursor of porphyrin biosynthesis, promotes plant growth and crop yields. Although ALA is known to promote carbon fixation and nitrogen assimilation in plants, the effects of ALA on sulfur assimilation have not been determined. In the present study, we analyzed the effect of ALA on sulfur assimilation. We used a fusion gene construct consisting of a promoter region of the high-affinity sulfate transporter SULTR1;2 from Arabidopsis and green fluorescent protein ([GFP] P SULTR1;2 -GFP) to determine whether ALA treatment influences the expression of the sulfur transport gene. The GFP levels in P SULTR1;2 -GFP plants were significantly increased by 0.3 and 1 mmol L−1 ALA under both sulfur-sufficient and sulfur-deficient conditions. Real-time reverse transcription-polymerase chain reaction experiments revealed that these concentrations of ALA also increased the mRNA levels of other key sulfur transport and assimilatory genes, such as SULTR, adenosine 5′-phosphosulfate reductases and serine acetyl transferase. Sulfate uptake was enhanced by ALA treatment under sulfur-sufficient conditions. In addition, ALA treatment increased the accumulation of cysteine and glutathione, particularly in the shoot. Our data demonstrated that exogenously applied ALA increases the transcript levels of some sulfur assimilatory genes, sulfate uptake, and the contents of cysteine and glutathione. We propose a new role for ALA in regulating the sulfur assimilatory pathway.
Introduction
5-Aminolevulinic acid (ALA) is a key precursor of porphyrin biosynthesis, including chlorophyll and heme. Treatment of higher plants with ALA promotes the greening of etiolated plants and increases the accumulation of several chlorophyll intermediates (CitationTanaka et al. 2005). Higher concentrations of ALA have herbicidal effects on plants (CitationKittsteiner et al. 1991; CitationRebeiz et al. 1990). It is assumed that the accumulated chlorophyll intermediates act as a photosensitizer for the formation of reactive oxygen, triggering photodynamic damage of ALA-treated plants (CitationAskira et al. 1991; CitationChakraborty and Tripathy 1992). In addition to these effects, ALA treatment promotes the growth and yield of several crops and vegetables (CitationHotta et al. 1997a,b; CitationTanaka et al. 2005). Under these conditions, ALA promotes carbon fixation through the stimulation of photosynthesis (CitationHotta et al. 1997b). In addition, ALA elevates nitrogen assimilation in plants through the induction of nitrate reductase and nitrite reductase activity (CitationMishra and Srivastava 1983). However, the effects of ALA on other metabolic pathways in plants have not been determined.
Sulfur is one of the essential macronutrients required by plants. Plants use sulfate as a major sulfur source and synthesize the sulfur-containing amino acids cysteine and methionine (CitationCrawford et al. 2000; CitationLeustek et al. 2000; CitationSaito 2004). Sulfur assimilation starts from the uptake of external sulfate by the activity of sulfate transporter (SULTR) in the roots. Sulfate taken up by plant roots is activated by ATP sulfulyrase and then reduced by two-step reactions catalyzed by adenosine 5′-phosphosulfate reductase (APR) and sulfite reductase to produce sulfide. Then with cysteine synthase, sulfide reacts with O-acetyl-L-serine, which is produced from serine by serine acetyltransferase (Serat) activity, and is turned into cysteine. Glutathione (GSH), methionine and many types of sulfur-containing compounds are produced from cysteine. Glutathione is synthesized from cysteine in a two-step reaction (CitationNoctor and Foyer 1998). A dipeptide γ-glutamylcysteine (γ-EC) is generated from cysteine and glutamate by the activity of γ-glutamylcysteine synthetase (γ-ECS). Then GSH synthesis is completed by the addition of glycine to γ-EC by GSH synthetase activity. Sulfur assimilation is highly regulated by the availability of sulfur in the environment. It has been reported that SULTR, APR and Serat are significantly activated in plants under sulfur-deficient conditions (CitationGutierrez-Marcos et al. 1996; CitationShibagaki et al. 2002; CitationSmith et al. 1995, 1997; CitationTakahashi et al. 1997, 2000; CitationYoshimoto et al. 2002). The mRNA levels of these three enzymes are also affected by the exogenous application of the metabolites of sulfur assimilatory pathways; that is, the addition of cysteine and GSH decrease mRNA levels (CitationLappartient et al. 1999; CitationSmith et al. 1997; CitationVauclare et al. 2002), and the addition of O-acetyl-L-serine increases these levels (CitationHirai et al. 2003; CitationKoprivova et al. 2000; CitationMaruyama-Nakashita et al. 2004b; CitationSmith et al. 1997). Furthermore, studies of the metabolic regulation of SULTR and APR have indicated that the regulatory network of sulfur assimilation is influenced by the nitrogen and carbon status of the plants (CitationKopriva et al. 1999; CitationKoprivova et al. 2000; CitationMaruyama-Nakashita et al. 2004b; CitationWang et al. 2003). These findings suggest a tight connection of sulfur assimilation with nitrogen and carbon assimilation.
