Abstract
Objective
To determine the gene expression profiles of the JAK/STAT pathway members STAT3, STAT5A, STAT5B at both mRNA and protein levels in HL-60 and K-562 leukemia cells that were undergoing apoptosis following high-dose methylprednisolone (MP) treatment.
Methods
HL-60 cells were treated with 0.1 mM MP and K-562 cells were treated with 0.4 mM MP according to their IC50 values. STAT3, STAT5A, and STAT5B mRNA relative expression levels were determined by qRT-PCR whereas the protein levels were detected via western-blot analysis and apoptosis was evaluated by Annexin V method.
Results
A significant decrease was seen in STAT5A mRNA relative expression level at 48 hours of MP treatment (P < 0.05) both in HL-60 and K-562 cells. Other STATs showed a lower downregulation in their relative expressions at 48 hours at mRNA level for both of the cell lines. STAT proteins showed no expression change in K-562 cells in time course experiments but while STAT5A expression was downregulated; STAT5B showed an increase at 96 hours in HL-60 cells. Apoptosis was triggered by high-dose MP treatment that was evaluated by fluorescent microscopy.
Conclusion
The JAK/STAT pathway components may play an important role in the apoptosis mechanism of leukemic cells under MP treatment in HL-60 and K-562 cells. Other pathways may also be involved with a post-translational modification seen in the HL-60 cell line, with both upregulation and downregulation of protein expression levels of STAT5B and STAT5A, respectively.
Introduction
Methylprednisolone (MP) is a steroid hormone family member and a synthetic glucocorticoid (GC)Citation1 which is a strong suppressor of immune response and inflammation. GCs can act upon their receptor called glucocorticoid receptor (GR)-alpha which is present in the cytoplasm; bounded to heat shock proteins. When GCs enter the cell, they bind to their receptor that gives rise to the heat-shock proteins’ dissolution and translocation of GCR into the nucleus. In the nucleus, the GC–GR complex alters the transcription initiation rateCitation2,Citation3 and induces gene expression levels by binding to cognate DNA sequences (AGAACAnnnTGTTCT).Citation4
GC s are well-known inhibitors of immune system's T-cell functional activities such as proliferation, cytotoxicity, and cytokine production. High-dose MP treatment induces in vivo differentiation of myeloid leukemia cells to mature granulocytes in acute promyelocytic leukemia patients and some other subtypes of acute myeloblastic leukemia.Citation5,Citation6 The inhibition of proliferation is achieved via some signal transduction pathways mostly by interfering with the IL-2-induced JAK-STAT (Janus kinase – signal transducer and activator of transcription) signaling pathway, serine/threonine protein phosphatases, and with the transactivation of some other transcription factors.Citation7,Citation8
STATs both act as cytoplasmic signaling proteins and nuclear transcription factors leading to the activation of some malignant progression-related genes.Citation9–Citation12 STAT proteins are latent transcription factors that are located in the cytoplasm at the resting stage of the cell.Citation13 STAT proteins are activated by signals that are derived from the growth factors or peptide receptors and they transmit these signals into the nucleus.Citation14 When STATs are activated, following phosphorylation; they dimerize and migrate into the nucleus where they alter gene expression profiles of different genes by binding to the promoter region of that target gene.Citation13,Citation15
STATs are responsible for the regulation of some proteins’ expressions that function as cell living and survival, stimulation of cell proliferation, stimulation of angiogenesis, and protection of the cells from immune system. Because of these factors, their abnormal activation usually causes the transformation of the cells.Citation12,Citation16
There are seven family members of STATs named STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6. Among these, although STAT5A and STAT5B have similar protein sequences, they are encoded via different genes and are called as STAT5 together.Citation10 Overexpression of some significant transcription factors may trigger canceration by altering gene expression patterns and by activating some malignant genes. Especially STAT3 and STAT5 are overexpressed in tumor cells that stimulate angiogenesis, proliferate uncontrollably, and escape from apoptosis.Citation9,Citation12,Citation16 STAT3 is indispensable for the embryonic development and its expression leads to the inhibition of the cytokines and chemokine production of the tumor cells. STAT5A and STAT5B regulate cell cycle, cell proliferation, and apoptosis according to the gene transcription efficiency in different cells. Both STAT3 and STAT5 are activated in various types of hematooncologic malignancies and they trigger the formation of leukemia.Citation14
In our previous study we have shown that in HL-60 leukemia cell line, serine/threonine protein phosphatases play a major role in the signaling pathways that induce apoptosis following high-dose MP treatment.Citation17 In the current study, we evaluated the effect of STAT3, STAT5A, and STAT5B gene expression profiles both at mRNA and protein levels and their functions in MP induced HL-60 and K-562 cell differentiation and apoptosis therefore determining the importance of JAK/STAT pathway in this phenomenon.
