Abstract
Glutathione provides means of regulating protein function by the process of glutathionylation. Despite the role of oxidative stress biomarkers assumed recently by glutathionylated proteins in human diseases, so far no information is available on the intracellular distribution of glutathionylated proteins in human cell lines. In this study, we combined the specificity of monoclonal antibody labeling for protein-bound glutathione (GS-Pro) with the ability of confocal microscopy to localize molecules with high spatial resolution. We performed immunofluorescence analysis on dermal fibroblasts, both in steady state than in proliferative conditions, and on in situ extracted matrix samples. For the first time, we report the compartmentalization of constitutively glutathionylated proteins in different subcellular districts and we found a tight association between glutathione, nuclear lamina, and cytoskeleton. In proliferating cells, total GS-Pro fluorescence increases in the early phases of growth and significantly drops when cells reach confluence. Interestingly, a nuclear shift of GS-Pro was observed between 6 and 48 hours after plating, becoming homogeneous with the cytoplasm when growth slows. The ability to visualize a detailed intracellular distribution of this critical marker of protein oxidation may provide an additional tool to highlight pathways in turns ‘redox-activated’ and to identify new pathogenic pathways in human diseases.
Introduction
Glutathione is the principal non-protein thiol involved in the antioxidant cellular defence.Citation1,Citation2 It is a tripeptide composed of cysteine, glutamic acid and glycine, and its active group is represented by the thiol (–SH) of cysteine residue. Accumulating evidence suggests that glutathione provides means of regulating protein function by a mechanism, called glutathionylation, where protein thiol groups are reversibly bound to glutathione. S-glutathionylation is a signal transduction mechanism by which cells respond to redox inputs, and several studies have supported the fundamental role for glutathionylation in some patho-physiological processes.Citation3–Citation6
Many proteins may undergo glutathionylation.Citation2,Citation3,Citation5,Citation6 A major group are enzymes involved in various pathways of carbohydrate/energy metabolismCitation4,Citation7 and others belong to the class of cytoskeletal proteins.Citation8 Some proteins sustain different but important functions, such as nucleophosmin, that is involved in the assembly of ribosomal proteins, or cyclophilin, a chaperonin involved in the proteasomal degradation of proteins. Moreover, growing evidence indicates glutathionylation as a modulatory mechanism of many cellular pathways, able to regulate the activity of several transcription factors (Nrf2 and NF-kB) and to interfere with the phosphorylation/dephosphorylation mechanism by interacting with kinases (PKA and CK) and/or phosphatases (PP2A and PTEN).Citation9 In addition, protein glutathionylation seems to be involved in cell proliferation and differentiation by contributing to the mitotic spindle formation during cell division. Indeed, the supramolecular organization of microfilaments (MF) and microtubules (MT) depends on the presence of exposed –SH residues and it is potentially susceptible to glutathionylation.Citation10 Besides a physiological role in redox signaling, several proteins become glutathionylated under conditions of oxidative and nitrosative stress.Citation4 Glyceraldehyde-3-phosphate dehydrogenase, for instance, represents the major S-glutathionylated protein in endothelial cells exposed to hydrogen peroxide and in monocytes, during the endogenous oxidative burst.Citation11,Citation12 Creatine kinase and glycogen phosphorylase b are also targets for S-glutathionylation in myocytes and cardiac tissue during cyclic oxidative stress.Citation13,Citation14 Among the cytoskeletal proteins, actin has been found to be glutathionylated in human platelets,Citation15 erythrocytes,Citation16 hepatocytes and T lymphocytesCitation17 under different oxidative stress conditions, and its glutathionylation may be considered a redox-regulatory mechanism of polymerization.Citation18–Citation21 Also tubulin can be readily oxidized in vitro, and the extent of tubulin cysteine oxidation has been shown to correlate with the inhibition of microtubule polymerization.Citation6,Citation22,Citation23
