Abstract
Most of the biological actions of vitamin D are mediated by an intracellular receptor (VDR) in which several single nucleotide gene polymorphisms have been identified. Vitamin D deficiency is increasingly identified among thalassemic patients and recent evidence links it with myocardial iron accumulation. The aim of this work was to assess the distribution of the Fok‐I polymorphism of the VDR gene among Greek children and young adults with beta‐thalassemia major and to investigate its association with 25(OH)D3 and 1,25(OH)2D3 serum levels. Sixty‐nine thalassemic patients (35 females and 34 males), with a mean age of 23·05±6·07 years, participated in the study. Genotype frequencies of Fok‐I were similar to those previously reported for other populations; 44·9% of the patients were homozygotes for F allele, 43·5% were heterozygotes and 11·6% were homozygotes for the f allele. Low levels of serum 25(OH)D3 were recorded, as 41 patients (59·4%) were below the cut‐off limit of 50 nmol/l that determines deficiency, whereas, levels of 1,25(OH)2D3 showed wide variability ranging from deficiency (⩽50 pmol/l) in 34 patients (49·3%) to excess (⩾125 pmol/l) in 13 patients (18·8%). When stratifying patients according to serum 1,25(OH)2 D3 concentrations, a higher prevalence of the f allele was observed in the deficiency group (P = 0·03). A comparison of the serum concentrations of the two vitamin D metabolites produced a trend towards a negative correlation (r = −0·204, P = 0·09). Further studies are required to assess the genetic contribution to the regulation of vitamin D metabolites in the serum of patients with beta‐thalassemia major.
Introduction
Vitamin D deficiency is increasingly identified among patients with beta‐thalassemia major, especially during the winter period and with ageing.Citation1,Citation2 In addition to the well‐documented role of vitamin D in the maintenance of several organ systems, recent evidence suggests a linkage between vitamin D deficiency and myocardial iron overload in thalassemic patients.Citation3,Citation4 As iron‐induced cardiomyopathy represents the most frequent cause of death among patients with thalassemia,Citation5 vitamin D status in addition to its biological mechanisms of action requires more attention, particularly focused on this special group of patients.
Most of the biological actions of vitamin D are mediated by a high‐affinity intracellular receptor (VDR) that acts as a nuclear transcription factor, regulating the synthesis of proteins involved in bone mineral homeostasis and cell proliferation.Citation6 The VDR gene is located on chromosome 12 (q12–q14); several single‐nucleotide polymorphisms that could potentially modify the expression and activation of VDR, have been identified.Citation7 One of the most frequently studied VDR polymorphism is Fok‐I, located near the 5′ end coding region of the gene. The Fok‐I polymorphism is represented by two allelic variants of the VDR gene: the f allele leads to the production of a 3 amino acid longer and less functional VDR protein, compared to the product of that of F allele.Citation8,Citation9 Research is predominantly focused on associating allelic variants to diseases,Citation10 whereas few evidence supports the genetic contribution of VDR polymorphisms to 25(OH)D3 and 1,25(OH)2 D3 serum concentrations.Citation11–Citation13
The aim of this study was to measure the concentrations of the circulating metabolites of vitamin D, 25(OH)D3 and 1,25(OH)2D3, and consequently to investigate the degree of contribution that Fok‐I polymorphism may have on vitamin D status in patients with beta‐thalassemia major.
Methods
Sixty‐nine children and young adults (35 females and 34 males) with beta‐thalassemia major and a mean decimal age of 23·05±6·07 years (range: 9·25–38·45 years) were recruited for this study. All patients were treated conventionally with regular blood transfusions in order to maintain pre‐transfusional Hb levels above 9 g/dl, and adequate iron‐chelation therapy with deferiprone, deferasirox or combined therapy with deferiprone and desferrioxamine. None of them suffered from other disease entities or was receiving any medication known to affect vitamin D status.