In the present study, we examined the effects of ALA on sulfur assimilation by analyzing the mRNA levels of the key sulfur assimilatory genes SULTR, APR and Serat using Arabidopsis thaliana. Sulfate uptake activity and the contents of cysteine and GSH were analyzed under the same conditions. The results demonstrated that exogenously applied ALA increases the transcript levels of some sulfur assimilatory genes, sulfate uptake and the contents of cysteine and glutathione. To the best of our knowledge, this is the first report to analyze the effect of ALA on the sulfur assimilation pathway.
Materials and methods
Plant growth and treatments
Arabidopsis thaliana plants ecotype Columbia were grown at 22°C under 16-h light/8-h dark cycles. The plants were grown on mineral nutrient media (CitationHirai et al. 1995) containing 1% sucrose. For preparation of the agar medium, the agar was washed twice with 1 L of deionized water and vacuum filtrated. The −S agar medium was prepared by replacing the MgSO4 contained in the media with equivalent molar of MgCl2. For the ALA treatment, plants were vertically grown on the media for 7 days and then transferred to the media with or without ALA at the concentrations indicated in the figures.
Quantification of green fluorescent protein
The expression of GFP in intact P SULTR1;2 -GFP plants (CitationMaruyama-Nakashita et al. 2004a) was visualized using an image analyzer FluorImager 595 under 488 nm excitation (Molecular Dynamics, Sunnyvale, CA, USA). The GFP and auto-fluorescence of the plants were detected using 530DF30 and 610RG filters, respectively. The relative intensities of the GFP signals were quantified with ImageQuant software (Molecular Dynamics).
Quantitative reverse transcription-polymerase chain reaction
Root tissues were used for Reverse transcription-quantitative polymerase chain reaction (RT-PCR) analysis. The total RNA was isolated using Sepasol-RNA I (Nacalai tesque, Kyoto, Japan) and treated with DNaseI (Invitrogen, Carlsbad, CA, USA). Reverse transcription was carried out using the Omniscript RT kit (Qiagen, Hilden, Germany) priming with oligo-d(T)12–18. Real-time PCR was carried out using SYBR Premix Ex Taq (Takara Bio, Shiga, Japan) and iCycler iQ Real-Time Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The gene-specific primer pairs used were as follows: 1854F (5′-GGATCCAGAGATGGCTACATGA-3′) and 1956R (5′-TCGATGTCCGTAACAGGTGAC-3′) for SULTR1;2, 1576F (5′- ATTGTTCGAATCGATGCTCCC-3′) and 1676R (5′- CCCTTGCTTGTGTGTTTGTCG-3′) for SULTR4;2, 199F (5′- GCTTCAACTCTAATTGCTCCTGAAG-3′) and 372R (5′- CAATGCAACATCTTCAGCTCCACT-3′) for APR2, 195F (5′- GGAATCCATTGTT-GCTTCTGAGGT-3′) and 372R (5′- CAGAGCAACATCTTCAGCTCCACT-3′) for APR3, 241F (5′- ATTCACCACACCCAAATCGAAG-3′) and 364R (5′- GAT-GAGATGTGATCGAAGCGTAGTAG-3′) for Serat2;1, and 144F (5′-CCAAGATCCAGGACAAAGAAGGA-3′) and 372R (5′-TGGAGACGAGCATAACACTTGC-3′) for UBQ2 (accession no. J05508).
Measurement of sulfate uptake activity
The sulfate uptake experiments were carried out as described previously (CitationKataoka et al. 2004; CitationMaruyama-Nakashita et al. 2004a).