Materials and methods
Cells
A human promyelocytic leukemia cell line HL-60 and a human chronic myelogenous leukemia cell line K-562 were purchased from ECACC (European Collection of Cell Cultures). Cells were cultured in RPMI 1640 medium containing 10% (v/v) heat inactivated fetal calf serum, 100 units of penicilin–streptomycin per ml, 1% l-glutamine at 37°C in humidified air containing 5% CO2.
MP treatment
While HL-60 cells were treated with a concentration of 0.1 mM MP, K-562 cells were treated with 0.4 mM MP that stand for the determined IC50 values in our previous studies.Citation17,Citation18 The experimental set up was designed as 24–96 hours time course treatment of cells with MP as well as with a non-treated control group. All experiments were triplicated and the average of the results was taken.
Total RNA extraction and cDNA synthesis
After 24 and 48 hours treatment with MP, total RNAs were extracted from the cells according to the manufacturer's protocol (High Pure RNA Extraction Kit, Roche Diagnostics GmbH, Mannheim, Germany). The quality of the RNAs were measured by nanoDrop spectrophotometer. About 100 ng RNA was reverse transcripted into cDNA via First-Strand cDNA Synthesis Kit from Roche Diagnostics following the instructions.
Quantitative assessment of STAT mRNAs by real-time qRT-PCR
The primer pairs and probes used for the amplification of STAT3 (NM_003150), STAT5A (NM_003152), and STAT5B (NM_012447) are given in . Target cDNA sequences were amplified by quantitative PCR using a fluorescence-based real-time detection method (LightCycler ver:2.0) The thermal cycling profiles were as follows: denaturation step for 10′ at 95°C, amplification step for 10″ at 95°C, 11″ at 56°C, 11″ at 72°C with 45 cycles and a cooling step for 30″ at 40°C. ‘LightCycler h-G6PDH Housekeeping Gene Set’ (Roche Diagnostics) kit was used as a housekeeping gene according to the kit manual. The 10 µl PCR reaction mixture for STAT3, STAT5A, or STAT5B target genes contained 3 mM MgCl2, 0.5 pmol/μl of each primer, 0.2 pmol/μl each fluorogenic probes (TIB MOLBIOL), and 1 µl Fast Start DNA Master Hybridisation probes enzyme with 2.5 µl of cDNA. The 10 µl PCR reaction mixture for G6PGH house keeping reference gene contained 3 mM MgCl2, 1 µl of ready to use h-G6PDH detection mix, and 1 µl of Fast Start DNA Master Hybridisation probes enzyme with 2.5 µl of cDNA. The relative expression levels were determined by the proportion of the target value to a reference value.
Table 1. The primer and probe sequences used for the quantitative real-time RT-PCR of STATs
Protein extraction
HL-60 cells and K-562 cells (2 × 106/ml) were washed three times with ice cold Tris-buffered saline (TBS; pH 7.4) and then they were disrupted in 1 ml of homogenization buffer containing 20 mM Tris-HCI, pH 7.4, 2 mM dithiothreitol, 2 mM EDTA, 2 mM EGTA, 0.25 mM sucrose, protease inhibitors 0.1 mM leupeptin, and 0.02 mM TLCK (Nα-p Tosyl-l lysine chloro methyl ketone) by a glass to glass Potter Elvehjem homogenizer. The homogenates were centrifuged at 1000 g for 10 minutes at 4°C and the supernatant was centrifuged at 45 000 g for half an hour. The resulting supernatant was used as the cytosolic fraction.
Protein assay
Total protein concentrations in the cell culture extracts were measured by Bradford method via Bradford reagent (Fermentas). The assay was standardized with bovine serum albumin ranging between 0.25 and 2 mg/ml concentrations and protein levels were detected with NanoDrop spectrophotometer.
