Abstract
In order to elucidate the feature of T‐cell receptor (TCR) signal transduction in T‐cells from acute myeloid leukemia (AML), the expression levels of CD3gamma, delta, epsilon and zeta chain genes in CD3+ T cells were analyzed using real‐time PCR. CD3+ T cells sorted from peripheral blood of 10 AML patients and 10 healthy donors were used in the study. Significantly lower expression levels of all four CD3gamma, delta, epsilon, and zeta chain genes were found in the AML samples. The expression pattern of the four CD3 chains was epsilon>gamma>delta>zeta in CD3+ T cells from AML samples, which was different from the healthy control group. In conclusion, the results provide a global gene expression profile of CD3gamma, delta, epsilon, and zeta chains in AML patients. Deficiency of all four CD3 gene expression levels might represent the feature related to T‐cell immunodeficiency.
Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia affecting adults, and its incidence is expected to further increase as the population ages. Despite recent scientific advances in understanding the molecular biology of AML, the survival rates in AML, with the exception of acute promyelocytic leukemia, has not improved significantly over the last 30 years and are still below 30%.Citation1,Citation2 In patients with hematological malignancies, cell‐mediated immunity is often suppressed, being most profound in those with advanced disease. Such immune dysfunction, as demonstrated in many patients with leukemia, may be due to reduced thymic output, skewed expression of T‐cell receptor (TCR) repertoire, and/or altered expression of the TCR–CD3 complex.Citation3–Citation7 Most studies focused on the change of TCR Vbeta repertoire.Citation4–Citation5Citation8 However, little is known about the expression pattern of TCR–CD3 complex in these patients.
The TCR–CD3 complex is composed of subunits originating from six different genes: TCRalpha and TCRbeta (or TCRgamma and TCRdelta), CD3gamma, CD3delta, CD3epsilon, and CD3zeta.Citation9,Citation10 The CD3 complex is transmembrane proteins and plays a key role in TCR signal transduction; it consists of three distinct dimers, namely, CD3gammaepsilon, CD3deltaepsilon, and CD3zetazeta.Citation11,Citation12 All of the CD3 subunits carry the so‐called immunoreceptor tyrosine‐based activation motif (ITAM) in the intracytoplasmic region. The signals are initiated by the specific phosphorylation of two tyrosine residues located in the conserved ITAM. There are 10 ITAMs in the CD3 complex, six of which are present in the CD3zeta homodimer; CD3gamma, CD3delta, and CD3epsilon each have one ITAM.Citation13,Citation14
The absence of the CD3zeta chain not only influences TCR expression on the cell membrane and the number of single positive (CD4+ or CD8+) circulating T cells, but also impairs the proliferative response and level of activation of mature T cells. T cells from patients with systemic lupus erythematosus,Citation15 aplastic anemia,Citation16 and leukemiaCitation7,Citation17 are all functionally impaired as indicated by decreased CD3zeta chain expression. CD3zeta chain expression is significantly down‐regulated in all T‐cell subsets in more than 90% of chronic myeloid leukemia (CML) patients, and such abnormalities result in increased ex vivo susceptibility to apoptosis.Citation6 CD3zeta chain expression in CD4+ and CD8+ T‐cells from multiple myeloma patients was found to be markedly reduced, particularly in advanced stage multiple myeloma.Citation18 In addition, there was a significantly lower expression of the CD3epsilon chain in CML patients.Citation6
Patients with primary immunodeficiency diseases may exhibit a deficiency of CD3gamma, CD3delta, or CD3epsilon.Citation14 Homozygous mutation of CD3delta and CD3epsilon genes leads to a complete block of T‐cell development and thus to an early‐onset severe combined immunodeficiency phenotype.Citation14 Deficiency of CD3gamma may cause partial T‐cell immunodeficiency.Citation14 However, there is no information about the change in the expression levels of CD3gamma, CD3delta, or CD3epsilon in cancer patients.
Our previous studies have showed that skewed expression of TCR Vbeta subfamilies and decreased recent thymic output function are the common feature in patients with AML.Citation3,Citation4 To further examine the characteristics of immunodeficiency in these patients, we investigated the expression levels and pattern in all of four CD3 complex genes in CD3+ T cells from patients with AML.