Therefore, in light of all these experimental evidence, protein-S-glutathionylation may be considered a sensor of tissue oxidative stress and its extent could help to define a threshold of basal antioxidant status in patho-physiological conditions. Indeed, in recent years, several proteins have been studied as possible markers of oxidative stress in human diseases and the extent of protein glutathionylation has taken a diagnostic/prognostic value.Citation24 Significant increases in glutathionylated proteins, for instance, have been found in hyperlipidemia, chronic renal failure, and diabetes mellitus, where higher levels of glutathionylated hemoglobin were identified in patients with type 1 and type 2.Citation6 Furthermore, evidence of a dysfunction of the glutathione metabolism has been proposed for the pathogenesis of several neurodegenerative diseases, such as Parkinson's disease, Alzheimer's disease, Friedreich's ataxia, and amyotrophic lateral sclerosis.Citation10,Citation25,Citation26
Thus, given the increasing role of glutathionylated proteins as redox-sensitive biomarkers, in this study we have explored the intracellular compartmentalization of protein glutathionylation in human skin fibroblasts using confocal laser microscopy and biochemical tools. The intracellular compartmentalization of protein glutathionylation has been examined in cultured dermal fibroblasts both in steady state and under proliferative conditions. Several recent studies have analyzed the glutathione distribution in intact cells,Citation27–Citation29 but the possibility to visualize glutathionylated proteins in different cellular compartments is critical also in light of the importance of compartmentalization in the redox signaling.Citation30 Here, we also provide a clear morphological evidence of a tight association between glutathione, nuclear lamina, and cytoskeleton by the exam of in situ extracted matrix samples, and we show a high colocalization pattern between glutathione and the endoplasmic reticulum (ER).
Further, we investigated the effect of a pro-oxidant agent (i.e. hydrogen peroxide) on the intracellular distribution and immunoreactivity of protein-bound glutathione (GS-Pro), by Z-reconstructions of multiple consecutive optical sections of treated and untreated cells. Additionally, we support our morphological finding measuring the GS-Pro amounts in oxidized and untreated cells by high pressure liquid chromatography (HPLC) analysis.
Materials and methods
Antibodies
Alexa Fluor (AF) 488 or 555 conjugated secondary antibodies, AF633 ConcanavalinA (ConA), rhodamine phalloidin, Hoechst 33342, and Prolong antifade reagent were purchased from Invitrogen (Molecular Probes, Carlsbad, CA, USA). Antibodies used were: mouse anti-GS-Pro (Virogen, Watertown, MA, USA), sheep anti-tubulin (Cytoskeleton, Denver, CO, USA), and goat immunoglobulins anti-vimentin and anti-lamin B (Santa Cruz Biotechnology, Temecula, CA, USA).
Cell culture and H2O2 treatments
Skin biopsies were collected after appropriate informed consent from normal subjects without any neuromuscular pathology and obtained from orthopedic surgery. Primary dermal fibroblasts were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (25 units/ml penicillin, 25 µg/ml streptomycin, and 0.3 µg/ml amphotericin B), at 37°C in 5% CO2. To examine the GS-Pro distribution during proliferation process, cells (1 × 105) were plated in 35-mm2 tissue culture dishes, fixed in paraformaldehyde after 6 hours, 24 hours, 48 hours, 72 hours, 6 days, and processed for immunofluorescence (see below).
For hydrogen peroxide treatments, cells were incubated with (100 µM) (final concentration) H2O2 for 1 hour at 37°C in humidified atmosphere with 5% CO2.