Western-blot analysis for STAT3, STAT5A, and STAT5B
Thirty-five micrograms of HL-60 protein extracts and 28 mg of K-562 protein extracts were resolved on a 8% SDS–PAGE gel and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad) (methanol treatment for 5′) using a wet transfer system (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked for 1 hour at room temperature with blocking buffer ((5% (w/v) fatfree milk and 0.1% (v/v) Tween 20 on TBS, 0.05% sodium azide)), followed by overnight incubation at 4°C with 1:1000 diluted polyclonal STAT3 (06-596, Upstate) and β-Actin (#4967, Cell Signalling Technology, Inc. Danvers, MA, USA), 1:2000 diluted STAT5A (06-968, Upstate) and STAT5B (06-969 Upstate) antibodies in blocking buffer. After washing steps with TBS/T, second antibody IgG was applied with 1:2500 dilution for 90 minutes at room temperature. The protein levels of STATs in each fraction were detected by using the enhanced colorimetric detection kit (Amplified Alkaline Phosphatase Goat Anti-Rabbit Immun-Blot Assay Kit, Bio-Rad) according to manufacturer's instructions. The western-blot results were evaluated with gel imaging system (Chemi Smart 2000).
Apoptosis analyses
Apoptosis of the MP-induced cells was evaluated by Annexin V-EGFP and PI staining that allows detection of apoptosis and differentiates apoptosis from necrosis (Annexin V-EGFP Apoptosis Kit, Biovision, Catalog#: K104–100). For this purpose, 5 × 105 cells were taken and the kit procedure was applied. The number of living, apoptotic, and necrotic cells were evaluated under a fluorescence microscopy.
Statistical analyses
While the mRNA expression levels were evaluated with Student t-test referring to significance of P < 0.05; the apoptosis analyses were made with odds ratio and 95% confidence interval tests with a significance of P < 0.05 again.
Results
MP reduces STAT mRNA expression levels in HL-60 and K-562 cells
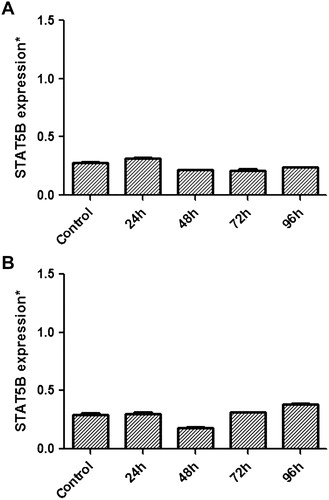
MP-treated cells were analyzed for STATs mRNA expression. After MP treatment, there was a significant decrease at mRNA levels of STAT proteins especially at 48 hours in both K-562 and HL-60 leukemic cells. While STAT3 expression was decreased significantly to 34.77% at 48 hours in HL-60 cells (P = 0.032), the value was 67.33% for K-562 cells at 72 hours (P = 0.058) when compared with the control group (A and B). STAT5A showed a significant expressional decrease at 48 hours with a rate of 14.89 and 19.06% for the same cell lines [(P = 0.019 and 0.014 respectively); A and B]. Among other STATs, STAT5B had the least expressional change with a decrease to 77.16% (P = 0.152) and 61.81% (P = 0.092) for K–562 and HL-60 cells, respectively, at 48th hour (A and B). We concluded that, under high-dose MP treatment, JAK/STAT pathway members STAT3 and STAT5A might play significant roles in the differentiation and apoptosis of leukemic cells at early (<48 hours) periods as suggested by the change in expression at mRNA levels of these transcription factors.
Figure 1. Relative mRNA expressional change of STAT3 in MP treated K-562 (A) and HL-60 (B) cells and also non-treated cells referring as control were evaluated by quantitative real-time RT-PCR (qRT-PCR). A significant decrease in STAT3 expression was seen especially at 48th hour (P < 0.05) in HL-60 cells.
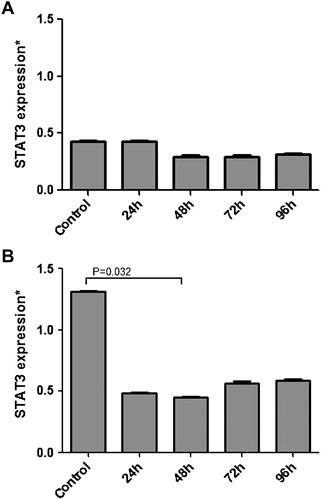
Figure 2. Relative mRNA expressional change of STAT5A in MP-treated K-562 (A) and HL-60 (B) cells, and also non-treated cells acting as control, were evaluated by quantitative real-time RT-PCR. A significant decrease in STAT5A expression was seen especially at 48th hour (P < 0.05) in both K-562 and HL-60 cells.
