Abstract
Background: Therapeutic plasma exchange (TPE) has been used for the treatment of patients with thrombotic thrombocytopenic purpura–hemolytic uremic syndrome (TTP–HUS). We report the 10‐year treatment results along with the risk factors analyses.
Methods: Retrospective analyses were performed on patients who were treated with TPE for TTP–HUS.
Results: Fifty‐two patients were included. Secondary causes were identified in 38 patients (73·1%). The others were classified as idiopathic. After a median five sessions of TPE, 26 patients (50·0%) achieved remission. Remission rate in patients with idiopathic and secondary TTP–HUS was 71·4 and 42·1%, respectively. Overall 30‐day mortality rate was 34·6% and median overall survival was 5·2 months. Patients with hematopoietic stem cell transplantation‐associated TTP–HUS did not respond and had poor overall survival. Males had a lower remission rate than females (P = 0·009).
Conclusions: TPE was an effective treatment in patients with idiopathic TTP–HUS. Treatment results were various according to etiology and gender.
Introduction
The mortality of patients with thrombotic thrombocytopenic purpura (TTP) was approximately 90% before the use of therapeutic plasma exchange.Citation1 The prognosis of these patients has dramatically improved with the implementation of therapeutic plasma exchange.Citation2–Citation4 When severe ADAMTS13 (an acronym for a disintegrin and metalloprotease with thrombospondin‐1‐like domains) deficiency is found in patients with classical TTP, therapeutic plasma exchange can remove the ADAMTS13 autoantibody from the plasma and recover normal ADAMTS13 activity.Citation5 In addition, previous studies demonstrated that many other factors such as drug‐dependent autoantibodies, factor V Leiden, and pregnancy also contributed to development of thrombotic thrombocytopenic purpura–hemolytic uremic syndrome (TTP–HUS)Citation6–Citation8 and that therapeutic plasma exchange was also effective for patients without severe ADAMTS13 deficiency.Citation9
The classical features of TTP include thrombocytopenia, microangiopathic hemolytic anemia, neurological symptoms, renal dysfunction, and fever.Citation1 Because of the high mortality, clinically suspected patients should be urgently treated with therapeutic plasma exchange even when not all of these clinical features are present. Currently both thrombocytopenia and microangiopathic hemolytic anemia without an alternative explanation are accepted as criteria for the clinical diagnosis.Citation2–Citation4 Because these criteria cannot distinguish TTP from HUS at an early stage of disease, the combined acronym TTP–HUS has frequently been used.Citation10
Although many cases of TTP–HUS are idiopathic, a number of causes have been identified: hematopoietic stem cell transplantation (HSCT), pregnancy, drugs, infectious diseases, autoimmune diseases, and so on.Citation11–Citation15 Therapeutic plasma exchange is the standard of care in most patients. However, in some forms of TTP–HUS which are associated with specific clinical conditions or drugs, the role of therapeutic plasma exchange is unclear or no longer recommended. The responses to therapeutic plasma exchange in patients with secondary TTP–HUS are various based on the cause of the TTP–HUS.Citation9,Citation11–Citation15
There have been many reports on the predictive and prognostic factors in patients who underwent therapeutic plasma exchange.Citation16–Citation19 However, many of the findings remain inconclusive and in some cases conflicting. The Rose severity scoring index was introduced to estimate the prognosis of patients with TTP–HUS.Citation16 However, in another study, the clinical use of this index was disappointing: fever was the only variable associated with 6‐month mortality.Citation17 In another report, advanced age and severe renal impairment (serum creatinine >2·0 mg/dl) were significant parameters associated with poor outcome.Citation18 There was a study concluding that renal impairment was not a significant prognostic factor and that neurological abnormalities were independent predictors of mortality.Citation19
The objectives of this study were to evaluate the clinical outcomes of therapeutic plasma exchange according to etiology of TTP–HUS and to elucidate factors related to treatment results in patients with TTP–HUS who received therapeutic plasma exchange.