Materials and Methods
Samples
Ten newly diagnosed, untreated AML cases were included in the study (seven males and three females with a median age of 36 years and a range of 13–65 years), classified according to the French–American–British criteria, two patients were classified as M1, three were M2, one was M3, three were M4, and one was M5. The clinical data of the patients are listed in . Ten healthy individuals (five males and four females with a median age of 38·5 years and a range of 24–54 years) served as control group.
Table 1. Clinical data of AML patients
Mononuclear cell isolation
Peripheral blood mononuclear cells were isolated from AML and healthy individuals by Ficoll‐Hypaque gradient centrifugation.
CD3+ T‐cell sorting
The CD3+ T cells from peripheral blood mononuclear cells from 10 AML cases and 10 healthy individuals were sorted using CD3 monoclonal antibodies and MACS® Magnetic Cell sorting technique (Miltenyi Biotec, Bergisch Gladbach, Germany).
RNA isolation and cDNA synthesis
RNA was extracted from the sorted CD3+ T cells using a Trizol RNA extraction buffer according to the manufacturer’s protocol (Trizol; Invitrogen, Carlsbad, CA, USA). The quality of RNA was analyzed in 0·8% agarose gel stained with ethidium bromide. Two microgram RNA was reversely transcribed into the first single‐stranded cDNA with random hexamer primers, using reverse transcriptase, of the Superscript II Kit (Gibco, Gaithersburg, MD, USA). The quality of cDNA was confirmed by RT‐PCR for beta2 microglobubin (beta2M) gene amplification (the primers of beta2M gene for RT‐PCR were listed in ).
Table 2. Sequences of primers used in PCR
Real‐time relative quantitative PCR for CD3gamma, delta, epsilon, and zeta genes
Real‐time PCR with SYBR Green I technique was used to examine CD3gamma, delta, epsilon, and zeta genes expression levels in cDNA of CD3+ T cells. The beta2‐microglobulin gene was used as an endogenous reference, and the fold‐change of CD3gamma, delta, epsilon, and zeta genes expression level were used by the ×100% method. The primers were purchased from Shanghai Invitrogen Biotechnology Co. Ltd (Shanghai, China; ).Citation19 PCR was performed according to Stams WAG which was described in our previous study.Citation19–Citation21 In brief, PCR of 25 μl total volume was performed with 1 μl cDNA, 0·5 μM of each primers (CD3gamma, delta, epsilon, zeta‐for and CD3gamma, delta, epsilon, zeta‐back primers for CD3gamma, CD3delta, CD3epsilon, and CD3zeta genes, respectively, beta2M‐forward and beta2M‐backward primers for beta2‐microglobulin gene amplification), 2·5×RealMastrMix 11·25 μl (Tiangen, Beijing, China). After initial denaturation at 95°C for 2 minutes, 45 cycles consisting of the following procedure was performed using MJ Research DNA Engine Opticon 2 PCR cycler (BIO‐RAD, USA): 15 seconds at 95°C; 1 minute at 58·9°C for beta2M and CD3gamma, 60°C for CD3zeta, 60·8°C for CD3delta, and 62°C for CD3epsilon; and 1 second at 82°C. The relative mRNA expression levels of CD3gamma, delta, epsilon, and zeta in each sample were calculated using the comparative cycle time (Ct) method.Citation20 Briefly, the target PCR Ct value (the cycle number at which emitted fluorescence exceeds 10×SD of baseline emissions) is normalized by subtracting the beta2M Ct value from the target PCR Ct value, which gives the ΔCt value. From this ΔCt value, the relative expression level of beta2M for each target PCR can be calculated using the following equation: Relative mRNA expression =
×100% (ΔCt =
)(x: anyone of CD3gamma, delta, epsilon, or zeta gene).Citation19,Citation20
Statistical analysis
The Mann–Whitney test was also used to compare the expression levels of CD3 genes between the two groups. Spearman’s correlation analysis was used to estimate the correlation between different gene expression levels from different CD3 genes. Only values with P<0·05 were considered statistically significant. Pearson correlation and linear regression analysis were used to estimate the correlation between age and expression levels of CD3 genes in T cells from AML patients.