Blood samples were collected during winter time (from December 2008 to February 2009) when ultraviolet exposure is inadequate and seasonally limited in our country. The concentrations of 25(OH)D3 and 1,25(OH)2D3 in the serum were quantified using commercial radio‐immuno assays (DRG Instruments GmbH, Marburg, Germany). Intra‐assay coefficients of variance were 3·3 and 9·3% for 25(OH)D3 and 1,25(OH)2D3, respectively. Inter‐assay coefficients of variance were 5·2 and 12·7% respectively. For the genotyping procedure, DNA was extracted from peripheral blood leukocytes with a DNA Extraction Kit (Promega, Madison, WI, USA) following the manufacturer’s instructions, and stored at −20°C. Primers used for genotyping the Fok‐I polymorphism were: forward, GATGCCAGCTGGCCCTGGCACTG; reverse, ATGGAAACACCTTGCTTCTTCTCCCTC. Polymerase chain reaction conditions included an initial denaturing step at 94°C for 5 minutes, followed by 25 cycles of 94°C for 30 seconds, 60–50°C touchdown (−0·5°C/cycle) for 30 seconds, 72°C for 45 seconds, and a final extension step at 72°C for 10 minutes. The amplified product was digested with the Fok‐I restriction enzyme (New England Bilolabs, Ipswich, MA, USA) producing fragments of 272 bp for the F allele, and 198 and 74 bp for the f allele. Digestion products were resolved on a 2% agarose gel stained with ethidium bromide and visualized with UV light.
The Statistical Package for the Social Sciences (SPSS) for Windows, version 15.0, was used for data analyses. Deviation from Hardy–Weinberg equilibriumCitation14 and differences in genotype distribution between groups were tested with the chi‐square test. The Kolmogorov–Smirnov test was employed to identify parameters with skewed distribution. Characteristics of the three groups defined by the Fok‐I genotypes were compared by using analysis of variance (ANOVA) with Bonferroni’s correction for multiple comparisons or the Kruskall–Wallis test for parameters with skewed distribution. Bivariate correlations were tested using Pearson’s or Spearman’s correlation coefficient for normally or skewed distributed parameters respectively. A P value<0·05 was considered statistically significant.
Results
Levels of vitamin D metabolites and genotype distribution of the Fok‐I VDR gene polymorphism in our study population, and following stratification based on sex, are shown in . No gender‐specific differences were observed with respect to either parameter. FF genotype was presented in 31 patients (44·9%), ff genotype in 8 patients (11·6%) and heterozygosity (Ff) was accounted for 30 patients (43·5%). There was no deviation of the genotype distribution from the Hardy–Weinberg equilibrium (P = 0·03). Stratification of vitamin D metabolite concentrations according to Fok‐I genotypes produced no statistically significant differences either, although patients with the ff genotype had apparently lower mean 25(OH)D3 and 1,25(OH)2D3 values compared to those carrying the F allele ().
Table 1. Distribution of serum vitamin D metabolite concentration and Fok‐I genotypes in the entire study group and following stratification according to sex
Table 2. Serum levels of vitamin D metabolites stratified according to Fok‐I genotypes: results are presented as mean±standard deviation (range) unless otherwise stated
The patients in our study were characterized by low levels of serum 25(OH)D3, as 41 out of 69 (59·4%) had values below the cut‐off limit of 50 nmol/l that determines deficiency, whereas 8 (11·6%) had borderline 25(OH)D3 levels (between 50 and 75 nmol/l). In contrast to the low levels of 25(OH)D3, levels of 1,25(OH)2D3 showed a wide variability ranging from deficiency (⩽50 pmol/l) in 34 patients (49·3%) to excess (⩾125 pmol/l) in 13 patients (18·8%). When patients were stratified according to their 25(OH)D3 status, no significant differences were observed regarding their Fok‐I genotypes (). On the contrary, when the same patients were stratified according to their 1,25(OH)2 D3 status, a higher prevalence of the f allele was recorded in the deficiency group (). Indeed, in patients with 1,25(OH)2 D3 deficiency, f allele heterozygosity (Ff) was found in 52·9% and homozygosity (ff) in 14·7% whereas in those with normal 1,25(OH)2 D3 levels, the percentages were 22·7 and 9·1% respectively for Ff and ff (P = 0·03; ). Finally, levels of 25(OH)D3 and 1,25(OH)2D3 showed a trend towards negative correlation (r = −0·204, P = 0·09; ).