Materials and Methods
Study design
Retrospective analyses were performed on patients who were treated with therapeutic plasma exchange for TTP–HUS. The diagnosis of TTP–HUS was made after confirmation of both thrombocytopenia (<150×109/l) and microangiopathic hemolytic anemia without an alternative explanation, which is consistent with many prior studies.Citation2–Citation4
Between August 1998 and October 2008, 52 consecutive patients with TTP–HUS received therapeutic plasma exchange in the Department of Internal Medicine and the Department of Laboratory Medicine of the Seoul National University Hospital. This population was selected for the present study.
The primary objective of this study was to evaluate the clinical outcomes of therapeutic plasma exchange. The secondary objectives were to analyze the overall survival, risk factors associated with treatment results, and adverse events during therapeutic plasma exchange.
Etiological classification of TTP–HUS
The etiological classification of patients was performed using the method reported by the Oklahoma TTP–HUS registry.Citation9 Patients with secondary TTP–HUS were hierarchically classified into six groups: (1) HSCT; (2) pregnancy; (3) drugs; (4) bloody diarrhea prodrome; (5) the presence of an additional or alternative disorder which may have caused the presenting features; and (6) idiopathic etiology.
Evaluation of complication of TTP–HUS
We defined major neurological abnormalities such as stroke, coma, seizure, or fluctuating focal signs. Less severe abnormalities such as headache, dizziness, or mental status changes with transient confusion were defined as minor abnormalities. Fever was defined as body temperature above 38·0°C at diagnosis. Renal dysfunction was defined as baseline serum creatinine level above 1·5 mg/dl.
Procedure of therapeutic plasma exchange
Therapeutic plasma exchange was performed with either a centrifugation method (COBE Spectra Apheresis System; CaridianBCT, Inc., Lakewood, CO, USA) or a membrane filtration method (Plasauto; Asahi Kasei Kuraray Medical, Tokyo, Japan). For each session, one estimated plasma volume of the patient was removed using these devices and then replaced with the same volume of fresh frozen plasma simultaneously. The estimated plasma volume was automatically calculated by the apheresis machine. The ACD‐A (anticoagulant citrate dextrose solution A) was used for anticoagulation during the centrifugation plasma exchange. When performing the membrane filtration plasma exchange, we used heparin for anticoagulation.
Measure of clinical outcomes
There is no consensus on response criteria after therapeutic plasma exchange. We defined a response to therapeutic plasma exchange as the achievement of a platelet count of 150×109/l or more, which is also used in Oklahoma TTP–HUS registry.Citation9 Exacerbation was defined as recurrent thrombocytopenia following a response plus resumption of therapeutic plasma exchange after 1 day or more but less than 30 days of no therapy. Relapse was defined as the recurrence of TTP–HUS following a remission. Remission was defined as no plasma exchange treatment for 30 days or more. The remission rate was defined as the percentage of patients who had a remission of TTP–HUS after therapeutic plasma exchange. The relapse‐free survival was defined as the length of time between remission and relapse or death among patients who achieved remission after therapeutic plasma exchange. The 30‐day mortality rate from the initiation of therapeutic plasma exchange was also calculated.
Statistical methods
Statistical analyses for categorical variables were performed using the Pearson’s chi‐square test or the Fisher’s exact test as appropriate. The survival data were calculated using the Kaplan–Meier method and comparisons between groups were made using the Cox proportional hazard model. Two‐sided P values below 0·05 were considered statistically significant. SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) software was used for all statistical analyses.
Ethical considerations
Informed consents were provided from patients or their relatives before therapeutic plasma exchange procedures. This study was approved by the Institutional Review Board of the Seoul National University Hospital.
Results
Baseline characteristics
The baseline characteristics of 52 patients are shown in . Renal dysfunction (serum creatinine ⩾1·5 mg/dl), fever (>38·0°C), and neurological abnormalities were observed in 40 patients (76·9%), 23 patients (44·2%), and 32 patients (61·5%), respectively. Major neurological abnormalities developed in 23 patients (44·2%): motor weakness in nine patients (17·3%), decreased consciousness in eight patients (15·4%), seizure in five patients (9·6%), and involuntary movement in one patient (1·9%). Minor neurological abnormalities occurred in nine patients (17·3%): headache in four patients (7·7%), dizziness in one patient (1·9%), and disorientation in four patients (7·7%).