Cysteine and glutathione analysis
Plant tissues were harvested and frozen in liquid nitrogen before extraction. Frozen tissues were homogenized in fivefold volume of 10 mmol L−1 HCl using Tissue Lyser (Retsch, Haan, Germany). After homogenization, the cell debris was removed by centrifugation to retain a cleared extract. The supernatant was used for the analysis. The cysteine and GSH contents were determined by monobromobimane (Invitrogen) labeling of thiols after reduction of the extracts by dithiothreitol (Nacalai tesque). The labeled products were separated by Waters 2695 High performance liquid chromatography using a Symmetry C18 column (4.6 mm × 150 mm; Waters, Milford, MA, USA) and detected with a scanning fluorescence detector 474 (Waters) monitoring the fluorescence of thiol-bimane adducts at 482 nm under excitation at 390 nm.
Results and discussion
ALA increased the mRNA levels of the sulfur assimilatory genes
To determine whether ALA treatment increases the expression of sulfur assimilatory related genes we used a transgenic A. thaliana P SULTR1;2 -GFP expressing GFP under the control of the SULTR1;2 promoter (CitationMaruyama-Nakashita et al. 2004a). In A. thaliana, sulfate uptake from the environment depends on the activity of SULTR1;1 and SULTR1;2, high-affinity sulfate transporters existing in the root epidermis and cortex, both of which are upregulated under sulfur-deficient (−S) conditions (CitationShibagaki et al. 2002; CitationTakahashi et al. 2000; CitationVidmar et al. 2000; CitationYoshimoto et al. 2002, 2007). Although SULTR1;2 was less responsive to sulfur limitation than SULTR1;1, SULTR1;2 was abundantly expressed under both sulfur sufficient (+S) and −S conditions (CitationYoshimoto et al. 2002, 2007). The influx of sulfate is substantially decreased in the sultr1;2 knockout even under +S conditions, but not in the sultr1;1 knockout, suggesting a large contribution of SULTR1;2 to the uptake of sulfate (CitationShibagaki et al. 2002; CitationYoshimoto et al. 2007). We have previously demonstrated that the GFP levels in P SULTR1;2 -GFP plants are good indicators of the mRNA levels of SULTR1;2 (CitationMaruyama-Nakashita et al. 2004a).
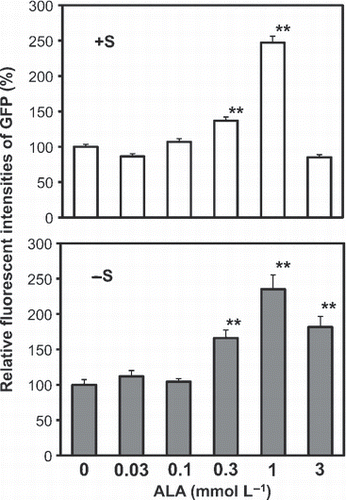
Seven-day-old P SULTR1;2 -GFP plants grown on 1500 (+S) or 15 μmol L−1 sulfate (−S) medium were transferred to the same media with or without ALA. Two days after the transfer, the accumulation of GFP in the roots was observed and quantified (). Under both +S and −S conditions, GFP accumulated significantly in the plants treated with 0.3 or 1 mmol L−1 ALA (). Only under the −S condition, 3 mmol L−1 ALA significantly increased the GFP accumulation, but the levels were lower than with 1 mmol L−1 ALA. The increase of GFP fluorescense in P SULTR1;2 -GFP plants treated by ALA indicated that ALA increases the mRNA accumulation of SULTR1;2.
Figure 1 Induction of green fluorescent protein (GFP) accumulation in PSULTR1;2-GFP plants by 5-aminolevulinic acid (ALA) treatment. The PSULTR1;2-GFP plants were grown for 7 days on control agar medium and then incubated for 2 days on the control medium or on medium containing 0.03, 0.1, 0.3, 1 or 3 μmol L−1 of ALA. The medium used for the experiment contained 1500 (+S) or 15 μ mol L−1 (−S) sulfate as a sulfur source. The relative fluorescent intensities in the roots were quantified by ImageQuant software. The relative values indicate comparisons with the control plants (0 mmol L−1 ALA). Error bars indicate the standard error. Statistically significant differences from the control with P-values <0.05 are shown as ** (n = 11).
