Abstract
Waldenström macroglobulinemia is a lymphoplasmacytic lymphoma. A serum monoclonal IgM protein is required to establish this diagnosis. The clinical features patients develop include normochromic normocytic anemia, thrombocytopenia, hepatosplenomegaly, lymphadenopathy and signs of hyperviscosity. The International Staging System for Waldenström macroglobulinemia divides patients prognostically based on age, hemoglobin, platelet count, IgM level, and beta2 microglobulin. Some patients with Waldenström macroglobulinemia have a smoldering form and can be observed without intervention. Active agents in the treatment of Waldenström macroglobulinemia include rituximab, chlorambucil, cyclophosphamide, fludarabine, bortezomib, lenalidomide, bendamustine, everolimus, and alemtuzumab. The current preferred Mayo Clinic non-study treatment is rituximab, cyclophosphamide, and dexamethasone. The median survival associated with this disease is now over 10 years.
Introduction
Waldenström macroglobulinemia is defined as lymphoplasmacytic lymphoma associated with a monoclonal IgM protein of any size. The most common presenting symptom for patients is fatigue related to progressive normochromic anemia. The median hemoglobin level at diagnosis is 100 g/l. The clinical findings include hepatomegaly in 20%, splenomegaly and lymphadenopathy in 15% each. Organomegaly is usually asymptomatic. Patients who present with a hemoglobin >100 g/l often do not require therapy since their fatigue is minimal. Only a small proportion of patients with Waldenström macroglobulinemia present with symptomatic hyperviscosity requiring intervention. There are a number of specific syndromes associated with an IgM monoclonal protein and include peripheral neuropathy, hemolytic anemia, and cryoglobulinemia ().
Table 1. Definitions of IgM-related phenomenon in macroglobulinemia (adapted from Gertz et al. PMID 21874060)
Disease Overview
In the original description of the disorder, hyperviscosity was supposedly present manifested by oronasal bleeding. These patients also have lymphadenopathy, anemia, thrombocytopenia, and an elevated sedimentation rate. The normal counterpart of the malignant cell is a post-germinal center B cell that has undergone somatic hypermutation but transforms before isotype class switching. There is intraclonal homogeneity. Waldenström’s is rare occurring in approximately three patients per million per year and is sporadic, although there is a reported increased incidence of hematologic malignancy in first-degree relatives. The only recurrent genetic abnormality identified by karyotype analysis is deletion 6q seen in nearly half of patients. The malignant cell infiltrates bone marrow and lymph nodes in a predominantly intertrabecular pattern. The cells express pan B markers including CD20 and are negative for CD3 and CD103. There is no correlation between symptoms and the size of the IgM monoclonal protein. Patients with an IgM protein of 5000 mg/dl may be completely asymptomatic and be observed. Conversely, patients can have IgM proteins of 500 mg/dl, massive marrow infiltration with pancytopenia, and require semi-urgent therapy. Finally, there are those patients who have an IgM monoclonal protein detectable by immunofixation only, have <10% lymphoplasmacytic cells in the bone marrow, yet need treatment because the IgM monoclonal protein has led to the deposition of amyloid or cold agglutinin hemolysis or a mixed cryoglobulin deposition.Citation1
In a large study of patients with MGUS, 17% had an IgM monoclonal protein. IgM MGUS is seen in 1 in 600 adults over age 50 in the United States. Patients with IgM MGUS require lifelong monitoring because the risk of transformation into lymphoplasmacytic lymphoma/Waldenström macroglobulinemia is 2% per year. An abnormal free light chain ratio helps predict the risk of transformation.Citation2 When a patient is seen with suspected Waldenström macroglobulinemia, the recommended evaluation is given in .
Table 2. Diagnostic approach to suspected Waldenström macroglobulinemia
Prognosis
Waldenström macroglobulinemia is distinct morphologically, cytogenetically, and by cell surface markers. As a consequence, it should come as no surprise that the International Prognostic Index for lymphoma and the follicular lymphoma International Prognostic Index are not used. gives the International Staging System for Waldenström and outcomes. Age has the greatest effect on outcome. A patient who is older than age 65 by definition cannot be in the low-risk category. The level of the monoclonal IgM protein does not become important until it exceeds 70 g/l. The level of the IgM protein does not appear to impact response rates. Unlike other forms of lymphoma, the lactate dehydrogenase is not part of the staging system, although it has been suggested that lactate dehydrogenase does discriminate between patients that are high-risk using standard criteria. The International Prognostic Scoring System is only used to assess prognosis in patients that require therapy by clinical criteriaCitation3 and is not used to determine whether a patient should be treated. The value of the prognostic scoring system has been validated in newly diagnosed and relapsed patients as well as patients treated with and without rituximab. It is important to note that patients with low-stage disease have a median survival of 12 years; and because of their advanced age at diagnosis, half of the patients succumb to non-disease-related mortality. Therefore, disease-specific mortality is longer than 12 years and must be kept in mind when selecting combination therapies for these patients.