Table 1. Baseline characteristics
Secondary causes of TTP–HUS were identified in 38 patients (73·1%): HSCT (19·2%), pregnancy (5·8%), drugs (25·0%), bloody diarrhea prodrome (3·8%), malignancies (11·5%), autoimmune diseases (5·8%), and surgery (1·9%). The other 14 cases (26·9%) were classified as idiopathic TTP–HUS.
Methods and schedules of therapeutic plasma exchange
Patients underwent a median five sessions of therapeutic plasma exchange (range: 1–32). Forty seven patients (90·4%) received 328 sessions of the therapy with the centrifugation method and five patients (9·6%) received 25 sessions with the membrane filtration method. Schedules of the therapy were as follows: 42 patients (80·7%) received the therapy everyday, seven patients (13·5%) every other day, and three patients (5·8%) just once because of the rapid progression of disease and patient deterioration.
Clinical outcomes of therapeutic plasma exchange
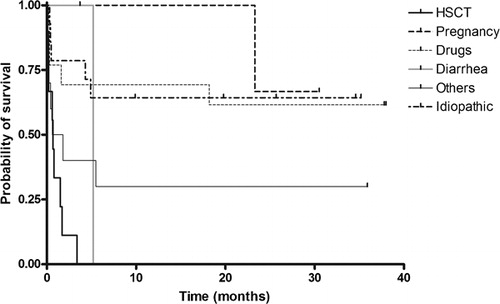
Twenty seven patients (51·9%) responded to the therapy. Exacerbation developed in three patients (5·8%) and relapse in one patient (1·9%) despite an initial response. Remission was achieved in 26 patients (50·0%) since one exacerbated patient did not respond to subsequent therapy. The remission rates in patients with idiopathic and secondary TTP–HUS were 71·4 and 42·1%, respectively. No patient with HSCT‐associated TTP–HUS responded to therapeutic plasma exchange (). The median serum lactate dehydrogenase (LDH) level was decreased to 402·5 (range: 141–9878) IU/l after finishing the therapy. In 20 patients (38·5%), serum LDH level (<250 IU/l) went to normal after the therapy.
Table 2. Analysis of factors associated with remission after therapeutic plasma exchange
The median duration of follow‐up was 64·1 months (range: 12·4–124·4 months). The median overall survival was 5·2 months [95% confidence interval (CI): 0·0–27·1 months). The overall survival varied by etiology (). The overall 30‐day mortality rate was 34·6%: 21·4% in idiopathic patients and 39·5% in patients with all secondary causes. The estimated 5‐year relapse‐free survival rate among patients with remission was 74·8%: 88·9% in idiopathic patients and 67·0% in secondary patients.
Analysis of factors associated with treatment outcomes
The remission rate varied by etiology (P = 0·003) and male patients had significantly high remission rate than female patients (P = 0·009; ). However, the difference in overall survival between male and female was not significant (P = 0·169; ).
Table 3. Analysis of risk factors associated with the overall survival
In the univariate analysis, patients with fever had a shorter overall survival than the others (P = 0·024). However, fever was not a significant factor (P = 0·081) in the multivariate analysis. Patients with HSCT‐associated TTP–HUS had a shorter overall survival than idiopathic patients (P = 0·001) not only in the univariate analysis but also in the multivariate analysis ().
Between patients treated with the centrifugation method and those treated with the membrane filtration method, the remission rate was not significantly different (51·1% versus 40·0%, P = 1·000). The overall survival between these methods was not different, either (P = 0·666).
Adverse events related to therapeutic plasma exchange
Therapeutic plasma exchange‐related adverse events were observed in 19 patients (36·5%) as described in . Abdominal discomfort includes five events (1·4%) of nausea or vomiting and one event (0·3%) of vague abdominal discomfort. Chest discomfort includes two events (0·6%) of palpitation and three events (0·8%) of vague chest discomfort.