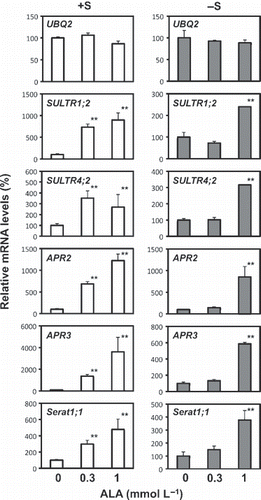
As 0.3 and 1 mmol L−1 ALA increased SULTR1;2 expression, we analyzed whether these concentrations of ALA would increase mRNA accumulation in a broader range of sulfur assimilatory genes (). In addition to SULTR1;2, the genes of several key enzymes in the sulfur assimilatory pathway were selected, including SULTR4;2, APR2, APR3 and Serat2;1. In brief, SULTR4;2 acts as a sulfate exporter from vacuoles to the cytosol (CitationKataoka et al. 2004); APR2 and APR3 are localized in plastids and catalyze the reaction from APS to sulfite (CitationGutierrez-Marcos et al. 1996); and Serat2;1 is localized in plastids and in the cytosol and catalyzes O-acetyl-L-serine synthesis from serine (CitationKawashima et al. 2005). The mRNA levels of all genes tested increased in response to ALA treatment (). Under the +S condition, both 0.3 and 1 mmol L−1 ALA significantly increased the mRNA levels of sulfur transport and assimilatory genes, whereas only 1 mmol L−1 ALA had a significant effect under the −S condition. These results suggested a possibility that exogenously applied ALA stimulates sulfur assimilation capacity in plants.
Figure 2 Induction of mRNA accumulation of sulfur assimilatory genes by 5-aminolevulinic acid (ALA) treatment. Plants were treated as described in Fig. 1 except that the plants used were wild-type Columbia and the ALA concentrations were 0, 0.3 and 1 mmol L−1. The mRNA contents of SULTR1;2, SULTR4;2, APR2, APR3, Serat2;1 and UBQ2 in the root tissues were determined by real-time reverse transcription-polymerase chain reaction. The relative values indicate comparisons with the control plants (0 mmol L−1 ALA). Statistically significant differences from the control with P-values <0.05 are shown as ** (n = 3).
ALA influenced sulfate uptake activity in plants
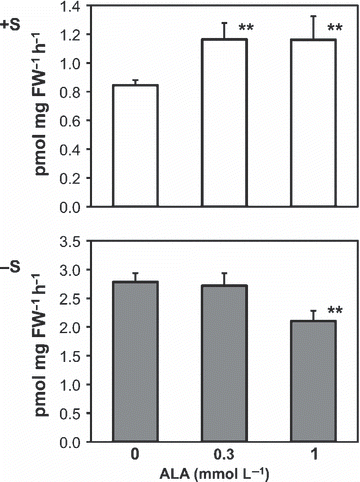
Treatment with ALA increased the mRNA level of SULTR1;2, which mainly facilitates sulfate uptake from the environment (CitationShibagaki et al. 2002; CitationYoshimoto et al. 2002). To determine whether ALA-mediated upregulation of SULTR1;2 mRNA results in modulation of sulfate uptake activity in the roots, the influx of [35S] sulfate was analyzed (). As shown in , application of 0.3 or 1 mmol L−1 ALA caused significant increases in sulfate uptake activities under the +S condition. Although the extent of upregulation in mRNA and sulfate uptake activity differed (), the positive effect of ALA on sulfate uptake activity could result from the increased levels of SULTR1;2 mRNA. In contrast, application of ALA under −S conditions did not show any positive effect on sulfate uptake activity and even caused a significant decrease with 1 mmol L−1 ALA (). The reason for this negative correlation between mRNA levels and sulfate uptake activity is not clear. It is possible that the sulfate uptake capacity is already saturated in the −S condition and that 1 mmol L−1 ALA may have some inhibitory effects on protein synthesis or maintenance of root tissues. The importance of post-transcriptional control in sulfate uptake has been reported by showing that sulfate uptake activity correlates with the SULTR1;2 protein level, but not with the mRNA level in SULTR1;2 over-expression plants (CitationYoshimoto et al. 2007). Post-transcriptional regulation could contribute to the different extent of upregulation between the mRNA level and sulfate uptake activity under +S conditions and the decrease in sulfate uptake activity under −S conditions by ALA treatment.
Figure 3 Effects of 5-aminolevulinic acid (ALA) treatment on the sulfate uptake activities in plants. Plants were treated as described in Fig. 2. The medium used for the experiment contained 1500 (+S) or 15 μ mol L−1 (−S) sulfate as a sulfur source. Statistically significant differences from the control with P-values <0.05 are shown as ** (n = 8). FW, fresh weight.