HPLC analysis
The cells were sonicated three times for 2 seconds in 0.1 ml of 0.1 M potassium-phosphate buffer (PBS), pH 7.2. After sonication, 50 µl of 12% sulfosalicylic acid was added, the protein pellet was dissolved in 150 µl of 0.1 N NaOH, and protein bound glutathione (GS-Pro) determined by HPLC as previously reported.Citation2 The derivatization and chromatography procedures were performed, with little modifications, as reported.Citation31
Immunofluorescence analysis
Cell samples were fixed with 4% formaldehyde in PBS (10 minutes), washed with PBS, permeabilized in 0.15% Triton X-100, and treated with 5% bovine albumin serum (BSA, 30 minutes). Then, cells were incubated with mouse anti-GS-Pro (1:100, overnight) and revealed with AF488 anti-mouse IgG. In double-labeling experiments, samples were incubated with a second primary antibody (against tubulin, vimentin, or lamin B) and the immunoreactions were revealed by incubation with specific AF555-conjugated immunoglobulins. Double- or triple staining with lectins was performed using rhodamine phalloidin (2 units/ml) or AF633 ConA (200 µg/ml dilution), respectively. Negative controls were performed using 1% PBS/BSA without the primary antibody. Nuclear staining was performed with Hoechst 33342. All experiments were repeated thrice.
In situ extracted matrix
Subconfluent primary dermal fibroblasts were processed as previously reportedCitation32 with some modifications: permeabilization with TBS-5 (10 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 5 mM MgCl2), 1% NP40, 2 mM sodium tetrathionate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 µg/mg leupeptin, and 10 µg/mg aprotinin (15 minutes); digestion with 0.01 mg/ml DNaseI (1 hours); double 2 M NaCl extraction in TBS-5 (5 minutes). Then, samples were double stained with anti-GS-Pro and lamin B antibodies, and incubated with AF488 and 555-conjugated antibodies. All experiments were repeated twice. Wide-field microscopy fluorescence images were collected with a Zeiss Axioskop 2 microscope and image processed using Photoshop software version 9.0 (Adobe Systems Inc., San Jose, CA, USA).
Imaging analysis
The confocal imaging was performed on Olympus Fluoview FV1000 confocal microscope equipped with FV10-ASW version 2.0 software, Multi Ar (458–488 and 515 nm), 2× He/Ne (543 and 633 nm), and 405-nm diode lasers, using 20× (0.75 numerical aperture, NA), 40× (0.90 NA), and 60× (1.42 NA oil) objectives. Optical single sections were acquired with a scanning mode format of 1024 × 1024 pixels, sampling speed of 40 µs/pixel, and 12 bits/pixel images. Fluorochromes’ unmixing was performed by acquisition of automated-sequential collection of multi-channel images, in order to reduce spectral crosstalk between channels. The pinhole aperture was 1 Airy unit. Colocalization analysis for dual-stained samples was carried out using the FV10-ASW software with the threshold-based approach.
The intensity average of fluorescence was calculated from cytometric measurements relative to nuclear, cytoplasmic, and total cell area, in six digital images randomly selected and analyzed for each immunolabeled cellular sample, using FV10-ASW Olympus software.
Z-reconstructions of serial single optical sections were performed with a scanning mode of 1024 × 1024 pixels with a 0.207 µm/pixel size, sampling speed of 40 µs/pixel, Z stack of 0.4 µs/slice, and 12 bits/pixel images. Images were processed using Photoshop software version 9.0 (Adobe Systems Inc.).
Statistical analysis
Statistical differences were calculated using the Student's t-test and the Wilcoxon/Mann–Whitney (U) test to assess the statistical significance of differences between groups, and data are presented as mean ± standard deviation (SD) or standard error of the mean (SEM). P < 0.05 was set as significant.
Results
Glutathionylated proteins are strictly associated with the nuclear lamina and colocalized with the ER
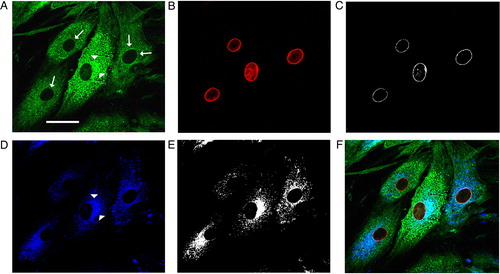
As shown in , the imaging analysis of subconfluent dermal fibroblasts revealed two different patterns of GS-Pro immunostaining: a granular cytoplasmic distribution of glutathionylated proteins reminiscent of organellar structures and ER cisternae, and microfilamentous staining underlining components of the cytoskeleton. In addition, a clear concentration of GS-Pro labeling around nuclei was also observed, that significantly colocalized with markers of the nuclear lamina. Colocalization analysis of cellular samples double stained with GS-Pro and lamin B antibodies revealed a significant number of colocalization areas (C). Further, the double labeling of GS-Pro with a marker of the ER, the ConcanavalinA (ConA), suggested that the glutathionylation is interested in ER compartments (E).