Table 4. Adverse events associated with therapeutic plasma exchange
Most of the events did not require treatments or needed only simple medication such as antihistamines, acetaminophen, and calcium infusion. Two patients who had dyspnea after therapeutic plasma exchange required escalation of care including mechanical ventilation in the intensive care unit (ICU). However, these patients received the other session of therapeutic plasma exchange in the ICU without any problem. One of them had a remission and another patient died of TTP–HUS without a response to therapeutic plasma exchange.
Between the centrifugation method and the membrane filtration method, the incidence of overall adverse events was not significantly different. Among patients receiving the centrifugation plasma exchange, paresthesia developed in nine of 328 sessions (2·7%) and paresthesia requiring calcium replacement in six sessions (1·8%). In contrast, paresthesia did not develop in the membrane filtration group. However, this difference was not statistically significant.
Discussion
The remission rate of patients with TTP–HUS who received therapeutic plasma exchange has been reported as 70–80% and the long term survival rate was 60·0–77·1%.Citation2,Citation3,Citation20 In the present study, the remission rate of all patients was 50·0% and the estimated 5‐year overall survival was 62·5%. Among them, the remission rate of idiopathic patients was 71·4% and the 5‐year survival was 62·9%. In this study, 10 of 38 patients with secondary TTP–HUS had history of previous HSCT and the other six patients were related to disseminated malignancies. High proportion of these patients might contribute to poor clinical results of our study.
No patient with HSCT‐associated TTP–HUS responded to therapeutic plasma exchange. These patients also had significantly shorter overall survival compared to patients with idiopathic TTP–HUS. These data are consistent with previous reports.Citation14,Citation21 Known possible pathogenesis of HSCT‐associated TTP–HUS is different from the ADAMTS13 autoantibody‐mediated mechanism of classical TTP. Various conditions such as drugs, mismatched donor, acute graft‐versus‐host disease, and sepsis may contribute to the injury of microvascular endothelial cells, which leads to the development of thrombotic microangiopathy.Citation22–Citation25 This difference in pathogenesis might contribute to poor outcome of patients with HSCT‐associated TTP–HUS. Since there is no efficacious option of treatment available for those patients yet,Citation26 we tried to treat them with therapeutic plasma exchange. However, our treatment results were also poor. Different therapeutic approaches would be necessary for these patients.
In addition, six patients of our study population had associated disseminated malignancies. The overall survival of these patients was extremely poor (median: 0·4 month). In a previous small case series, no patient with TTP associated with bone marrow metastasis responded to therapeutic plasma exchange alone and several patients responded to chemotherapy or chemotherapy plus plasma exchange.Citation27 Because our patients with disseminated malignancy had no other effective treatment option at that time, therapeutic plasma exchange was considered as an option. However, our results were also poor. It could also be considered that the malignancy was merely mimicking the clinical features of TTP–HUS, causing an incorrect diagnosis. Considering the poor prognosis of this group of patients despite therapeutic plasma exchange, other treatments such as anticancer chemotherapy might be preferred to therapeutic plasma exchange if indicated.
In the present study, the overall survival of patients with drug‐associated TTP–HUS was similar to that of patients with idiopathic TTP–HUS. However, the remission rate of drug‐associated patients (53·8%) tended to be lower than that of idiopathic patients (71·4%). The role of therapeutic plasma exchange in patients with drug‐associated TTP–HUS is still debated.Citation15 Therapeutic plasma exchange appeared to reduce the mortality in patients with ticlopidine‐induced TTP–HUS.Citation28,Citation29 However, the effect was uncertain or in doubt for patients with cyclosporine or mitomycin‐C‐induced TTP–HUS.Citation15,Citation30 Further studies are necessary.