Cysteine and glutathione contents in shoots were increased by ALA treatment
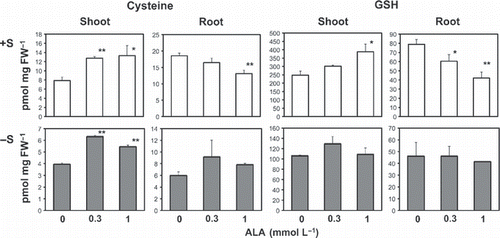
Upregulation of the transcript levels of sulfur assimilatory genes by ALA treatment suggests that exogenously applied ALA may stimulate cysteine and GSH synthesis in plants. To determine whether ALA increases the levels of these compounds, we analyzed the cysteine and GSH contents in the roots and shoots of wild-type plants treated with or without ALA (). Under +S conditions, treatment with ALA increased the levels of both cysteine and GSH in the shoots, whereas the levels in roots decreased. The cysteine contents in the shoots and roots of the ALA-treated plants were 5.42 pmol mg per fresh weight (FW) more and 5.44 pmol mg per FW less, respectively, than those of the non-treated plants (). The GSH contents in the shoots and roots of the ALA-treated plants were 139.9 pmol mg per FW more and 36.8 pmol mg per FW less, respectively, than those of the non-treated plants (). The fresh weights of the shoots were two–threefold more than those of the roots and the ALA treatment did not influence the fresh weights of these tissues (data not shown). Thus, these data indicated that the ALA treatment increased the cysteine and GSH contents in plants under the +S condition. Significant increases in the cysteine contents of the shoots in response to ALA treatment were also observed in plants under the −S condition, whereas the GSH content in the shoots did not increase. In contrast, no decreases in either the cysteine or GSH contents were detected in the ALA-treated plants under the −S condition. These data indicate that ALA increased the cysteine or GSH contents more effectively in shoots compared with roots and under the +S condition compared with the −S condition.
The increased levels of cysteine and GSH support the usefulness of ALA as a fertilizer. Cysteine is the first organic compound in the sulfur assimilation process and is further converted to methionine, an essential amino acid for animals (CitationCrawford et al. 2000; CitationLeustek et al. 2000; CitationSaito 2004). In addition, GSH functions not only as the major reservoir of non-protein reduced sulfur, but also as a cellular protectant and a signaling molecule in plants (CitationFoyer et al. 2001; CitationNoctor and Foyer 1998). By its anti-oxidant activity, GSH regulates the redox potential of the cell. Glutathione takes part in the sequestration of xenobiotics from cytosol to vacuole through formation of GSH conjugates by glutathione-S-transferase. In addition, GSH plays an important role in heavy metal detoxification. Thus, ALA’s effect on the upregulation of cysteine and GSH contents, as shown here, can be used to increase the nutritional value of crops and to increase their tolerance to environmental stresses.
Figure 4 Effects of 5-aminolevulinic acid (ALA) treatment on cysteine and glutathione (GSH) accumulation in plants. Plants were treated as described in Fig. 2. The medium used for the experiment contained 1500 (+S) or 15 μ mol L−1 (−S) sulfate as a sulfur source. Statistically significant differences from the control are shown. *P < 0.1; **P < 0.05 (n = 4). FW, fresh weight.
The patterns of cysteine and GSH accumulation in shoots and roots in response to ALA treatment differed (), which was unexpected considering the gene expression analysis in the roots. Under the +S condition, ALA treatment induced enhanced expression of sulfur transport and assimilation genes clearly in the roots; however, it increased the levels of cysteine and GSH in the shoots, but not in the roots (). Cysteine and GSH are synthesized in both photosynthetic and non-photosynthetic tissues (CitationCrawford et al. 2000; CitationLeustek et al. 2000; CitationNoctor and Foyer 1998; CitationNoctor et al. 2002; CitationSaito 2004). It has been reported that cysteine and GSH are translocated from roots to shoots through xylem flow and vice versa from the shoot to the roots through the phloem (CitationHerschbach et al. 1998; CitationLi et al. 2006; CitationRauser et al. 1991; CitationTausza et al. 2004). We speculate that the ALA treatment induced the biosynthesis of cysteine and GSH in the roots and the transfer of cysteine and GSH to the shoots, probably because ALA treatment activates the translocation mechanism only from roots to shoots. In contrast, under the −S condition, the effects of ALA on the metabolite levels were not so significant compared with the +S condition, although the cysteine contents in the shoots did increase. Taken together, ALA should be used as an effective fertilizer under +S conditions and still has the potential to be exploited under −S conditions.