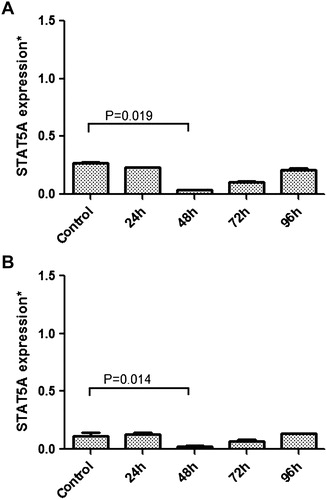
Figure 3. Relative mRNA expressional change of STAT5B in MP-treated K-562 (A) and HL-60 (B) cells and also non-treated cells referring as control were evaluated by quantitative real-time RT-PCR. A decrease in STAT5B expression was seen especially at 48th hour (P > 0.05) in both K-562 and HL-60 cells.
Effects of MP induction on STATs’ protein levels
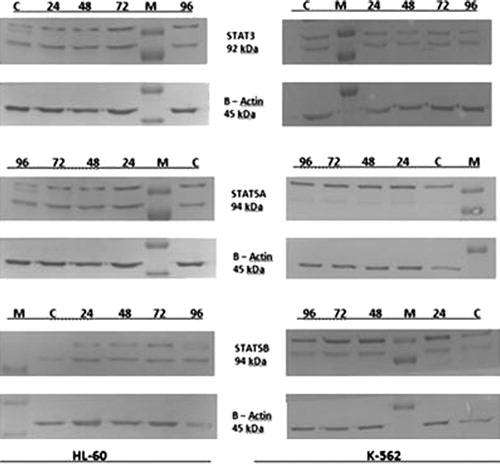
The western-blot results did not show any change in any of the target STAT proteins in K-562 cells that were treated with high-dose MP. Therefore, we concluded that JAK/STAT pathway components STAT3, STAT5A, and STAT5B probably do not play any major role at protein level in the MP effect mechanism that induced the apoptosis of K-562 leukemic cells.
In the HL-60 cells that were treated with MP, some discrepancies were observed at the end of our western-blot analyses. While STAT3 expression did not differ in our time course experiment at 24–48 and 72 hours when compared with non-treated control cells; STAT5A showed a decrease in its expression at 96th hour of MP treatment. An extra band was detected in STAT5A at 96 hours that was identified as an increase in the phosphorylated form of STAT5A protein giving rise to the conclusion that a post-translational modification was seen in STAT5A expression. The STAT5B protein level was also changed with an increase in expression at 96 hours when compared with the control group. This upregulation was referred to as a post-translational modification in STAT5B owing to the reduced association between STAT5B protein expression and MP treatment both at 72 and 96 hours in HL-60 cells ().
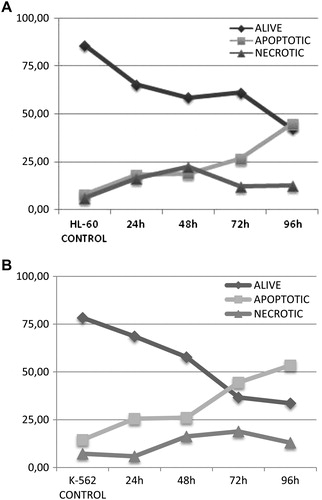
Figure 4. HL-60 and K-562 cells were cultured for 96 hours in the presence of MP (0.1 and 0.4 mM, respectively) also with non-treated control groups. Cell extracts were analyzed by western-blot analysis everyday in order to assess the content of STATs, β-actin was used as a loading control. The target STAT proteins showed no expressional change in K-562 cells but while STAT5A expression downregulated, STAT5B showed an increase at 96th hour in HL-60 cells. M: marker, C: control group, 24–96: time interval-study period.
MP induces apoptosis in HL-60 and K-562 cells
Although it had been shown in the HL-60 cell line that high-dose MP induced apoptosis by morphology and by Ethidium Bromide/Acridin Orange staining, it was not clear for K-562. In our study, we evaluated both apoptosis and necrosis for both of the cell lines by a different technique that had not been reported in MP treatment before. We showed that MP induced apoptosis in these leukemic cell lines. While a decrease was shown in the percentage of the living cells, a significant increase was seen in the apoptotic cells in the time course experiment. For HL-60, a statistically significant 11.395-fold increase of apoptotic cells were seen at 96 hours when compared with the control group (P < 0.001) with a statistically significant 8.599-fold increase for K-562 cells at the same time line (P < 0.01). Necrosis was seen at the IC-50 concentration value of MP treatment on leukemic cells (A and B).