Figure 1. Glutathionylated proteins colocalized with the nuclear lamina, ER, and cytoskeleton. Immunofluorescence of glutathionylated proteins in human dermal fibroblasts (A) and multiple labeling with markers of the nuclear lamina (lamin B, in B) or rough ER (ConA pseudocolored in blue, in D), and their colocalization masks (C and E). GS-Pro was thickened around nuclei (arrows) and showed a strong colocalization with the nuclear lamina (white spots in C). The granular GS-Pro distribution was perinuclearly concentrated (arrowheads), interspersed in the cytoplasm, and significantly overlapped with the ER (colocalization mask in E, and merge signal in F). Scale bar, 40 µm.
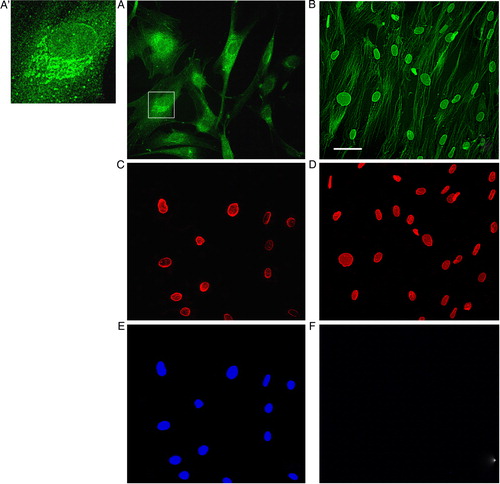
As GS-Pro staining of the perinuclear rim was a frequent and recurring finding (A and 2A′), we continued studying the association between glutathione and the nuclear lamina, by the analysis of samples obtained from in situ extraction of matrix (). This techniqueCitation32 (see Materials and methods) allows discarding cytosol, organuli, ER cisternae, nucleoplasm, and DNA, saving nuclear lamina and cytoskeleton only (B). By this method it is possible to reveal antigenic sites, amplifying the intensity of GS-Pro labeling, and investigating about the nature of GS-Pro binding. The immunofluorescence analysis of these extracted matrix samples detected a brilliant signal by glutathionylated proteins in correspondence to the nuclear lamina, thus confirming the tight association of GS-Pro with the cytoskeleton (B).
Figure 2. The glutathionylation process is strictly associated with the nuclear lamina and the cytoskeleton. Epifluorescence analysis of control unextracted (A, C, E) and extracted fibroblasts (B, D, F) using GS-Pro (A, B) and lamin B (C, D) antibodies. In normal conditions, glutathionylated proteins were concentrated around nuclei, interspersed in subcellular compartments, and in correspondence to cytoskeletal filaments (A). Immunolabeling of structures like cisternae was observed in several cells (inset in A, high magnification in A′). After matrix extraction, that preserves nuclear lamina and cytoskeleton and dissolves cytosol, organuli, ER cisternae, nucleoplasm and DNA, samples showed a clear and brilliant staining of cytoskeletal filaments and of the nuclear rim (B), as supported by double labeling with lamin B antibody (D). Hoechst staining for nuclei (E, F) was negative in extracted cells treated with DNAse (F), following DNA digestion. Scale bar, 50 µm.
In subcellular districts where glutathione shows a granular and punctate distribution, we revealed a recurrent association between glutathione and ER (E, A, and 2A′). We further confirmed the ER distribution of glutathionylated proteins by double immunolabeling of GS-Pro and protein disulfide isomerase, a protein specifically localized in the ER (data not shown).