Table 3. ISSWM: International Staging System for Waldenström macroglobulinemia factors associated with prognosis in the IWMSS
Management of Hyperviscosity Syndrome
Hyperviscosity syndrome is now seen uncommonly in newly diagnosed patients because of increased recognition. Symptomatic hyperviscosity is rare in patients whose IgM level is <40 g/l, and viscosity measurements are not required until the IgM levels exceed this. Symptoms of hyperviscosity are usually oronasal bleeding and visual disturbance related to retinal hemorrhages. Caution should be exercised in attributing nonspecific symptoms such as lightheadedness and generalized fatigue as a manifestation of hyperviscosity. The normal serum viscosity is 1·8. Symptoms rarely develop until the serum viscosity is >4.
When hyperviscosity is present, plasma exchange should be used but is only to be used until systemic chemotherapy lowers the IgM level and reduces the level of serum viscosity.Citation4 Long-term plasma exchange is generally not required unless patients are refractory to multiple lines of chemotherapy and cytoreductive therapy is no longer effective.
Systemic Chemotherapy to Reduce Tumor Mass
When assessing responses in patients with Waldenström, it is important to recognize that with some agents, responses can be delayed and result in an underestimate of response. With the use of single-agent fludarabine, 17% of responses are not achieved until 6 months following first exposure. Patients should not be prematurely considered therapy failures until sufficient time has elapsed to allow the IgM level to fall. With the use of single-agent rituximab, patients who achieve a minor response (a 25–50% reduction) in IgM level can do just as well as those patients who have a >50% response. It is important to recognize that patients who receive rituximab without cytotoxic chemotherapy are at the risk of a flare phenomenon where the IgM level may rise and can precipitate hyperviscosity.Citation5 Rituximab as a single agent is not a particularly good choice for the treatment of patients with Waldenström since the response rate to rituximab as a single agent is only 55%. As a single agent, pulse chlorambucil is far more effective producing a response rate of 88%; and in five published studies of alkylating agents, the response rate ranged from 31 to 82%. Combining rituximab with the subcutaneous use of cladribine results in a PR or greater rate of 88%. The use of rituximab as a single agent is only recommended for those patients who have isolated peripheral neuropathy associated with anti-myelin-associated glycoprotein. Single-agent fludarabine can produce durable responses. Ninety-eight patients with no more than one risk factor had an eight-year survival of 55%. By comparison, 20 patients with over two risk factors had an eight-year survival of only 5%.
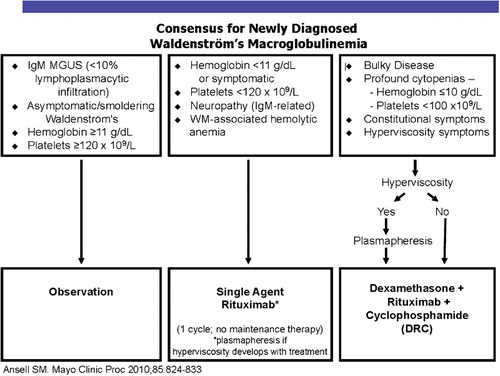
Single-agent chemotherapy is not recommended for the majority of patients with Waldenström, and most patients receive combination chemotherapy. Cyclophosphamide, doxorubricin, vincristine, and prednisone plus rituximab (R-CHOP) treatment has at least a 90% response rate. Rituximab combined with cyclophosphamide and dexamethasone has a response rate of 83% and minimal toxicity. Two-year disease-specific survival for this regimen is 90% and is currently the non-study standard for symptomatic patients with a new diagnosis of Waldenström at Mayo Clinic ().Citation6
Table 4. DRC regimen
Myelodysplasia is seen with the use of chlorambucil, cyclophosphamide, and fludarabine. It has also been associated with an increased risk of transformation to large-cell lymphoma. Fludarabine reduces the ability to adequately mobilize stem cells and is not a desirable agent for patients who are eligible for stem cell transplantation.
Stem cell transplantation is an underutilized technique for Waldenström macroglobulinemia.Citation1 Treatment-related mortality is 3·8%. Reported 5-year progression-free and overall survival rates are 39·7 and 68·5%, respectively. A good outcome with high-dose therapy should be expected in a disease such as Waldenström with a low proliferative rate and a normal cytogenetic profile in the majority.
