Abstract
Since 1965, when antithrombin deficiency was identified as the first congenital defect of hemostasis able to increase the risk of thrombosis, we have assisted in a substantial evolution of thrombophilia. From the original monogenic view, it has been demonstrated that thrombosis is a polygenic and complex disorder that involves potentially hundreds of polymorphisms and rare mutations, as well as multiple acquired and triggering factors. From the enthusiasm of searching prothrombotic polymorphisms that might contribute to the risk of each individual to have a thrombotic episode, to the frustration of considering that thrombophilic tests might have no clinical relevance. Also the methods used in thrombophilic analysis have significantly changed from original simple analysis to recent and complex technological approaches. It is time to analyze carefully, without any pressure, the real state of the art and to moderate the conclusions, separating clinical use and research of inherited thrombophilic conditions.
The Starters: A Monogenic View
In 1965, the Norwegian hematologist O. Egeberg identified a family with high incidence of thrombosis that had deficiency of antithrombin, a key endogenous anticoagulant. A rare mutation affecting the gene encoding antithrombin explained the deficiency and was associated with a high risk of thrombosis. In the 1980s, the identification of a similar picture associated with deficiency of other anticoagulant proteins, protein C and protein S, suggested that at least some cases of venous thrombosis might be considered as a monogenic disease. However, the penetrance of these defects is variable, and the incidence of all these defects collectively is low, even among thrombophilic patients (5–10%). These data, together with the fact that >60% of the variation in susceptibility to common thrombosis is attributable to genetic factors,Citation1 strongly suggested the presence of additional prothrombotic genetic risk factors.
Identification of Prothrombotic Polymorphisms: the Polygenic View
The search of genetic factors involved in thrombosis changed from rare mutations associated with high risk to common polymorphisms with moderate thrombotic risk, following the attractive hypothesis that ‘common genetic variants are involved in common diseases’. Certainly such interesting hypothesis was validated in thrombosis by the group of R. Bertina, who in 1994 and 1996 identified two functional polymorphisms, the first impairing the inactivation of FV (FV Leiden R506Q) and the second associated with moderately high levels of prothrombin (PT G20210A), which significantly increased the risk of thrombosis.Citation2
The Search of Prothrombotic Polymorphisms
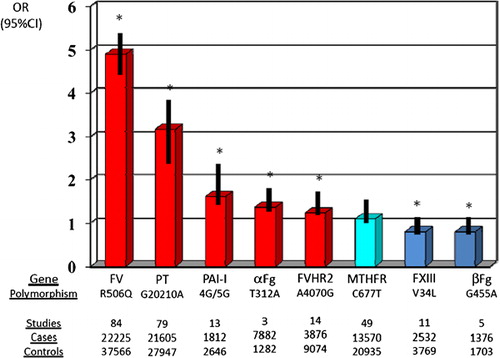
These reports clearly sustained that venous thrombosis is a polygenic disorder and sustained the search of new prothrombotic polymorphisms, particularly since heritable risk factors for venous thrombosis can be identified only in 30–50% of affected patients.Citation2 This search was originally restricted to candidate genes, mainly from the hemostatic system using case–control studies. The simplicity of such studies facilitated the sudden increase in thrombophilic studies (a PubMed search using ‘polymorphisms and thrombosis’ renders more than 2000 entries). However, the final conclusion of these studies is quite frustrating since most studies found no significant association, and those with a positive association, which was restricted to some specific group of patients or population, had conflictive results when validating in independent studies, and in any case, the risk of thrombosis associated with any polymorphism was always moderate (OR<2). A recent meta-analysis including ∼126 525 cases and 184 068 controls derived from 173 case–control studies that had evaluated 21 genes (28 polymorphisms) found that only few polymorphisms were mildly associated with the risk of thrombosis ().Citation3 In this framework, the thrombotic relevance of common polymorphisms responsible for the ABO blood group must be pointed out. Since it was described in 1969, many independent studies consistently found that non-O blood group increases almost two-fold the risk of venous thrombosis. However, an unexplained predisposition has excluded ABO genotyping from thrombophilic studies.
Figure 1. Polymorphisms that is significantly associated with the risk of thrombosis in Caucasians. Data have been obtained from the meta-analysis of Gohil and co-workers.Citation3
With the development of new technologies of genotyping, the design of new experiments to identify new prothrombotic polymorphisms changed, leading firstly to massive analysis of hundreds of polymorphisms located on candidate genes, many of which were not restricted to the hemostatic system, and then to genome wide association analysis (GWAS) that genotyped up to 551 141 single nucleotide polymorphisms (SNPs) covering the whole genome in 1542 cases and 1110 controls.Citation4 Unfortunately, these expensive studies only revealed classical associations (F5, ABO, F11, and FGG), but surprisingly failed to identify signals in F2, and identified potential thrombotic relevance of few polymorphisms located in HIVEP1, PROCR, or STAB2, always with very mild effect.Citation4 Probably, the main limitations of these studies lie on the statistical methods required to discard false associations typical of multiple testing, since these methods also reject true associations of low prevalent SNPs, probably those with stronger functional and thrombotic effect.
Clinical Relevance
Since the identification of FV Leiden and PT G20210A, these polymorphisms have been included together with severe anticoagulant deficiencies in routinely thrombophilic screening. Actually, this screening has been massively required (more than 25 000 requirements/year of FV Leiden determination in the UK), probably due to the high rate of success (>20% of patients with venous thrombosis may carry a thrombophilic defect) and by the absence of clear guidelines.