There were some discrepancies among the mRNA levels of sulfur assimilatory genes and cysteine or GSH contents, particularly under the −S condition. The transcript level of SULTR1;2 increased with 1 mmol L−1 of ALA treatment under the −S condition, but sulfate uptake activity decreased and, in turn, the cysteine contents in the shoots increased in the same condition (–). The cysteine contents in the shoots were significantly increased by 0.3 mmol L−1 of ALA treatment under the −S condition, whereas mRNA levels of sulfur assimilatory genes in roots increased under the same condition (,). There have been several reports demonstrating the post-transcriptional control of sulfur assimilatory enzymes, in which changes in APR mRNA levels and APR protein levels by some treatments are not correlated (CitationKoprivova et al. 2008). Cysteine synthesis is controlled by the protein–protein interaction of cysteine synthase and Serat (CitationKumaran et al. 2009; CitationWirtz and Hell 2007). Synthesis of γ-EC is controlled by cellular redox state through the modification of thiol bases in the glutamate–cysteine ligase (CitationHicks et al. 2007). Thus, levels of cysteine and GSH could be controlled through these post-transcriptional regulation mechanisms for adequate maintenance of sulfur levels in plants.
The positive effects of ALA on the transcript levels of sulfur assimilatory genes were determined in the present study (,). Stimulation of carbon fixation and nitrogen assimilation by ALA has been reported (CitationHotta et al. 1997b; CitationMishra and Srivastava 1983), and the positive effects of carbon and nitrogen availability on the mRNA levels of sulfur assimilatory genes have been reported (CitationKopriva et al. 1999; CitationKoprivova et al. 2000; CitationMaruyama-Nakashita et al. 2004b). It is not clear whether the effects of ALA on mRNA levels of sulfur assimilatory genes and cysteine and GSH contents result from the increased level of carbon and nitrogen assimilation or the direct influence of ALA on the sulfur assimilatory genes; this is an interesting question with regard to the regulatory mechanism of the sulfur assimilatory pathway. The tested concentrations of ALA in the present study did not increase the plant growth of A. thaliana; however, the new roles of ALA demonstrated in the present study possibly contribute to ALA-mediated stimulation of plant growth.
Acknowledgments
We thank Eri Inoue for the HPLC operation in the thiol analysis. This work was supported in part by a Special Postdoctoral Fellowship of RIKEN and Grants-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan to A. Maruyama-Nakashita.
References
- Askira , Y , Rubin , B and Rabinowitch , HD . 1991 . Differential response to the herbicidal activity of δ-aminolevulinic acid in plant with high and low sod activity . Free Radic. Res. Commun , 12-13 : 837 – 843 .
- Chakraborty , N and Tripathy , BC . 1992 . Involvement of singlet oxygen in 5-aminolevulinic acid-induced photodynamic damage of cucumber (Cucumis sativus L.) chloroplasts . Plant Physiol. , 98 : 7 – 11 .
- Crawford , NM , Kahn , ML , Leustek , T and Long , SR . 2000 . “ Nitrogen and sulfur ” . In Biochemistry & Molecular Biology of Plants , Edited by: Buchanan , BB , Gruissem , W and Jones , RL . 824 – 849 . Rockville, MD : American Society of Plant Biologists .
- Foyer , CH , Theodoulou , FL and Delrot , S . 2001 . The functions of inter- and intracellular glutathione transport systems in plants . Trends Plant Sci. , 6 : 486 – 492 .
- Gutierrez-Marcos , JF , Roberts , MA , Campbell , EI and Wray , JL . 1996 . Three members of a novel small gene-family from Arabidopsis thalianaable to complement functionally an Escherichia colimutant defective in PAPS reductase activity encode proteins with a thioredoxin-like domain and “APS reductase” activity . Proc. Natl Acad. Sci. USA , 93 : 13377 – 13382 .
- Herschbach , C , Jouanin , L and Rennenberg , H . 1998 . Overexpression of {gamma}-glutamylcysteine synthetase, but not of glutathione synthetase, elevates glutathione allocation in the phloem of transgenic poplar trees . Plant Cell Physiol. , 39 : 447 – 451 .