Results
The expression levels of CD3gamma, CD3delta, CD3epsilon, and CD3zeta genes were quantitatively assessed by real‐time PCR in CD3+ T cells from both AML and healthy samples. All of four genes could be detected in every sample. The PCR products from all of the four genes were sent to sequence and the sequencing results were confirmed by blast analysis as compared with data from GeneBank in our previous study.Citation19
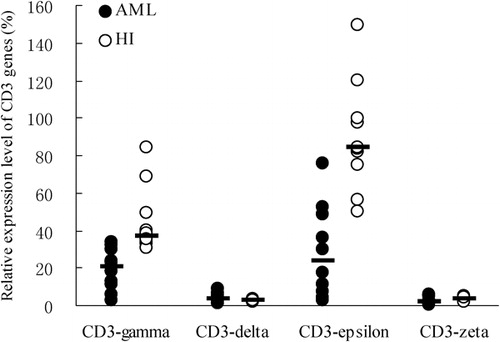
The relative mRNA expression levels of all four CD3gamma, delta, epsilon, and zeta chain genes (mean rank = 6·0, 13·3, 5·9, and 7·6%) in CD3+ T cells from AML samples were significantly lower than those from healthy individuals (mean rank = 15·0, 7·7, 15·1, and 13·4%) (P = 0·001, P = 0·034, P = 0·001, and P = 0·028 respectively) (). The expression pattern of the four CD3 chains was epsilon>gamma>delta>zeta in CD3+ T cells from AML samples. In contrast, that in healthy control CD3+ T cells was epsilon>gamma>zeta>delta ().
Figure 2. The pattern of CD3 gene expression levels in CD3+ T cells from 10 cases with AML and healthy individuals (HI).
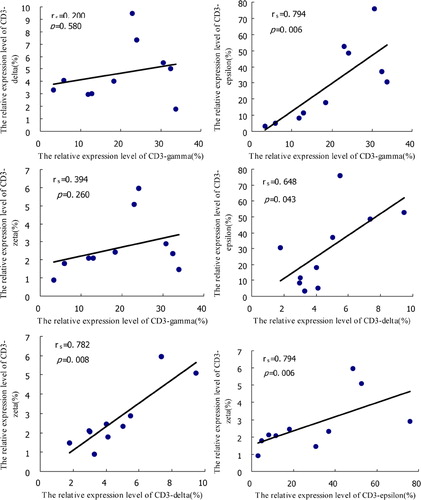
Spearman’s correlation analysis was performed for the relative expression levels of the CD3gamma, delta, epsilon, and zeta chain genes. There was a significant positive correlation between the relative expression levels of CD3gamma and CD3epsilon (rs = 0·794, P = 0·006), CD3delta and CD3epsilon (rs = 0·648, P = 0·043), CD3delta and CD3zeta (rs = 0·782, P = 0·008), and CD3epsilon and CD3zeta (rs = 0·794, P = 0·006). No significant correlation of gene expression levels was found in CD3gamma and CD3delta (rs = 0·200, P = 0·580), and CD3gamma and CD3zeta (rs = 0·394, P = 0·263) ().
Figure 3. The correlation analysis among the relative expression levels of CD3gamma, delta, epsilon, and zeta chain genes in CD3+ T cells from AML patients.
No statistically significant correlation was found between the age and the expression levels of all four CD3 genes in AML T‐cell group (r = −0·020, P = 0·957; r = −0·515, P = 0·128; r = −0·102, P = 0·780; and r = −0·500, P = 0·141, respectively) by Pearson correlation and linear regression analysis.
Discussion
In hematological malignancies, poor cellular immune function may relate to carcinogenic processes and to worse prognosis.Citation22,Citation23 The abnormal expression of the TCR repertoire and altered expression of the TCR–CD3 complex may be the primary reasons for T‐cell immunodeficiency.Citation22 Previous research showed that skewed expression of TCR Vbeta repertoire and the reduced recent thymic output function are the common feature in patients with leukemia.Citation3,Citation4 To gain more insight into the TCR–CD3 complex and the transduction of TCR signals, which is important for T‐cell activation, we analyzed the expression level of all four CD3 genes (CD3gamma, CD3delta, CD3epsilon, and CD3zeta) in sorted CD3+ T cells from AML patients. Significant low expression of all CD3 genes were found, indicating that lower capability of transduction of TCR signals might be the common feature in AML patients. An essential role of CD3 genes in T‐cell development has been identified in mice by using gene‐specific mutations,Citation24 and down‐expression of CD3 might play an important role in mechanism of T‐cell immunodeficiency in AML patients.