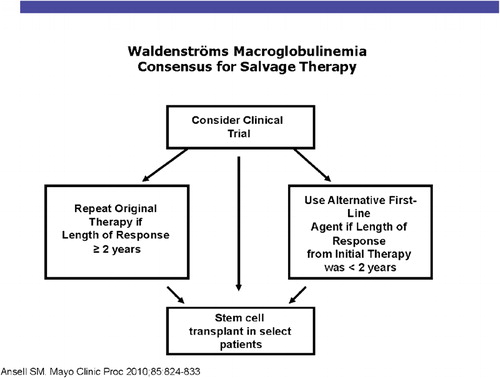
The recent introduction of novel agents has provided important benefits for patients with Waldenström macroglobulinemia. Rituximab with thalidomide produced a 72% response rate. Rituximab with lenalidomide produced a 50% response rate, although it tends to aggravate the anemia in a large proportion of patients.Citation7 Thalidomide and lenalidomide have important activity. Bortezomib has high levels of activity in the management of Waldenström macroglobulinemia in schedules of twice-weekly, 2 weeks of 3.Citation8 Response rates range from 81 to 96%, but the neurotoxicity is an issue in patients who have a high prevalence of subclinical neuropathy.Citation9 Everolimus, an inhibitor of the mammalian target of rapamycin (mTor) produces a response rate of 70% in relapsed refractory Waldenström macroglobulinemia. The AKT inhibitor, perifosine, shows a response rate of 35%, but gastrointestinal toxicity is a serious problem. Bendamustine has been combined with rituximab in a prospective randomized study compared with R-CHOP in low-grade lymphoma. Forty-one of these patients had Waldenström macroglobulinemia, 22 received bendamustine-rituximab, and 19 received R-CHOP. The median progression-free survival is significantly longer with bendamustine 36 months vs not reached (P<0·001). Bendamustine with rituximab was better tolerated with less hematotoxicity, a lower frequency of infection, and a lower instance of neuropathy. The figures provide the Mayo Clinic algorithm for the recommended treatment of Waldenström patients with newly diagnosed disease (). includes our treatment recommendations for relapsing patients.
Conclusion
When Waldenström is diagnosed before the development of symptoms, patients may be observed. Symptomatic patients required chemotherapy. Multi-agent regimens are superior to single-agent regimens. The Mayo Clinic preferred option is rituximab, cyclophosphamide, and dexamethasone. Stem cell transplantation is a highly active underutilized treatment for Waldenström macroglobulinemia. A focus on reducing long-term morbidity for a population destined to live a long time is increasingly important.
References
- Gertz MA, Reeder CB, Kyle RA, Ansell SM. Stem cell transplant for Waldenstrom macroglobulinemia: an underutilized technique. Bone Marrow Transplant. 2011 Aug 29. [Epub ahead of print]
- Gertz MA. Waldenstrom macroglobulinemia: 2011 update on diagnosis, risk stratification, and management. Am J Hematol. 2011;86:411–6.
- Dimopoulos MA, Gertz MA, Kastritis E, Garcia-Sanz R, Kimby EK, Leblond V, et al.. Update on treatment recommendations from the Fourth International Workshop on Waldenstrom’s Macroglobulinemia. J Clin Oncol. 2009;27:120–6.
- Treon SP. How I treat Waldenstrom macroglobulinemia. Blood. 2009;114:2375–85.
- Issa GC, Leblebjian H, Roccaro AM, Ghobrial IM. New insights into the pathogenesis and treatment of Waldenstrom macroglobulinemia. Curr Opin Hematol. 2011;18:260–5.
- Ansell SM, Kyle RA, Reeder CB, Fonseca R, Mikhael JR, Morice WG, et al.. Diagnosis and management of Waldenstrom macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin Proc. 2010;85:824–33.
- Kastritis E, Terpos E, Dimopoulos MA. Emerging drugs for Waldenstrom's macroglobulinemia. Expert Opin Emerg Drugs. 2011;16:45–57.
- Dimopoulos MA, Chen C, Kastritis E, Gavriatopoulou M, Treon SP. Bortezomib as a treatment option in patients with Waldenstrom macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2010;10:110–7.
- Baehring JM, Hochberg EP, Raje N, Ulrickson M, Hochberg FH. Neurological manifestations of Waldenstrom macroglobulinemia. Nat Clin Pract Neurol. 2008;4:547–56.