Figure 5. MP-induced HL-60 and K-562 cells were analyzed for apoptosis. While the dark arrow shows live cells, the gray arrow shows apoptotic and necrotic cells. As a result, a decrease was seen in the percentage of live cells with an increase in the percentage of apoptotic cells, indicating that high-dose MP induced programmed cell death of both HL-60 and K-562 leukemic cells when compared with untreated control cells (P < 0.001 and P < 0.01, respectively).
Discussion
It has been known that the abnormalities seen in the JAK/STAT pathway not only involved in the development of solid cancers, such as lung, breast, head, and neck, but also impacts in leukemia and lymphoma.Citation19,Citation20 In the treatment of leukemia, a widely used synthetic corticosteroid MP affects metabolism in various ways and modifies the immune system and it is used to achieve prompt suppression of inflammation.Citation21 The observation by Hiçsönmez et al.Citation22 showing that childhood acute myeloblastic leukemia treated with high-dose MP induced apoptosis stimulated many further studies. It was also found that high-dose MP treatment causes differentiation in acute promyelocytic leukemia and acute myelogenous leukemia with blast cells becoming mature granulocytes in vivo. As a well-tolerated anti-inflammatory drug, it is widely used in treatment combinations. In one of our previous studies, we have shown that MP triggered apoptosis with Ethidium Bromide/Acridine Orange staining method.Citation17 Based on these observations, in the current study we investigated whether MP induced apoptosis in HL-60 and K-562 leukemic cell lines via JAK/STAT pathway components STAT3, STAT5A, and STAT5B. Because these transcription factors are overexpressed in tumor cells that proliferate uncontrollably, escape from immune system, avoid apoptosis, and stimulate angiogenesis;Citation23 the change in expression of these STATs both at mRNA and protein levels were investigated after MP treatment of the two leukemia cell lines. We determined the apoptosis with a novel technique for MP studies. Our study seems to be one of the first to evaluate whether the effect of MP on leukemia treatment is via the JAK/STAT signaling pathway. Previously, it has been considered that high-dose MP triggered the apoptosis of leukemic cells upon serine/threonine protein phosphatases.Citation24–Citation26
We have shown that high-dose MP treatment reduced STAT mRNA relative expression levels in both HL-60 and K-562 leukemic cells. A significant downregulation was detected on STAT5A mRNA expression level with a decrease to 14.89% for K-562 and 19.06% for HL-60 after 48 hours of the MP treatment. So it can be concluded that STAT5A may play a major role in the effect mechanism of MP upon leukemia highlighting the potential importance of JAK/STAT pathway. The current study is the first to suggest that MP may impact via the JAK/STAT pathway member STAT5A in leukemia. We also considered that expression of STAT5B may also be downregulated following MP treatment but we have observed that STAT5B had the least decrease in expression among our STATs. These two STAT proteins that are 96% conserved at protein level are encoded by two different genes and a difference in their functional phenotypes is observed in their ‘knock-out’ mutant mice.Citation27 So we concluded that it is possible that one of the STATs is more effective under MP treatment; the other has little effect and a more detailed examination of the role of STAT5B is needed. In support of our hypothesis Xu et al. reported that MP was used for the clinical therapy of white matter diseases in the nervous system to prevent oligodendrocytes undergoing apotosis via the activation of the GR and the upregulation of bcl-XL. In their study, they examined whether the GC signaling pathway interacted with STAT5 to upregulate bcl-XL and protect oligodendrocytes and they concluded that the GR and STAT5 complex were present on the STAT5 binding site of the bcl-x promoter region in oligodendrocytes. When the active form of STAT5 was overexpressed, it induced cell death but when STAT5 gene was knocked down, apoptosis was not seen again. So they concluded that STAT5A but not STAT5B mediated the anti-apoptotic effects of MP on oligodendrocytes.Citation28
According to our western-blot analyses, the target STAT proteins showed no change in expression in K-562 cells but while STAT5A expression was downregulated, STAT5B showed an increase at 96 hours in HL-60 cells referring to a post-translational modification. We concluded that while STAT5A might have an essential role in the MP effector mechanism upon leukemic cells, STAT5B had the least effect among other STATs owing to the upregulation in protein expression profiles. Analyzing apoptosis we showed that MP induced cell death of K-562 and HL-60 cells increased with a concomitant reduction in living cells.
In summary, we have shown that MP might induce apoptosis of leukemic cells via the JAK/STAT pathway and that STAT5A played a significant role in this phenomenon. Our findings have important implications in the treatment of leukemia with STAT5A as a new potential candidate target of therapy. Our study may also play a key role in the understanding of the underlying mechanism of the significant signaling pathways in the pathogenesis of leukemia.