Dysregulation of CD3zeta gene expression has been identified in several types of leukemia, including CML, T‐ALL, and B‐CLL (B cell‐chronic lymphocytic leukemia), and lower expression of the CD3epsilon chain was found in CML patients.Citation6–Citation7,Citation18,Citation25 However, no information exists regarding the down‐regulation of CD3gamma, and CD3delta in hematological malignancies or solid tumors. There are no comparisons of the expression patterns of all CD3 genes in leukemia or other diseases. In the present study, we analyzed the expression pattern of all four CD3 genes, not only CD3zeta and CD3epsilon displayed lower expression levels, but also the other two CD3 genes: CD3gamma and CD3delta, showed down‐regulation. Moreover, the results did not show a complementary phenomenon of expression levels in different CD3 genes, but rather a negative correlation of the expression pattern. Thus, differences in activation of TCR signal transduction might be found in different individuals. The result is similar to our previous finding in cord blood and healthy donor samples. It showed positive correlation in most CD3 genes in both CD4+ T cells and CD8+ T cells from cord blood.Citation19 This means that higher expression levels of all CD3 genes might exist in some cases, while lower expression levels might exist in others. This result seems a different expression pattern from the research model of CD3 immunodeficiency, which showed that in the absence of TCR zeta chain, the pre‐TCR complex could conceivably contain the FcepsilonRIgamma chain, which is expressed in early thymocytes and is theoretically capable of substituting for the zeta chain.Citation26 In CD3delta immunodeficiency patients, a marked reduction in CD3gamma, CD3epsilon, and CD3zeta was found.Citation27,Citation28
In the present study, the expression pattern of the CD3 chains was epsilon>gamma>delta>zeta in CD3+ T cells from AML samples, which was different from those in healthy individuals CD3+ T cells with epsilon>gamma>zeta>delta pattern. However, the significance of the CD3 gene expression profiles is unclear, and whether these gene expression patterns are typical in CD3+ T cells from AML patients and healthy individuals remains an open question.
The recent thymic output function and the expression frequency of TCR Vbeta repertoire show age‐related decreased.Citation3 However, the expression levels of CD3 genes did not showed age‐related difference. The expression levels of CD3 genes which are corresponding to the frequency of CD3 molecular expressed on surface of T cells are related to the activity of TCR signal transduction, as well as the T‐cell function; thus, functional deficiency of T cells in AML may be affected by the disease, rather an age‐dependent decline.
In conclusion, the present study provides a global gene expression profile of CD3gamma, delta, epsilon, and zeta chains in CD3+ T cells from AML patients. To the best of our knowledge, this study is the first attempt to analyze the expression pattern of the CD3 complex in AML patients. All of four low expression of CD3 complex in AML patients might relate to T‐cell immunodeficiency.
The study was supported by grants from the Key project of Natural Science Foundation of Guangdong Province, China (no. 9251063201000001) and Medical Science Foundation of Guangdong Province (no. A2008341).
References
- Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer 2006;107:2099–107.
- Erba HP. Prognostic factor in elderly patients with AML and the implications for treatment. Hematology Am Soc Hematol Educ Program 2007;2007:420–8.
- Li Y, Yin Q, Yang L, Chen S, Geng S, Wu X, et al.. Reduced levels of recent thymic emigrants in acute myeloid leukemia patients. Cancer Immunol Immunother 2009;58:1047–55.
- Li Y, Chen S, Yang L, Zhou Y, Wu X, Huang M, et al.. Clonal expanded TCR Vβ T cells in patients with APL. Hematology 2005;10:135–9.
- Rezvany M., Jeddi-Tehrani M, Osterborg A, Kimby E, Wigzell H, Mellstedt H. Oligoclonal TCR BV gene usage in B-cell chronic lymphocytic leukemia: major perturbations are preferentially seen within the CD4 T-cell subset. Blood 1999;94:1063–9.
- Chen X, Woiciechowsky A, Raffegerst S, Schendel D, Kolb HJ, Roskrow M. Impaired expression of the CD3-zeta chain in peripheral blood T cells of patients with chronic myeloid leukaemia results in an increased susceptibility to apoptosis. Br J Haematol 2000;111:817–25.