Figure 1. Increased prevalence of ff and Ff genotypes (14·7 and 52·9, respectively) in patients with 1,25(OH)2 D3 deficiency compared to 9·1 and 22·7% for prevalence of ff and Ff genotypes in patients with normal levels of 1,25(OH)2 D3. This difference is statistically significant (P = 0·03).
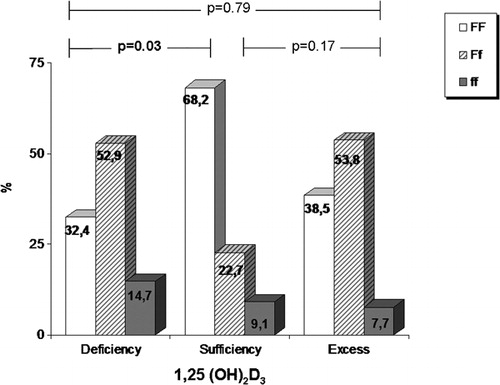
Figure 2. Scatterplot showing levels of 25(OH)D3 plotted against levels of 1,25(OH)2D3 in patients with FF (white triangles), Ff (white circles) and ff genotypes (dark triangles). Values showed a negative correlation approaching statistical significance in the totality of patients (r = −0·204, P = 0·09).
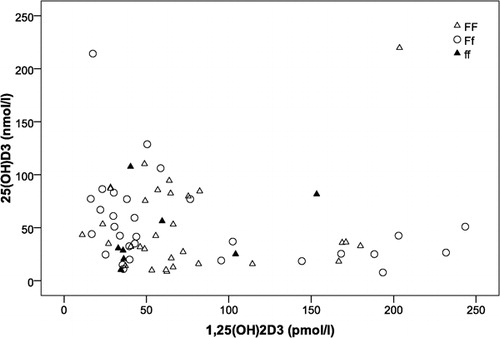
Table 3. Distribution of Fok‐I genotypes among patients following stratification according to their 25(OH)D3 status
Table 4. Distribution of Fok‐I genotypes among patients following stratification according to their 1,25(OH)2D3 status
Discussion
In our study the distribution of Fok‐I genotypes among Greek beta‐thalassemia major patients did not differ considerably from that of other Greek patient groups, different ethnic populations or the population from the HapMap project.Citation15
The discovery of genetic variants of VDR gene has led to studies associating various polymorphisms with increased susceptibility to diseases. Fok‐I and other polymorphisms of VDR gene have been associated with altered calcium homeostasis and impaired bone metabolism.Citation16 In addition, polymorphisms of the VDR gene have been linked with susceptibility to diabetes, cancer, cardiovascular and renal disease, even to autoimmune diseases, as they are extensively reviewed by Valdivielso and Fernandez.Citation10 However, the results obtained so far have been conflicting and the role of VDR polymorphisms in the pathogenesis of various diseases still remains obscure. Similarly, in patients with thalassemia, Tantawy et al.Citation17 showed that homozygosity for the F allele of Fok‐I polymorphism is associated with higher bone mineral density (BMD) at the femoral neck, in contrast to the study by Ferrara et al.Citation18 in which lower BMD at both lumbar spine and femoral neck were observed in patients with the FF genotype. Finally, decreased lumbar BMD in patients with thallasemia has been associated with the presence of homozygosity for another polymorphism of the VDR gene (BsmI) in either both sexesCitation19 or females only.Citation18
Recently, considerable scientific interest has been generated in the association between vitamin D deficiency and myocardial iron overload in patients with beta‐thalassemia major.Citation3,Citation4 The proposed pathogenetic mechanism linking vitamin D and cardiac iron uptake involves the transport of non‐transferrin bound iron in cardiomyocytes through L‐type calcium channels, as was initially proposed by Tsushima et al.Citation20 and was latter supported by studies on mouse models.Citation21,Citation22 Although, most of the biological activities of the 1,25(OH)2D3 are mediated by VDR, which acts as a ligand‐activated transcription factor,Citation10 there has been only a little direct investigation into the involvement of VDR gene polymorphisms in the regulation of vitamin D metabolites concentrations. In 2009, Smolders et al.Citation13 studied the association between Fok‐I polymorphism and serum levels of 25(OH)D3 and 1,25(OH)2D3 in both general population and in patients with multiple sclerosis (MS). In that study the FF genotype was associated with low 25(OH)D3 levels, in apparent contrast to our results, where a trend towards lower 25(OH)D3 serum concentrations was observed in thalassemic patients harboring the ff genotype. On the other hand, our results are in agreement to those reported by Orton et al.,Citation23 where the ff genotype was associated with lower 25(OH)D3 concentrations, in patients with MS. Moreover, our study showed an association between 1,25(OH)2D3 deficiency and the presence of the f allele in patients with beta‐thalassemia major, whereas Smolders et al.Citation13 found increased levels of 1,25(OH)2D3 in MS patients carrying the FF genotype.
