Abstract
Background: Thrombophilias have been suggested as a possible cause of recurrent pregnancy loss (RPL).
Objective: Testing for the association of factor V Leiden (FVL) and prothrombin (FII) mutations with RPL among cases from the Nile Delta region of Egypt.
Subjects and methods: Participants included 72 cases having a history of two or more events of unexplained RPL and 70 controls with a good obstetric history. Detection of FVL (G1691A) and FII (G20210A) mutations was carried out using PCR with sequence specific primers.
Results: Cases showed a significantly higher frequency of FVL GA (OR = 21·38, P<0·0001) and FII GA (OR = 36·7, P<0·0001) genotypes. Cases with two or more risk factors had significant higher frequency of both mutant genotypes, while no significant difference could be elicited related to primary or secondary infertility, number of fetal losses, or phase of pregnancy loss.
Conclusion: Screening for thrombophilic mutations may help in the prevention of unexplained RPL.
Introduction
Recurrent pregnancy loss (RPL), defined as two or more spontaneous abortions, affects approximately 5% of women of reproductive age. Although several causes of RPL have been established, more than 50% of cases remain unexplained. Recently, thrombophilias have been suggested as a possible cause of RPL.Citation1,Citation2
Factor V Leiden (FVL; G1691A) and prothrombin (FII; G20210A) gene mutations were considered the two most common causes implicated as risk factors of hereditary thrombophilias acting early or late during pregnancy, i.e. before or after the twentieth week of gestation leading to possible fetal loss.Citation3,Citation4
The gene for factor V is located on chromosome 1(q21 to q25) and its FVL variant arises as a result of a point mutation at nucleotide position 1691 (G/A), resulting in an arginine to a glutamine substitution at position 5064 of the translated polypeptide chain.Citation5–Citation7 FVL is responsible for 20–40% of isolated thrombotic events and 40–45% of familial thrombophilias. Studies investigating the relationship between FVL and RPL, found an association, with odds ratios (ORs) ranging from 0·5 to 18.Citation3,Citation8,Citation9
On the other hand, the human FII gene has been localized to chromosome 11 (11p11–q12). Sequence variation of a G–A transposition in position 20210 of the prothrombin gene recently was identified as a genetic risk factor for thrombosis. The FII gene mutation was found in 4–9% of women with recurrent pregnancy loss, compared with 1–2% of those with uncomplicated pregnancies, with odds ratios ranging from 2 to 9.Citation10,Citation11
In Egypt, early and late pregnancy wastage constitutes a great problem especially among low socioeconomic class and high‐parity groups.Citation12 To our knowledge, the data pertinent to the contribution of genetically based thrombophilia to pregnancy loss among Egyptian women are relatively lacking. This study was planned in order to check for the association of genetic mutations related to FVL and FII genes with unexplained RPL among affected women from the Nile Delta region of Egypt.
Subjects and Methods
This is a case–control study including 142 females with an age ranging from 19 to 38 years taken randomly from those admitted and followed up in the Departments of Obstetrics and Gynecology and Genetics, Mansoura University Hospitals, Mansoura, Egypt. Of these, 72 had a history of two or more events of fetal loss in the form of abortion, miscarriage or stillbirth in any phase of pregnancy. The other 70 were clinically healthy unrelated women with a good obstetric history assigned as a control group. Cases that were documented to have genetic or chromosomal aberrations, anatomic anomalies, or endocrine (hormonal) abnormalities were excluded from the study.