References
- Unterberger C, Staples KJ, Smallie T, Williams LM, Foxwell B, Schaefer A, et al. Role of STAT3 in glucocorticoid-induced expression of the human IL-10 gene. Mol Immunol. 2008;45:3230–7
- Meier CA. Regulation of gene expression by nuclear hormone receptors. J Recept Signal Transduct Res. 1997;17:1319.
- Encio IJ, Detera-Wadleigh SD. The genomic structure of the human glucocorticoid receptor. J Biol Chem. 1991;266:7182–8.
- Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 2004;125:697–706.
- Hicsonmez G, Tuncer AM, Güler E, Tan E, Tekelioğlu M. The potential role of high-dose methylprednisolone on the maturation of leukemic cells in children with acute promyelocytic leukemia (APL[]. Exp Hematol. 1993;21:599–601.
- Hiçsönmez G, Tuncer M, Toksoy HB, Yenicesu I, Çetin M. Differentiation of leukemic cells induced by short-course high-dose methylprednisolone in children with different subtypes of acute myeloblastic leukemia. Leuk Lymph. 1999;33:573–80.
- Clark AR, Lasa M. Crosstalk between glucocorticoids and mitogen-activated protein kinase signaling pathways. Curr Opin Pharmacol. 2003;3:404–11.
- Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–45.
- Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21.
- Darnell JE. STATs and gene regulation. Science. 1997;277:1630–5.
- Bromberg J, Darnell JE. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–73.
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–88.
- Igaz P, Tóth S, Falus A. Biological and clinical significance of the JAK-STAT pathway; lessons from knockout mice. Inflamm Res. 2001;50:435–41.
- Yu H, Jove R. The STATs of cancer – new molecular targets come on age. Nature Reviews. 2004;4:97–105.
- Williams JG. Serpentine receptors and STAT avtivation: more than one way to twin a STAT. Trends Biochem Sci. 1999;24:333–4.
- Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–54.
- Omay SB, Saydam G, Aydın H, Selvi N, Öktem G, Sanli UA, et al. Potential involvement of calcineurin in regulating the state of differentiation and apoptosis of HL-60 cells during methylprednisolone treatment. Turk J Haematol. 2003;20:143–51.
- Uzunoglu S, Uslu R, Tobu M, Saydam G, Buyukkececi F, Omay SB. Augmentation of methylprednisolone-induced differentiation of myeloid leukemia cells by serine/threonine protein phosphatase inhibitors. Leuk Res. 1999;5:507–12.
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19(21):2474–88.
- Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8(4):945–54.
- Dan AE, Thygesen TH, Pinholt EM. Corticosteroid administration in oral and orthognathic surgery: a systematic review of the literature and meta-analysis. J Oral Maxillofac Surg. 2010;68(9):2207–20.
- Hiçsönmez G, Erdemli E, Tekelioglu M, Tuncer AM, Ozbek N, Cetin M, et al. Morphologic evidence of apoptosis in childhood acute myeloblastic leukemia treated with high-dose methylprednisolone. Leuk Lymphoma. 1996;22(1–2):91–6.
- Yu H, Jove R. The STATs of cancer – new molecular targets come of age. Nat Rev Cancer. 2004;4(2):97–105.
- Aydın HH, Selvi N, Saydam G, Tobu M, Uzunoğlu S, Uslu R, et al. Up-regulation of serine/threonine protein phosphatase type 2A regulatory subunits during methylprednisolone-induced differentiation of leukaemic HL-60 cells. Clin Lab Haem. 2000;22:271–4.
- Saydam G, Aydin HH, Sahin F, Selvi N, Oktem G, Terzioglu E, Buyukkececi F, Omay SB. Involvement of protein phosphatase 2A in interferon-α-2b-induced apoptosis in K562 human chronic myelogenous leukaemia cells. Leuk Res. 2003;27:709–17.
- Omay SB, Saydam G, Aydin HH, Selvi N, Oktem G, Sanlı UA, et al. Potential involvement of calcineurin in regulating the state of differentiation and apoptosis of HL-60 cells during methylprednisolone-treatment. Turk J Haematol. 2003;20(3):143–51.
- Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–21.
- Xu J, Chen S, Chen H, Xiao Q, Hsu CY, Michael D, et al. STAT5 mediates anti-apoptotic effects of methylprednisolone on oligodendrocytes. J Neurosci. 2009;29(7):2022–6.