- Hicks , LM , Cahoon , RE , Bonner , ER , Rivard , RS , Sheffield , J and Jez , JM . 2007 . Thiol-based regulation of redox-active glutamate-cysteine ligase from Arabidopsis thaliana . Plant Cell , 19 : 2653 – 2661 .
- Hirai , MY , Fujiwara , T , Awazuhara , M , Kimura , Y , Noji , M and Saito , K . 2003 . Global expression profiling of sulfur-starved Arabidopsisby DNA macroarray reveals the role of O-acetyl-L-serine as a general regulator of gene expression in response to sulfur nutrition . Plant J. , 33 : 651 – 663 .
- Hirai , MY , Fujiwara , T , Chino , M and Naito , S . 1995 . Effects of sulfate concentrations on the expression of a soybean seed storage protein gene and its reversibility in transgenic Arabidopsis thaliana . Plant Cell Physiol. , 36 : 1331 – 1339 .
- Hotta , Y , Tanaka , T , Takaoka , H , Takeuchi , Y and Konnai , M . 1997a . New physiological effects of 5-aminolevulinic acid in plants: The increase of photosynthesis, chlorophyll content, and plant growth . Biosci. Biotechnol. Biochem. , 61 : 2025 – 2028 .
- Hotta , Y , Tanaka , T , Takaoka , H , Takeuchi , Y and Konnai , M . 1997b . Promotive effects of 5-aminolevulinic acid on the yield of several crops . Plant Growth Regul. , 22 : 109 – 114 .
- Kataoka , T , Watanabe-Takahashi , A Hayashi , N . 2004 . Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis . Plant Cell , 16 : 2693 – 2704 .
- Kawashima , CG , Berkowitz , O , Hell , R , Noji , M and Saito , K . 2005 . Characterization and expression analysis of a serine acetyltransferase gene family involved in a key step of the sulfur assimilation pathway in Arabidopsis . Plant Physiol. , 137 : 220 – 230 .
- Kittsteiner , U , Mostowska , A and Redinger , W . 1991 . The greening process in cress seedlings. I. Pigment accumulation and ultrastructure after application of 5-aminolevulinate and complexing agents . Physiol. Plant. , 81 : 139 – 147 .
- Kopriva , S , Muheim , R Koprivova , A . 1999 . Light regulation of assimilatory sulphate reduction in Arabidopsis thaliana . Plant J. , 20 : 37 – 44 .
- Koprivova , A , North , KA and Kopriva , S . 2008 . Complex signaling network in regulation of adenosine 5′-phosphosulfate reductase by salt stress in Arabidopsis roots . Plant Physiol. , 146 : 1408 – 1420 .
- Koprivova , A , Suter , M , Op den Camp , R , Brunold , C and Kopriva , S . 2000 . Regulation of sulfur assimilation by nitrogen in Arabidopsis . Plant Physiol. , 122 : 737 – 746 .
- Kumaran , S , Yi , H , Krishnan , HB and Jez , JM . 2009 . Assembly of the cysteine synthase complex and the regulatory role of protein-protein interactions . J. Biol. Chem. , 284 : 10268 – 10275 .
- Lappartient , AG , Vidmar , JJ , Leustek , T , Glass , ADM and Touraine , B . 1999 . Inter-organ signaling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound . Plant J. , 18 : 89 – 95 .
- Leustek , T , Martin , MN , Bick , J and Davies , JP . 2000 . Pathways and regulation of sulfur metabolism revealed through molecular genetic studies . Annu. Rev. Plant Physiol. Plant Mol. Biol. , 51 : 141 – 166 .
- Li , Y , Dankher , OP , Carreira , L , Smith , AP and Meagher , RB . 2006 . The shoot-specific expression of gamma-glutamylcysteine synthetase directs the long-distance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic . Plant Physiol. , 141 : 288 – 298 .
- Maruyama-Nakashita , A , Nakamura , Y , Yamaya , T and Takahashi , H . 2004a . A novel regulatory pathway of sulfate uptake in Arabidopsisroots: implication of CRE1/WOL/AHK4-mediated cytokinin-dependent regulation . Plant J. , 38 : 779 – 789 .