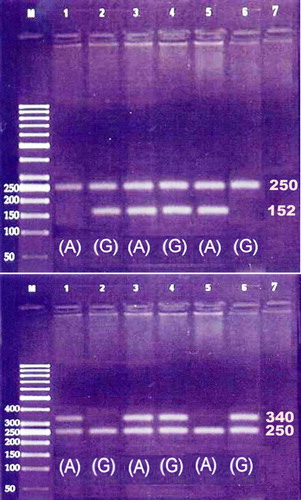
After obtaining approval of local scientific and ethical committees as well as an informed consent from all cases and controls, 3 ml venous blood sample was collected from each subject for DNA extraction and purification (Gentra Systems, Minneapolis, MN, USA). Detection of FVL (G1691A) and FII (G20210A) mutations were done using polymerase chain reaction technique with sequence specific primers. In addition, an amplification of a segment of factor IX gene was done and used as an internal control. Each polymerase chain reaction was performed with 300 ng of DNA, 200 mmol/l of each dNTP, 500 nmol/l of each primer, and 2·5 units of Taq DNA polymerase (Amplitaq Gold; PerkinElmer Cetus, Norwalk, CT, USA). DNA was initially denatured for 10 minutes at 95°C; followed by 10 cycles each in the form of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute; followed by 25 cycles each in the form of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute; then completed with a final extension at 72°C for 7 minutes. Amplification resulted in 152 base pair (bp) product for factor V gene, 340 bp product for FII gene and 250 bp product for FIX. The amplified products will be then electrophoresed in 2% agarose gel, stained with ethidium bromide, and visualized under UV transilluminator ().Citation13,Citation14 The sequences of primers for FVL gene were: FVL (common) 5′‐GGA CTA CTT GAC AAT TAC TGT TCT CTT G‐3′; FVL (wild type): 5′‐GCA GAT CCC TGG ACA GAC G‐3′; FVL (mutant): 5′‐GCA GAT CCC TGG ACA GAC A‐3′; and for FII were: FII (common) 5′‐TCT AGA AAC AGT TGC CTG GCA G‐3′; FII (wild type) 5′‐GCA CTG GGA GCA TTG AGG ATC‐3′ and FII (mutant) 5′‐GCA CTG GGA GCA TTG AGG ATT‐3′; and for FIX (control) were: FIX‐F 5′‐CTC CTG CAG CAT TGA GGG AGA TGG ACA TT‐3′ and FIX‐R: 5′‐CTC GAA TTC GGC AAG CAT ACT CAA TGT AT‐3′.
Figure 1. Amplification of FVL (above) and FII (down) using primers for mutant A alleles (lanes 1, 3, and 5) and normal G allele (lanes 2, 4, and 6) showing positive bands of size 152 bp for FVL and 340 bp for FII, whereas the 250 bp band corresponds to amplified segment of FIX serving as internal control. Lane M corresponds to DNA size marker and lane 7 to the negative control.
Statistical analysis
Data were analyzed using SPSS Statistical Package version 13. Testing association and risk related to FVL and FII was done by comparing genotype and allele frequencies in cases and controls using Fisher’s exact test together with OR and 95% confidence intervals. Hardy–Weinberg test of genetic equilibrium was applied using Chi‐square test to assure that there was no significant difference between observed and expected genotype frequencies.