Compartmentalization of protein-S-glutathionylation under proliferative conditions
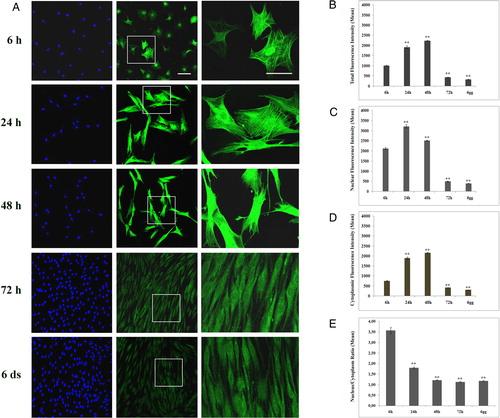
We evaluated the changes of GS-Pro immunostaining compartmentalization in different phases of the cell cycle. Six hours after plating, dermal fibroblasts showed a progressive intensification of the total immunoreaction both in nuclei and in cytoplasm up to 48 hours of growth (A). Then, GS-Pro immunofluorescence significantly decreased upon cells reaching the confluence (72 hours to 6 days; A). The quantification of the GS-Pro intensity in proliferating cells showed a maximal peak in whole cell at 48 hours after plating (2240 ± 25.28; n = 198), increasing of 2.2-fold with respect to 6 hours (1007 ± 27.47; n = 156) (A and B). When cells reached confluence (433.51 ± 7.49; n = 182) at 72 hours, it decreased of about 57% if compared to 6 hours of growth (A and B). Thus, we analyzed the intensity fluorescence values both in nuclei and cytoplasm during cell proliferation, finding a more concentrated nuclear GS-Pro localization in the early phases of the cell cycle (C and D). In view of these results, we determined nucleus/cytoplasm ratios in proliferative conditions (E). Interestingly, they reached a maximum (3.57 ± 0.15) at 6 hours after plating and then decreased to values close to 1 after 48 hours (1.21 ± 0.02) of cell growth (E). These findings highlight a nuclear shift of the GS-Pro in the early phases of cell proliferation, whereas it redistributed uniformly between the nucleus and cytoplasm at late stages (1.12 ± 0.03).
Figure 3. Protein glutathionylation is a process depending on the cell proliferative status. (A) Confocal analysis of glutathionylated proteins in human proliferative dermal fibroblasts examined at 6 hours, 24 hours, 48 hours, 72 hours, and 6 days after plating. GS-Pro immunostaining (green) was clearly detected in nuclei at the early phases of growth (6 hours) with few cells showing a brilliant cytoplasmic signal. The high magnification of insets was showed in the right column, whereas Hoechst staining for nuclei was reported in the left column. Up to 48 hours of growth, GS-Pro labeling showed a progressive intensification both in nuclear and in cytoplasmic districts, then it significantly decreased (72hours and 6 days). Magnification bars: 50 µm (middle column) and 30 µm (right column). (B, E) Total (B), nuclear (C), and cytoplasmic (D) GS-Pro fluorescence intensities detected at the different phases of the cell cycle. (E) Nucleus/cytoplasm GS-Pro ratios during cell proliferation. Data are presented as the mean ± SEM (**P < 0.001 versus values at 6 hours).
Cytoskeletal distribution of glutathionylated proteins
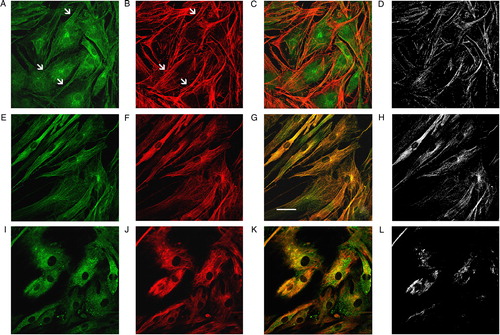
To better characterize the cytoskeletal distribution of glutathionylated proteins, we used specific cytoskeletal markers, such as F-actin, tubulin, and vimentin. As displayed in , the colocalization analysis showed an overlap of GS-Pro with microfilamentous actin (MF, B) in correspondence to stress fibers (D). Immunoprecipitation and western blotting analyses confirmed the glutathionylation of actin in fibroblasts (data not shown). MT, stained by an anti-tubulin antibody (F), exhibited a complete and significant overlap in all distribution points (G and H), whereas vimentin intermediate filaments (J) showed colocalization areas confined to central and perinuclear districts only (H and L).