- Maruyama-Nakashita , A , Nakamura , Y , Yamaya , T and Takahashi , H . 2004b . Regulation of high-affinity sulfate transporters in plants: towards systematic analysis of sulfur signaling and regulation . J. Exp. Bot. , 55 : 1843 – 1849 .
- Mishra , SN and Srivastava , HS . 1983 . Stimulation of nitrate reductase activity by delta amino levulinic acid in excised maize leaves . Cell. Mol. Life Sci. , 39 : 1118 – 1120 .
- Noctor , G and Foyer , CH . 1998 . ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control . Annu. Rev. Plant Physiol. Plant Mol. Biol. , 49 : 249 – 279 .
- Noctor , G , Gomez , L , Vanacker , H and Foyer , CH . 2002 . Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling . J. Exp. Bot. , 53 : 1283 – 1304 .
- Rauser , WE , Schupp , R and Rennenberg , H . 1991 . Cysteine, {gamma}-glutamylcysteine, and glutathione levels in maize seedlings. Distribution and translocation in normal and cadmium-exposed plants . Plant Physiol. , 97 : 128 – 138 .
- Rebeiz , CA , Reddy , KN , Nandihalli , UB and Velu , J . 1990 . Tetrapyrrole-dependent photodynamic herbicides . Photochem. Photobiol. , 52 : 1099 – 1117 .
- Saito , K . 2004 . Sulfur assimilatory metabolism. The long and smelling road . Plant Physiol. , 136 : 2443 – 2450 .
- Shibagaki , N , Rose , A McDermott , JP . 2002 . Selenate-resistant mutants of Arabidopsis thalianaidentify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots . Plant J. , 29 : 475 – 486 .
- Smith , FW , Ealing , PM , Hawkesford , MJ and Clarkson , DT . 1995 . Plant members of a family of sulfate transporters reveal functional subtypes . Proc. Natl Acad. Sci. USA , 92 : 9373 – 9377 .
- Smith , FW , Hawkesford , MJ Ealing , PM . 1997 . Regulation of expression of a cDNA from barley roots encoding a high affinity sulfate transporter . Plant J. , 12 : 875 – 884 .
- Takahashi , H , Watanabe-Takahashi , A , Smith , FW , Blake-Kalff , M , Hawkesford , MJ and Saito , K . 2000 . The roles of three functional sulfate transporters involved in uptake and translocation of sulfate in Arabidopsis thaliana . Plant J. , 23 : 171 – 182 .
- Takahashi , H , Yamazaki , M Sasakura , N . 1997 . Regulation of sulfur assimilation in higher plants: a sulfate transporter induced in sulfate starved roots plays a central role in Arabidopsis thaliana . Proc. Natl Acad. Sci. USA , 94 : 11102 – 11107 .
- Tanaka , T , Iwai , K , Watanabe , K and Hotta , Y . 2005 . Development of 5-aminolevulinic acid for agricultural uses . Regul. Plant Growth Dev. , 40 : 22 – 29 .
- Tausza , M , Pilcha , B , Rennenberg , H , Grilla , D and Herschbach , C . 2004 . Root uptake, transport, and metabolism of externally applied glutathione in Phaseolus vulgaris seedlings . J. Plant Physiol. , 161 : 347 – 349 .
- Vauclare , P , Kopriva , S Fell , D . 2002 . Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5′-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols . Plant J. , 31 : 729 – 740 .
- Vidmar , JJ , Tagmount , A , Cathala , N , Touraine , B and Davidian , JCE . 2000 . Cloning and characterization of a root specific high-affinity sulfate transporter from Arabidopsis thaliana . FEBS Lett. , 475 : 65 – 69 .
- Wang , R , Okamoto , M , Xing , X and Crawford , N . 2003 . Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism . Plant Physiol. , 132 : 556 – 567 .
- Wirtz , M and Hell , R . 2007 . Dominant-negative modification reveals the regulatory function of the multimeric cysteine synthase protein complex in transgenic tobacco . Plant Cell , 19 : 625 – 639 .
- Yoshimoto , N , Inoue , E , Watanabe-Takahashi , A , Saito , K and Takahashi , H . 2007 . Posttranscriptional regulation of high-affinity sulfate tansporters in Arabidopsis by sulfur nutrition . Plant Physiol. , 145 : 378 – 388 .
- Yoshimoto , N , Takahashi , H , Smith , FW , Yamaya , T and Saito , K . 2002 . Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsisroots . Plant J. , 29 : 465 – 473 .