A guide for the management of inherited thrombophilia mainly directed to genetics professionals have been recently published, showing not only the benefits but also the pitfalls of thrombophilia testing.Citation5 The clinical utility of thrombophilia screening has been condensed to two main objectives, once universal screening or even not-directed screening in high risk situations such as oral contraceptive use or pregnancy, has been discarded:Citation6
to reduce the risk of recurrence of patients with venous thrombosis by offering long-term rather than short-term anticoagulation in the index case;
to reduce the risk of a first venous thrombotic event in asymptomatic relatives.
However, an increasing opinion is now considering that thrombophilia testing has no clinical utility at all. This negative attitude has been strongly influenced by the review of the clinical utility of thrombophilia testing published in 2008, which concluded that testing for heritable thrombophilia serves a limited purpose and should not be performed on a routine basis.Citation7 Thus, despite that all congenital thrombophilic defects (anticoagulant deficiencies, FV Leiden, and PT G20210A) significantly increase the relative risk of thrombosis, the absolute risk is, in general, low (lower than other risk factors for venous thrombosis such as surgery, trauma, immobilization, pregnancy, or oral contraceptive use).Citation8 Moreover, while thrombophilia testing identified defects associated with an increased risk of a first thrombotic event, they do not particularly increase the risk of recurrence, which has been described as the thrombophilia paradox. Actually, this is not the only paradox identified when dealing with thrombophilia. Carriers of FV Leiden have high risk of venous thrombosis but not of pulmonary embolism. All these paradoxes only reflect that thrombophilia has been considered with a very simplistic view.Citation9 We use a limited dichotomous testing and evaluating strategy (carrier of a single defect versus non-carrier). Moreover, in many studies, all thrombophilic defects are joined to get stronger statistical power, despite that rare mutations have stronger consequences than common polymorphisms. Even among anticoagulant deficiencies, there is a considerable clinical heterogeneity that has to be considered. Additionally, in order to reduce the number of cases with inappropriate diagnosis, it is necessary to improve the methods used to diagnose thrombophilic defects or even the thrombotic events. But the main limitation in this field is the absence of randomized controlled trials or controlled clinical trials that had assessed the benefit(s) of testing for thrombophilia on the risk of primary or recurrent venous thrombosis. Only few recent studies suggest that for prevention of both a first thrombosis in asymptomatic carriers, and recurrence in symptomatic carriers, thrombophilia testing is helpful, although only for severe anticoagulant deficiencies.Citation10 Further studies increasing the size of the sample are required to verify whether this conclusion might also be extrapolated to homozygous or compound heterozygous of common prothrombotic polymorphisms.
Future Perspectives
The new high throughput sequencing methods already available together with the expected reduction in their cost will allow, in the next few years, by exome or even whole genome sequencing of patients with idiopathic venous thrombosis, the identification of new mutations involved in this pathology. More importantly from our point of view, this approach, together with genotype–phenotype GWAS analysis will distinguish new genes involved in the hemostatic system and risk of thrombosis. Additionally, the compilation of classical case–control GWAS might increase the statistical power that might allow the identification of low prevalent prothrombotic SNPs.Citation4 Actually, common variants could explain ∼35% of the genetic variance underlying venous thrombosis susceptibility, among which 3% could be attributable to the mainly identified venous thrombosis loci. Finally, new elements not evaluated so far must be considered according to new evidences. Thus, miRNA, small RNA molecule with a post-translational regulatory function, has been recently identified to play a role in the hemostatic system. SNPs or mutations affecting these elements, which hardly might be detected using current methods, might also play a role in thrombosis.
But the main challenge of this field is to achieve clinical application. In this framework, we cannot forget the polygenic and multifactorial nature of thrombosis. The risk of thrombosis of any subject will finally depend on the individual SNP profile, the presence of mutations, and the combination with other thrombotic risk factors. Therefore, it is necessary to generate algorithms that considering multiple genetic variations might calculate the risk of thrombosis, and combine this prediction with functional tests that evaluate the status of the whole hemostatic system (such as the thrombin generation or thromboelastography methods), and well-defined markers of thrombosis such as the D-dimer. Only a complex view might give data useful for clinicians to deal with a complex disease.
References
- Souto JC, Almasy L, Borrell M, Blanco-Vaca F, Mateo J, Soria JM, et al.. Genetic susceptibility to thrombosis and its relationship to physiological risk factors: the GAIT study. Genetic analysis of idiopathic thrombophilia. Am J Hum Genet. 2000;67:1452–9.
- Bertina RM. Genetic approach to thrombophilia. Thromb Haemost. 2001;86:92–103.
- Gohil R, Peck G, Sharma P. The genetics of venous thromboembolism. A meta-analysis involving approximately 120,000 cases and 180,000 controls. Thromb Haemost. 2009;102:360–70.
- Germain M, Saut N, Greliche N, Dina C, Lambert JC, Perret C, et al.. Genetics of venous thrombosis: insights from a new genome wide association study. PLoS One. 2011;6(9):e25581.
- Varga E, Kujovich J. Management of inherited thrombophilia: guide for genetics professionals. Clin Genet. 2011;81(1):7–17.
- Baglin T, Gray E, Greaves M, Hunt BJ, Keeling D, Machin S, et al.. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149:209–20.
- Keeling D. Thrombophilia screening or screaming. J Thromb Haemost. 2010;8:1191–2.
- Middeldorp S. Evidence-based approach to thrombophilia testing. J Thromb Thrombol. 2011;31:275–81.
- Corral J, Roldán V, Vicente V. Deep venous thrombosis or pulmonary embolism and factor V Leiden: enigma or paradox. Haematologica. 2010;95:863–6.
- Lijfering WM, Brouwer JL, Veeger NJ, Bank I, Coppens M, Middeldorp S, et al.. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood. 2009;113:5314–22.