Figure 4. The cytoskeleton-S-glutathionylation concerns actin MF and MT. Immunofluorescence analysis of the cytoskeleton glutathionylation. GS-Pro (A) and phalloidin (B) double staining showed a codistribution (C) in correspondence to stress fibers (arrows in A and B), with a significant colocalization pattern as visualized by their colocalization mask (D). Double immunolabeling of protein-S-glutathionylation (E) in association with tubulin (F), exhibited a complete overlap degree between GS-Pro and MT (yellow signal in G) in all distribution points (colocalization mask in H). Protein-S-glutathionylation (I) and intermediate vimentin filaments (J) showed a partial codistribution particularly in central and perinuclear areas (K), as pointed out by their colocalization mask (L). Scale bar, 40 µm.
Protein glutathionylation is affected by pro-oxidant treatment
In order to verify whether changes in cellular redox status are reflected in changes of the level of protein-S-glutationylation, we treated cultured fibroblasts with hydrogen peroxide (H2O2), a pro-oxidant agent. Treated fibroblasts showed an increase of the GS-Pro immunoreaction (A, d–f), confirmed by HPLC analysis detecting a 36% rise of GS-Pro in oxidized fibroblasts (B).
Figure 5. Protein glutathionylation is depending on cellular redox status. (A) GS-Pro immunolabeling of untreated (a) and H2O2 treated (d) fibroblasts showing an increase of the fluorescence intensity, owing to pro-oxidant treatment. Z-reconstructions of cell nuclei (arrows in a and d) and their X- and Y-axis projections revealed a negative or faint intranuclear reaction in control cells (b, c), whereas H2O2 treated fibroblasts exhibited a higher GS-Pro immunoreaction (e, f). Nuclei are counterstained with Hoechst (c, f). Scale bars in (d): 20 µm (for (a) and (d)); in (c): 50 µm (for (b, c) and (e, f)). (B) HPLC measurement of GS-Pro levels in control and H2O2 treated cell lysates. Results represent the mean (± SD) of three independent determinations. *P < 0.05. (C) GS-Pro fluorescence intensity in the nuclear compartment under oxidized conditions (data are representative of three separate experiments, and are expressed as mean ± SD; **P < 0.001).
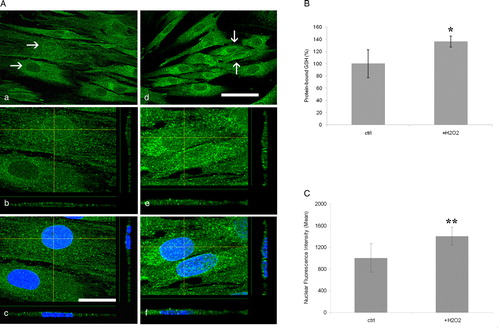
The GS-Pro distribution in the lateral and axial dimensions of treated cells, as visualized by XZ- and YZ-axis projections, revealed a higher GS-Pro concentration in oxidized fibroblasts particularly in nuclei (1409.77 ± 26.85; n = 38), than untreated cells (1007.49 ± 48.26; n = 29), corresponding to a 40% increase of GS-Pro nuclear staining (A, e and f).
Discussion
In the present work, we firstly describe the compartmentalization of constitutively glutathionylated proteins in different subcellular structures in human dermal fibroblasts by combining the specificity of monoclonal antibody labeling for GS-Pro, with the ability of confocal microscopy to analyze intracellular localization of fluorescently labeled molecules with high spatial resolution by optical sectioning. Furthermore, we investigated the protein glutathionylation in proliferative conditions and the reactivity of glutathionylated proteins to an oxidant by in vivo treatment.