The risk factors associated with treatment outcomes have been reported in many prior studies. However, the results are conflicting.Citation16–Citation19 In our study, female gender and etiology of TTP–HUS were significant factors for remission after therapeutic plasma exchange. Patients with low baseline serum creatinine level (⩾1·5 mg/dl) and fever tended to have a low remission rate. In addition, patients with fever (>38·0°C) had a shorter overall survival than those without fever in the univariate analysis and this association was not significant in the multivariate analysis. Our results on the risk factors associated with therapeutic plasma exchange are limited by the different etiologies and small sample size of each etiology among patients with secondary TTP–HUS. Given that all the studies on risk factor analysis were performed retrospectively and that the pathogenic mechanisms of secondary TTP–HUS vary according to etiology, these results must be reinterpreted in the context of the cause of TTP–HUS and further study with a larger prospective design is necessary.
There has been no report regarding the difference in treatment efficacy and adverse events between methods of therapeutic plasma exchange in patients with TTP–HUS. In the present study, there was no significant difference in efficacy and adverse events between these two methods, although the membrane filtration group was relatively small. Because ACD‐A was used for anticoagulation during the centrifugation plasma exchange, hypocalcemia and related symptoms such as paresthesia may develop. No event of paresthesia developed in the membrane filtration group. In contrast, paresthesia developed in 2·7% of sessions in the centrifugation group although this difference was not significant.
This study has several limitations. First, this study was conducted retrospectively. Second, because some subtypes such as pregnancy or bloody diarrhea‐associated TTP–HUS consisted of a very small sample, statistical analysis was impossible in these groups. In addition, because there is still no consensus on the diagnosis and the etiologic classification of TTP–HUS, some might disagree with our patient selection and classification methods which have been used in Oklahoma TTP–HUS registry.
Conclusions
Therapeutic plasma exchange was an effective treatment in patients with idiopathic TTP–HUS and might be considered as an adjunctive therapy for patients with drug‐associated TTP–HUS. However, therapeutic plasma exchange was not effective in HSCT‐ or malignancy‐associated patients. Male gender was related to low remission rate. Therapeutic plasma exchange‐related adverse events were manageable.
The authors wish to acknowledge the efforts of all the staff, especially Ms Yanghyun Kim, in the Apheresis Unit of the Seoul National University Hospital, for performing the therapeutic plasma exchange for patients. This study was supported by Grant no. 0520100020 from the SNUH Research Fund and by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (0405‐BC02‐0604‐0004).
References
- Amorosi EL, Ultmann JE. Thrombotic thrombocytopenic purpura: report of 16 cases and review of the literature. Medicine 1966;45:139–59.
- Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al.. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med 1991;325:393–7.
- Lara PNJr, Coe TL, Zhou H, Fernando L, Holland PV, Wun T. Improved survival with plasma exchange in patients with thrombotic thrombocytopenic purpura–hemolytic uremic syndrome. Am J Med 1999;107:573–9.
- von Baeyer H. Plasmapheresis in thrombotic microangiopathy-associated syndromes: review of outcome data derived from clinical trials and open studies. Ther Apher 2002;6:320–8.
- Moake JL. Thrombotic microangiopathies. N Engl J Med 2002;347:589–600.
- Kojouri K, Vesely S, George JN. Quinine-associated thrombotic thrombocytopenic purpura–hemolytic uremic syndrome: frequency, clinical features, and long-term outcomes. Ann Intern Med 2001;135:1047–51.
- Raife TJ, Lentz SR, Atkinson BS, Vesely SK, Hessner MJ. Factor V Leiden: a genetic risk factor for thrombotic microangiopathy in patients with normal von Willebrand factor-cleaving protease activity. Blood 2002;99:437–42.
- McMinn JR, George JN. Evaluation of women with clinically suspected thrombotic thrombocytopenic purpura–hemolytic uremic syndrome during pregnancy. J Clin Apheresis 2001;16:202–9.
- Vesely SK, George JN, Lämmle B, Studt JD, Alberio L, El-Harake MA, et al.. ADAMTS13 activity in thrombotic thrombocytopenic purpura–hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood 2003;102:60–8.