Results
Compared to controls, cases of unexplained pregnancy loss showed a significantly higher frequency of FVL GA (23·61% versus 1·43%, OR = 21·38, P<0·0001) and FII GA (34·72% versus 1·43%, OR = 36·7, P<0·0001) genotypes with significantly lower frequency of the normal genotypes: FVL GG and FII GG (P<0·0001). The mutant alleles FVL A and FII A showed also a significantly higher frequency among cases compared to controls (P<0·0001). ()
Table 1. Frequencies of FVL and FII gene polymorphic genotypes and alleles among cases of pregnancy loss compared to controls
Analyzing potential risk factors, it was noted that 24 cases were exposed to passive smoking, 22 cases had positive ELISA test for perinatal infection (TORCH), eight had rhesus negative blood group, and 12 had positive test of anti‐phospholipid and anti‐cardiolipin antibodies. Testing the frequency of mutations associated with each risk item showed non‐significant difference; nonetheless, cases with two or more risk factors showed significant higher frequency of both mutant genotypes than other cases (21·4% versus 3·4%, P<0·05). ()
Table 2. Association of potential risk factors related to recurrent abortions and individual cases FVL and FII combined genotypes
Cases showed also significantly higher frequency of combined mutant genoyptes of both FVL and FII genes compared to controls (8·3% versus 0·0%, P<0·001). Cases with primary infertility differed from cases with secondary infertility having higher frequency of FII mutations but this was statistically non‐significant. The same was observed among cases with higher number of fetal losses (more than four times) and was also statistically non‐significant. Regarding the phase of pregnancy loss, cases did not differ regarding the frequency of mutations and stage of pregnancy loss whether this was in the first or second trimester (early loss) or in the third trimester (late loss) or during both phases. ()
Table 3. Association of combined FVL and FII gene mutations related to infertility, number of abortions, and stage of pregnancy in which abortion occurred
Discussion
Thrombophilia is considered still a debated problem sometimes common among women with unexplained recurrent pregnancy loss, with a prevalence as high as 65% in selected populations.Citation11 Inherited thrombophilias as that of FVL G1691A and FII G20210A have been implicated with a variety of adverse pregnancy outcomes including RPL, pre‐eclampsia, intrauterine growth restriction, placental abruption, and stillbirth.Citation9,Citation10,Citation15,Citation16
This study showed a higher risk of unexplained pregnancy loss among Egyptian cases carrying FVL and FII mutations manifested by high ORs compared to controls. On the other hand, the frequencies of mutant FVL and FII genes among Egyptian healthy controls (1·4 and 1·43%, respectively) were found lower than was reported before by Ulu et al. (9·6 and 6·4%, respectively).Citation17 The difference may be due to the fact that their sample was mainly from Cairo including both male and female subjects.
In a previous meta‐analysis, a combined ORs for the association between FVL and FII with RPL were approximately 2·0.Citation3 The same was also reported by Brenner et al. in a study among Jewish women who found FVL and FII were more common in cases compared to controls (32% versus 10% and 8% versus 4%, respectively, P<0·0001) noting that 7% out of them were homozygous mutant to FII compared to none of the controls (P = 0·012).Citation18 Foka et al. also found significant increases in FVL and FII mutations in Greek cases compared to controls (19% versus 4%, P = 0·003, OR = 5·5 and 9% versus 2%, P = 0·038, OR = 4·6, respectively).Citation19
Other studies have revealed also a relatively high mutation rate of FVL like that done by Zammiti et al. and Mtiraoi et al. who reported that FVL mutations but not FII mutation were significantly higher in Tunisian cases versus controls.Citation20,Citation21 Also, Grandone et al. reported a high FVL mutation rate among Italian affected women (16·28% versus 4·24%, P = 0·011).Citation22 In contrast, Sehirali et al. observed that FII mutation rather than FVL was significantly higher in Turkish women with RPL compared to controls.Citation23 In contrast, some other studies denied the presence of an association between FVL and FII mutations and RPL as reported by Raziel et al. and Carp et al. among Jewish,Citation8,Citation24 Sottilotta et al. among Italians,Citation25 Sotiriadis et al. and Mougiou et al. among Greeks,Citation26,Citation27 and Altinatas et al. among Turkish cases.Citation28
Analyzing potential risk factors as smoking, rhesus negative blood group, perinatal infection, and positive immune antibodies showed that cases with two or more risk factors had significant higher frequency of both mutant genotypes that other cases (21·4% versus 3·4%, P<0·05). No significant difference could be elicited among cases subgroups related to primary or secondary infertility, number of fetal losses or phase of pregnancy loss whether being early or late. This is in partial agreement with that of the meta‐analysis done by Rey et al. reporting that FVL mutation was associated with both early and late pregnancy loss, while FII mutation was mainly associated with early pregnancy loss.Citation29 Other studies have reported a higher frequency of FVL mutations mainly among cases with late pregnancy loss as that reported by Kovacheva et al. among Bulgarians,Citation30 Grandone et al. and Martinelli et al. among ItaliansCitation22,Citation31 In contrast, Krause et al. reported a mainly higher frequency of FVL mutations among German cases with early pregnancy lossCitation15 Other studies supporting that FII gene mutations was associated with late pregnancy loss include that of Martinelli et al. among ItaliansCitation31 and Krause et al. among German cases,Citation15 Kovacheva et al. among BulgariansCitation30 and Pihusch et al. among German cases,Citation32 and Brenner et al. among Jewish cases.Citation18 In this respect, taking into consideration the other risk factors, we can speculate that both mutations could affect both pregnancy phases which might depend on the effectiveness of other interacting risk factors.