- Chen S, Yang L, Chen S, Li Y. TCR ζ chain expression in T cells from patients with CML. Hematology 2009;14:95–100.
- Wang L, Zhu K, Zha X, Chen S, Yang L, Chen S, et al.. Evolution of T-cell clonality in a patient with Ph-negative acute lymphocytic leukemia occurring after interferon and imatinib therapy for Ph-positive chronic myeloid leukemia. J Hematol Oncol 2010;3:14.
- Call ME, Wucherpfenning KW. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol 2005;23:101–25.
- Clevers H, Alarcon B, Wileman T, Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol 1988;6:629–62.
- Call ME, Pyrdol J, Wucherpfenning KW. Stoichiometry of the T-cell receptor-CD3 complex and key intermediates assembled in the endoplasmic reticulum. EMBO J 2004;23:2348–57.
- Arnett KL, Harrison SC, Wiley DC. Crystal structure of a human CD3-epsilon/delta dimmer in complex with a UCHT1 singlechain antibody fragment. Proc Natl Acad Sci USA 2004;101:16268–73.
- Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signaling paradigm. Nat Rev Immunol 2004;4:301–8.
- Fischer A, de Saint Basile G, Le Deist F. CD3 deficiens. Curr Opin Allergy Clin Immunol 2005;5:491–5.
- Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest 1998;101:1448–57.
- Solomou EE, Wong S, Visconte V, Gibellini F, Young NS. Decreased TCR zeta-chain expression in T cells from patients with acquired aplastic anaemia. Br J Haematol 2007;138:72–6.
- Torelli GF, Paolini R, Tatarelli C, Soriani A, Vitale A, Guarini A, et al.. Defective expression of the T-cell receptor-CD3 zeta chain in T-cell acute lymphoblastic leukaemia. Br J Haematol 2003;120:201–8.
- Mozaffari F, Hansson L, Kiaii S, Ju X, Rossmann ED, Rabbani H, et al.. Signalling molecules and cytokine production in T cells of multiple myeloma-increased abnormalities with advancing stage. Br J Haematol 2004;124:315–24.
- Chen S, Yang L, Lu X, Li B, Chan JY, Cai D, et al.. Gene expression profiling of CD3γ, δ, ϵ and ζ chains in CD4+ and CD8+ T-cells from human umbilical cord blood. Hematology 2010;15:230–5.
- Stams WA, den Boer ML, Beverloo HB, Meijerink JP, Stigter RL, van Wering ER, et al.. Sensitivity to L-asparaginase is not associated with expression levels of asparagine synthetase in t(12;21)+ pediatric ALL. Blood 2003;101:2743–7.
- Li Y, Chen S, Yang L, Li B, Chan JY, Cai D. TRGV and TRDV repertoire distribution and clonality of T cells from umbilical cord Blood. Transpl Immunol 2009;20:155–62.
- Hadden JW. Immunodeficiency and cancer: prospects for correction. Int Immunopharmacol 2003;3:1061–71.
- Costello RT, Rey J, Fauriat C, Gastaut JA, Olive D. New approaches in the immunotherapy of haematological malignancies. Eur J Haematol 2003;70:333–45.
- DeJarnette JB, Sommers CL, Huang K, Woodside KJ, Emmons R, Katz K, et al.. Specific requirement for CD3epsilon in T cell development. Proc Natl Acad Sci USA 1998;95:14909–14.
- Kiaii S, Choudhury A, Mozaffari F, Kimby E, Osterborg A, Mellstedt H. Signaling molecules and cytokine production in T cells of patients with B-cell chronic lymphocytic leukemia (B-CLL): comparison of indolent and progressive disease. Med Oncol 2005;22:291–302.
- Shores EW, Love PE. TCRζ chain in T cell development and selection. Curr Opin Immunol 1997;9:380–9.
- Dadi HK, Simon AJ, Roifman CM. Effect of CD3δ deficiency on maturation of α/β and γ/δ T-cell lineages in severe combined immunodeficiency. N Engl J Med 2003;349:1821–8.
- Roifman CM. CD3δ immunodeficiency. Curr Opin Allergy Clin Immunol 2004;4:479–84.