- George JN. How I treat patients with thrombotic thrombocytopenic purpura–hemolytic uremic syndrome. Blood 2000;96:1223–9.
- Vesely SK, Li X, McMinn JR, Terrell DR, George JN. Pregnancy outcomes after recovery from thrombotic thrombocytopenic purpura–hemolytic uremic syndrome. Transfusion 2004;44:1149–58.
- Lesesne JB, Rothschild N, Erickson B, Korec S, Sisk R, Keller J, et al.. Cancer-associated hemolytic–uremic syndrome: analysis of 85 cases from a national registry. J Clin Oncol 1989;7:781–9.
- Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005;365:1073–86.
- Kojouri K, George JN. Thrombotic microangiopathy following allogeneic hematopoietic stem cell transplantation. Curr Opin Oncol 2007;19:148–54.
- Medina PJ, Sipols JM, George JN. Drug-associated thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Curr Opin Hematol 2001;8:286–93.
- Rose M, Rowe JM, Eldor A. The changing course of thrombotic thrombocytopenic purpura and modern therapy. Blood Rev 1993;7:94–103.
- Wyllie BF, Garg AX, Macnab J, Rock GA, Clark WF. Thrombotic thrombocytopenic purpura/haemolytic uraemic syndrome: a new index predicting response to plasma exchange. Br J Haematol 2006;132:204–9.
- Dervenoulas J, Tsirigotis P, Bollas G, Pappa V, Xiros N, Economopoulos T, et al.. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS): treatment outcome, relapses, prognostic factors. A single-center experience of 48 cases. Ann Hematol 2000;79:66–72.
- Pene F, Vigneau C, Auburtin M, Moreau D, Zahar JR, Coste J, et al.. Outcome of severe adult thrombotic microangiopathies in the intensive care unit. Intensive Care Med 2005;31:71–8.
- Altuntas F, Aydogdu I, Kabukcu S, Kocyigit I, Cikim K, Sari I, et al.. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci 2007;36:57–67.
- Teruya J, Styler M, Verde S, Topolsky D, Crilley P. Questionable efficacy of plasma exchange for thrombotic thrombocytopenic purpura after bone marrow transplantation. J Clin Apher 2001;16:169–74.
- Hahn T, Alam AR, Lawrence D, Ford L, Baer MR, Bambach B, et al.. Thrombotic microangiopathy after allogeneic blood and marrow transplantation is associated with dose-intensive myeloablative conditioning regimens, unrelated donor, and methylprednisolone T-cell depletion. Transplantation 2004;78:1515–22.
- George JN. Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med 2006;354:1927–5.
- Nakamae H, Yamane T, Hasegawa T, Nakamae M, Terada Y, Hagihara K, et al.. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol 2006;81:525–1.
- Cohen H, Bull HA, Seddon A, Enayat MS, Hill FG, Woolf N, et al.. Vascular endothelial cell function and ultrastructure in thrombotic microangiopathy following allogeneic bone marrow transplantation. Eur J Haematol 1989;43:207–14.
- Batts ED, Lazarus HM. Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: real progress or are we still waiting? Bone Marrow Transplant 2007;40:709–19.
- Chang JC, Naqvi T. Thrombotic thrombocytopenic purpura associated with bone marrow metastasis and secondary myelofibrosis in cancer. Oncologist 2003;8:375–80.
- Bennett CL, Weinberg PD, Rozenberg-Ben-Dror K, Yarnold PR, Kwaan HC, Green D. Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases. Ann Int Med 1998;128:541–4.
- Bennett CL, Davidson CJ, Raisch DW, Weinberg PD, Bennett RH, Feldman MD. Thrombotic thrombocytopenic purpura associated with ticlopidine in the setting of coronary artery stents and stroke prevention. Arch Intern Med 1999;159:2524–8.
- Cantrell JE, Phillips TM, Schein PS. Carcinoma-associated hemolytic–uremic syndrome: a complication of mitomycin C chemotherapy. J Clin Oncol 1985;3:723–4.