In conclusion, this strong association between the thrombophilic mutations related to FVL and FII genes and unexplained RPL in Egyptian cases probably warrants the adoption of a screening program for an adequate prevention of such a drastic outcome.
We are grateful to the staff of Obstetrics and Gynecology Department and Genetics Unit of Mansoura University Hospital, Egypt.
References
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V Leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002;126:1304–18.
- Belinda C, Gavin S, Lesley R. Recurrent miscarriage: pathophysiology and outcome. Obstet Gynacol 2005;17:591–7.
- Kovalevsky G, Gracia CR, Jesse A, Berlin JA, Sammel MD, Barnhart KT. Evaluation of the association between hereditary thrombophilias and recurrent pregnancy loss. A meta-analysis. Arch Intern Med 2004;164:558–63.
- Rosendorff A, Dorfman DM. Activated protein c resistance and factor V Leiden: a review. Arch Pathol Lab Med 2007;131:866–71.
- Dahlback B. Procoagulant and anticoagulant properties of coagulation factor V. Factor V Leiden (APC resistance) causes hypercoagulability by dual mechanisms. J Lab Clin Med 1999;133:415–22.
- Nicolaes GA, Dahlback B. Factor V and thrombotic disease: description of a janus-faced protein. Arterioscler Thromb Vasc Biol 2002;22:530–8.
- Dahlback B. Blood coagulation and its regulation by anticoagulant pathways: genetic pathogenesis of bleeding and thrombotic diseases. J Intern Med 2005;257:209–23.
- Raziel A, Kornberg Y, Friedler S, Schachter M, Sela BA, Ron-El R. Hypercoagulable thrombophilic defects and hyperhomocysteinemia in patients with recurrent pregnancy loss. Am J Reprod Immunol 2001;45:65–71.
- Rai R, Tuddenham E, Backos M, Jivraj S, El’Gaddal S, Choy S, et al.. Thromboelastography, whole blood haemostasis and recurrent miscarriage. Hum Reprod 2003;18:2540–3.
- Gerhardt A, Scharf RE, Beckmann MW, Struve S, Bender HG, Pillny M, et al.. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
- Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol 2004;191:412–24.
- Serour G, Younis N, Hefnawi F, Daghistani H, El-bahy M, Nawara M, et al.. Pregnancy wastage. Popul Sci 1981;(2):57–69.
- Ripoll L, Paulin D, Thomas S, Drouet LO. Multiplex PCR-mediated site-directed mutagenesis for one-step determination of factor V Leiden and G20210A transition of the prothrombin gene. Thromb Haemost 1997;78:960–1.
- Hezard N, Cornillet L, Lefebvre P, Gillot L, Potron G, Nguyen P. Multiplex ASA PCR for a simultaneous determination of factor V Leiden gene G-A20210 Prothrombin gene and C-T677 MTHFR gene mutations. Thromb Haemost 1998;79:1054–5.
- Krause M, Zwinge B, Vigh TH, Scharrer I. Important role of FV G1691A in women with pregnancy loss without apparent causes. J Thromb Haemost 2003;1:12–8.
- Pabinger I, Vormittag R. Thrombophilia and pregnancy outcomes. J Thromb Haemost 2005;3:1603–10.
