Abstract
The thrombocytopenia ensuing during acute graft‐versus‐host disease (GVHD) is multifactorial and may significantly compromise the prognosis of the patient; non‐immune persistent thrombocytopenia has been considered as an adverse prognostic factor in GVHD. We describe here the case of a 10‐year‐old girl who developed steroid‐refractory thrombocytopenia and who responded promptly to the subcutaneous delivery of romiplostin. To the best of our knowledge, this is the first description of the usefulness of the peptibody in the setting of GVHD.
Introduction
Thrombopoietin (TPO) is the key cytokine involved in thrombopoiesis, and is the endogenous ligand for the TPO receptor that is expressed on the surface of platelets, megakaryocytes, and megakaryocytic precursors. First‐generation thrombopoietic agents were recombinant forms of human TPO, but their development was discontinued after prolonged thrombocytopenia due to neutralizing auto‐antibodies cross‐reacting with endogenous TPO was observed. Second‐generation thrombopoiesis‐stimulating molecules are now available, which have unique pharmacological properties and no sequence homology to endogenous TPO. Two of these new agents, romiplostim and eltrombopag, have already completed phase III trials in primary immune thrombocytopenia and have been granted marketing authorization for use in this disease. Phase II and III trials with these novel drugs are ongoing in other conditions characterized by thrombocytopenia, such as chemotherapy, chronic liver disease, the myelodysplastic syndromes, and other conditions.Citation1,Citation2
Allogeneic hematopoietic stem cell transplantation (HSCT) is an important therapeutic option for various malignant and nonmalignant conditions. As allogeneic HSCT continues to increase, greater attention is given to improvements in supportive care, infectious prophylaxis, immunosuppressive medications, and DNA‐based tissue typing. However, graft‐versus‐host disease (GVHD) remains the most frequent and serious complication following allogeneic HSCT and limits the broader application of this important therapy.Citation3 GVHD is a consequence of interactions between the donor and host and their innate and adaptive immune responses. The fundamental interaction for induction of GVHD is the interaction of donor T cells with antigen‐presenting cells and that this interaction is regulated positively or negatively by a plethora of cytokines, chemokines, and several immune cell subsets. The hematological features of acute GVHD include pancytopenia and sometimes complete marrow failure occurs, which usually resolves if the GVHD is controlled.Citation4 Non‐immune persistent thrombocytopenia has been considered as an adverse prognostic factor in GVHD.Citation5 We present here the case of a 10‐year‐old girl who developed refractory thrombocytopenia during acute GVHD which finally resolved with the use of romiplostin. To the best of our knowledge, this is the first case of GVHD in which romiplostin has been successfully used.
Case history
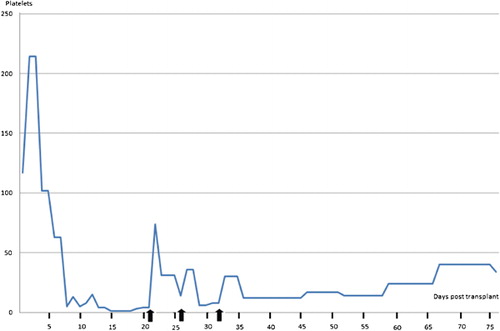
This 10‐year‐old girl was diagnosed at age 3 as B‐cell acute lymphoblastic leukemia; she was treated with chemotherapy and went into a complete remission. After two relapses and refractory to chemotherapy, with 6% blast cells in the peripheral blood, she was allografted from her HLA compatibleCitation5,Citation6 sibling on 6 June 2010, employing the ‘Mexican Method’ of reduced‐intensity conditioning.Citation6 On day +15, she presented a cutaneous rash and a perianal vesicle which extended rapidly and whose histological study was consonant with acute GVHD; despite recovery of granulocytes above 0·5×109/l, she had by then 3×109/l platelets and was treated with prednisone, which limited the growth of the ulcer but did not resolve the thrombocytopenia (). The patient became a full chimera on day +22 and minimal residual disease assessed by flow cytometryCitation7 since then has been consistently negative. On day 21 after the allograft, the patient remained with 3×109/l platelets despite the use of prednisone (1 mg/kg/day). Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome were ruled out. At this point, subcutaneous romiplostin (5 μg/kg) was delivered the platelets rapidly increasing (). Romiplostin was delivered every 7 days for a total of three doses and the platelet count has remained in hemostatic levels until day +75 post‐allograft, the patient remaining a full chimera with 0% minimal residual disease.