The finding that our patient group was characterized by high prevalence of 25(OH)D3 deficiency is perhaps worth noting, as this metabolite is considered the best index of vitamin D status.Citation24 The fact that blood sampling in our study was performed during winter time could partly have affected 25(OH)D3 levels, as seasonal variability of vitamin D metabolites is well documented in the literature.Citation25 However, low levels of vitamin D in patients with beta‐thalassemia major have been frequently described previously,Citation1,Citation3,Citation26–Citation29 even during summer time.Citation1 On the other hand, levels of 1,25(OH)2 D3 showed a wide range in our study, in agreement to previous reports.Citation1,Citation3,Citation26 The serum concentration of 1,25(OH)2D3 is mainly the result of a balance between 1alpha‐hydroxylation of 25(OH)D3 in the kidneys and deactivation of 1,25(OH)2D3 by 24‐hydroxylation in its target tissues.Citation30 A negative feedback mechanism is suggested by several papers showing that increasing levels of 1,25(OH)2D3 limit the formation of its precursor 25(OH)D3.Citation30 The latter is supported by the negative correlation that was observed between the two metabolites in our study.
Up until now, research has been focused on the environmental influences into the regulation of vitamin D metabolite concentrations, whereas there has been little investigation regarding a possible genetic contribution to that. On the other hand, genotyping studies are predominately focused on polymorphism–disease associations and most of them do not relate the presence of polymorphisms to concentrations of vitamin D metabolites. Our study showed significantly decreased levels of 25(OH)D3 in patients with beta‐thalassemia major and a linkage between 1,25(OH)2D3 and the presence of f allele of the Fok‐I polymorphism of the VDR gene. More studies are thus required to confirm and further extent our findings as vitamin D deficiency has been lately associated with unfavorable sequelae in patients with beta‐thalassemia major.
References
- Moulas A, Challa A, Chaliasos N, Lapatsanis PD. Vitamin D metabolites (25-hydroxyvitamin D, 24,25-dihydroxyvitamin D and 1,25-dihydroxyvitamin D) and osteocalcin in beta-thalassaemia. Acta Paediatr 1997;86:594–9.
- Vogiatzi MG, Macklin EA, Trachtenberg FL, Fung EB, Cheung AM, Vichinsky E, et al.. Differences in the prevalence of growth, endocrine and vitamin D abnormalities among the various thalassaemia syndromes in North America. Br J Haematol 2009;146:546–56.
- Wood JC, Claster S, Carson S, Menteer JD, Hofstra T, Khanna R, et al.. Vitamin D deficiency, cardiac iron and cardiac function in thalassaemia major. Br J Haematol 2008;141:891–4.
- Dimitriadou M, Christoforidis A, Economou M, Tsatra I, Vlachaki E, Fidani L, et al.. Elevated serum parathormone levels are associated with myocardial iron overload in patients with betathalassaemia major. Eur J Haematol 2010;84:64–71.
- Borgna-Pignatti C, Rugolotto S, de Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al.. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004;89:1187–93.