- Ulu A, Elsobky E, Elsayed M, Yidiz Z, Tekin M, Akar N. Frequency of five thrombophilic polymorphisms in the Egyptian population. Turk J Hematol 2006;23:100–3.
- Brenner B, Sarig G, Weiner Z, Younis J, Blumenfeld Z, Lanir N. Thrombophilic polymorphisms in women with fetal loss. Thromb Haemost 1999;82:6–9.
- Foka ZJ, Lambropoulos AF, Saravelos H, Karas GB, Karavida A, Agorastos T, et al.. Factor V Leiden and prothrombin G20210A mutations, but not methylenetetrahydrofolate reductase C6777T, are associated with recurrent miscarriages. Hum Reprod 2000;15:458–62.
- Zammiti W, Mtiraoui N, Mercier E, Abboud N, Saidi S, Mahjoub T, et al.. Association of factor V gene polymorphisms (Leiden; Cambridge; Hong Kong and HR2 haplotype) with recurrent idiopathic pregnancy loss in Tunisia. A case–control study. Thromb Haemost 2006;95:612–7.
- Mtiraoui N, Borgi L, Hizem S, Nsiri B, Finan RR, Gris JC, et al.. Prevalence of antiphospholipid antibodies, factor V G1691A (Leiden) and prothrombin G20210A mutations in early and late recurrent pregnancy loss. Eur J Obstet Gynecol Reprod Biol 2005;119:164–70.
- Grandone E, Margaglione M, Colaizzo D, d’Addedda M, Cappucci G, Vecchione G, et al.. Factor V Leiden is associated with repeated and recurrent unexplained fetal losses. Thromb Haemost 1997;77:822–4.
- Sehirali S, Inal MM, Yildirim Y, Balim Z, Kosova B, Karamizrak T, et al.. Prothrombin G20210A mutation in cases with recurrent miscarriage: a study of the Mediterranean population. Arch Gynecol Obstet 2005;273:170–3.
- Carp H, Salomon O, Seidman D, Dardik R, Rosenberg N, Inbal A. Prevalence of genetic markers for thrombophilia in recurrent pregnancy loss. Hum Reprod 2002;17:1633–37.
- Sottilotta G, Oriana V, Latella C, Luise F, Piromalli A, Ramirez F, et al.. Genetic prothrombotic risk factors in women with unexplained pregnancy loss. Thromb Res 2006;117:681–4.
- Sotiriadis A, Vartholomatos G, Pavlou M, Kolaitis N, Dova L, Stefos T, et al.. Combined thrombophilic mutations in women with unexplained recurrent miscarriage. Am J Reprod Immunol 2007;57:133–41.
- Mougiou A, Androutsopoulos G, Karakantza M, Theodori E, Decavalas G, Zoumbos N. Inherited thrombophilia screening in Greek women with recurrent fetal loss. Clin Exp Obstet Gynecol 2008;35:172–4.
- Altintas A, Pasa S, Akdeniz N, Cil T, Yurt M, Ayyildiz O, et al.. Factor V Leiden and G20210A prothrombin mutations in patients with recurrent pregnancy loss: data from the southeast of Turkey. Ann Hematol 2007;86:727–31.
- Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet 2003;361:901–8.
- Kovacheva K, Ivanov P, Konova E, Simeonova M, Komsa-Penkova R. Genetic thrombophilic defects (factor V Leiden, prothrombin G20210A, MTHFR C677T) in women with recurrent fetal loss. Akush Ginekol (Sofiia) 2007;46:10–6.
- Martinelli I, Taioli E, Cetin I, Marinoni A, Gerosa S, Villa MV, et al.. Mutations in coagulation factors in women with unexplained late fetal loss. N Engl J Med 2000;343:1015–8.
- Pihusch R, Buchholz T, Lohse P, Rübsamen H, Rogenhofer N, Hasbargen U, et al.. Thrombophilic gene mutations and recurrent spontaneous abortion: prothrombin mutation increases the risk in the first trimester. Am J Reprod Immunol 2001;46:124–31.