Discussion
Romiplostin is a 60 Da recombinant peptibody, produced by linking several copies of an active TPO‐binding peptide sequence to a carrier Fc fragment; it has been shown to be effective in ameliorating thrombocytopenia in patients with chronic idiopathic thrombocytopenic purpura and other thrombocytopenic conditions, is well tolerated and does not elicit cross‐reacting antibodies.Citation8 Romiplostin is now being shown to be useful in other thrombocytopenic conditions such as dengue hemorrhagic fever (Rodríguez‐Mejorada, personal communication), myelodysplasia,Citation9 and chemotherapy‐induced thrombocytopenia.Citation10
The thrombocytopenia ensuing in patients with GVHD may stem from several mechanisms: impaired platelet production, increased platelet destruction, and combination of these mechanisms. The usefulness of romiplostin in this case may stem from the fact that the patient may have had a combined origin of the refractory thrombocytopenia: on the one hand, the impaired platelet production by a bone marrow which is recovering and damaged by the graft‐versus‐host reaction. and on the other hand, the mechanisms responsible for thrombocytopenia in GVHD. Additional studies are needed to confirm the usefulness of romiplostin in the setting of GVHD‐associated thrombocytopenia.
References
- Stasi R, Bosworth J, Rhodes E, Shannon MS, Willis F, Gordon‐Smith EC. Thrombopoietic agents. Blood Rev 2010;24:179–90.
- de la Cruz‐Vicente F, Alonso‐Rosa D, Urbano‐Ispizua A. Empleo del romiplostin como agente trombopoyético en la púrpura trombocitopénica inmune. Rev Hematol Méx 2010;11:101–4.
- Ruiz‐Argüelles GJ, Ruiz‐Delgado GJ. Enfermedad de injerto contra huésped. Rev Hematol Méx 2010;11(Suppl. 1):31–2.
- Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft‐versus‐host disease. Immunol Alergy Clin North Am 2010;30:75–101.
- Sohn SK, Kim DH, Kim JG, Lee NY, Suh JS, Lee KS, et al.. Transplantation outcome in allogeneic PBSCT patients according to a new chronic GVHD grading system, including extensive skin involvement, thrombocytopenia, and progressive‐type onset. Bone Marrow Transplant 2004;34:63–8.
- Gómez‐Almaguer D, Ruiz‐Argüelles GJ, Tarín‐Arzaga LC, González‐Llano O, Jaime‐Pérez JC, López‐Martínez B, et al.. Reduced‐intensity stem cell transplantation in children and adolescents: the Mexican experience. Biol Blood Marrow Transplant 2003;9:157–61.
- Ruiz‐Argüelles GJ, Fernández‐Lara D, Estrada‐Gómez R, Manzano C, Ruiz‐Delgado GJ, Pérez‐Romano B, et al.. Minimal residual disease testing in acute leukemia by flow cytometry immunophenotyping: prognostic significance. Lab Hematol 2007;13:22–6.
- Molineux G, Newland A. Development of romiplostim for the treatment of patients with chronic immune thrombocytopenia: from bench to bedside. Br J Haematol 2010;150:9–20.
- Newland A. Romiplostim: a breakthrough treatment for the management of immune thrombocytopenic purpura. Eur J Haematol Suppl 2009;(71):20–5.
- Kantarjian HM, Giles FJ, Greenberg PL, Paquette RL, Wang ES, Gabrilove JL, et al.. Phase II study of romiplostim in patients with low‐ or intermediate‐risk myelodysplastic syndrome receiving azacitidine therapy. Blood 2010;116:3163–70.