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol 2006;92:4–8.
- Uitterlinden AG, Fang Y, van Meurs JB, Pols HA, van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004;338:143–56.
- Whitfield GK, Remus LS, Jurutka PW, Zitzer H, Oza AK, Dang HT, et al.. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol 2001;177:145–59.
- Gross C, Krishnan AV, Malloy PJ, Eccleshall TR, Zhao XY, Feldman D. The vitamin D receptor gene start codon polymorphism: a functional analysis of FokI variants. J Bone Miner Res 1998;13:1691–9.
- Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta 2006;371:1–12.
- Hunter D, de Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, et al.. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res 2001;16:371–8.
- Wjst M, Altmuller J, Braig C, Bahnweg M, Andre E. A genome-wide linkage scan for 25-OH-D3 and 1,25- (OH)2-D3 serum levels in asthma families. J Steroid Biochem Mol Biol 2007;103:799–802.
- Smolders J, Damoiseaux J, Menheere P, Tervaert JW, Hupperts R. Fok-I vitamin D receptor gene polymorphism (rs10735810) and vitamin D metabolism in multiple sclerosis. J Neuroimmunol 2009;207:117–21.
- Mayo O. A century of Hardy–Weinberg equilibrium. Twin Res Hum Genet 2008;11:249–56.
- The International HapMap Project. Nature 2003;426:789–96.
- Keen RW, Major PJ, Lanchbury JS, Spector TD. Vitamin-D-receptor-gene polymorphism and bone loss. Lancet 1995;345:990.
- Tantawy AA, El Kholy M, Moustafa T, Elsedfy HH. Bone mineral density and calcium metabolism in adolescents with beta-thalassemia major. Pediatr Endocrinol Rev 2008;6 Suppl 1:132–5.
- Ferrara M, Matarese SM, Francese M, Borrelli B, Coppola A, Coppola L, et al.. Effect of VDR polymorphisms on growth and bone mineral density in homozygous beta thalassaemia. Br J Haematol 2002;117:436–40.
- Dresner Pollack R, Rachmilewitz E, Blumenfeld A, Idelson M, Goldfarb AW. Bone mineral metabolism in adults with beta-thalassaemia major and intermedia. Br J Haematol 2000;111:902–7.
- Tsushima RG, Wickenden AD, Bouchard RA, Oudit GY, Liu PP, Backx PH. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Circ Res 1999;84:1302–9.
- Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, et al.. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med 2003;9:1187–94.
- Oudit GY, Trivieri MG, Khaper N, Husain T, Wilson GJ, Liu P http://www.ncbi.nlm.nih.gov/pubmed?term=%22Sole%20MJ%22%5BAuthor%5D", et al.. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation 2004;109:1877–85.
- Orton SM, Morris AP, Herrera BM, Ramagopalan SV, Lincoln MR, Chao MJ, et al.. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr 2008;88:441–7.
- Hollis BW. Assessment of vitamin D nutritional and hormonal status: what to measure and how to do it. Calcif Tissue Int 1996;58:4–5.
- Stamp TC, Round JM. Seasonal changes in human plasma levels of 25-hydroxyvitamin D. Nature 1974;247:563–5.
- Napoli N, Carmina E, Bucchieri S, Sferrazza C, Rini GB, Di Fede G. Low serum levels of 25-hydroxy vitamin D in adults affected by thalassemia major or intermedia. Bone 2006;38:888–92.
- Tsitoura S, Amarilio N, Lapatsanis P, Pantelakis S, Doxiadis S. Serum 25-hydroxyvitamin D levels in thalassaemia. Arch Dis Child 1978;53:347–8.
- Aloia JF, Ostuni JA, Yeh JK, Zaino EC. Combined vitamin D parathyroid defect in thalassemia major. Arch Intern Med 1982;142:831–2.
- Dandona P, Menon RK, Houlder S, Thomas M, Hoffbrand AV, Flynn DM. Serum 1,25 dihydroxyvitamin D and osteocalcin concentrations in thalassaemia major. Arch Dis Child 1987;62:474–7.
- Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol 2005;289:F8–28.