S-glutathionylation is known to be implicated in the regulation of several signaling and metabolic pathways;Citation3–Citation6 thus the ability to visualize differently glutathionylated cellular districts may provide an important tool for following the oxidation states of individual organelles and for analyzing the ‘activation’ of specific redox pathways under patho-physiological conditions. Furthermore, the colocalization analysis allows correlating the spatial localization of two proteins occupying the same volume, making possible the identification of proteins mostly involved in a cellular response.
Association of glutathionylated proteins with nuclear lamina and ER
Our immunofluorescence analysis of dermal fibroblasts and extracted matrix samples detected a high signal of glutathionylated proteins in correspondence to the nuclear lamina. Besides to a barrier function, accumulating evidences reveal a role for the nuclear envelope as a ‘node’ of regulating signal transduction pathways in eukaryotic cells.Citation33,Citation34 In this context, the glutathionylation of nuclear lamina becomes a critical factor in redox signaling, and nuclear envelope acquires a crucial function in maintaining the cellular redox compartmentalization. Indeed, as evidenced by Markovic et al.,Citation35 the nuclear reduced status may induce heterochromatin formation, whereas glutathionylation of nuclear proteins regulate DNA compaction, cell cycle, and DNA repair.
In subcellular structures we observed an association between glutathione and ER. The ER is the first intracellular compartment for processing secretory and transmembrane proteins. Such processing may include a series of modifications, notably glycosylation and disulfide bond formation, and depends on distinct redox conditions provided within the ER. Moreover, glutathionylation may constitute an additional reversible mechanism regulating proper protein folding within the ER.
Protein-S-glutationylation in proliferative conditions
We investigated the intracellular compartmentalization of protein-S-glutathionylation in cultured dermal fibroblasts both in steady state and under proliferative conditions. Indeed, recent studies have reported a different distribution of glutathione during cell proliferation, with its recruitment into the nucleus in early phases of the cycle.Citation27–Citation29 An impairment of cell proliferation was further observed after depletion of nuclear GSH in 3T3 fibroblasts.Citation36 Our data showed a significant increase of total GS-Pro fluorescence in the whole cell at the early phases of growth and a strong reduction of the signal when cells approached to confluence. Interestingly, a nuclear shift of GS-Pro was observed at 6 hours after plating when cells started to grow, whereas the GS-Pro distribution became homogeneous throughout the cell when growth slowed. The accumulation of GS-Pro in the nucleus was also estimated by calculating the nuclear/cytoplasmic ratios during the culture progression. The results show a high nuclear/cytoplasmic ratio (3.6 ± 0.15) for GS-Pro very early after plating (6 hours), thus strongly supporting a regulating role for glutathionylation during the cell cycle.
Glutathionylation of cytoskeletal proteins
Our data displayed a strong colocalization between glutathionylated proteins and cytoskeleton components. Cytoskeletal glutathionylation is not surprising as the supramolecular organization of MF and MT depends on the presence of exposed –SH residues, thus being potentially susceptible to bind glutathione. Glutathionylation may modulate MF and MT assembly leading to interfere with important cellular functions, such as proliferation, migration, and differentiation. Actin, for instance, has been proposed as a target for redox regulation in several cells under oxidative stress, and its glutathionylation may be considered a physiological regulatory mechanism of G-actin polymerization.Citation18–Citation21 Actin as a redox target has been reported in some neurodegenerative diseases, such as in brain extracts of patients with Alzheimer's disease and in fibroblasts of patients with Friedreich's ataxia.Citation2,Citation37 By immunohistochemistry, a constitutive glutathionylation of the neuronal cytoskeleton has also been reported in human central nervous system.Citation8
Protein glutathionylation under oxidative conditions
Protein glutathionylation is affected by pro-oxidant treatment, as evidenced by immunofluorescence and HPLC analyses that revealed a higher GS-Pro amount in fibroblasts treated with a pro-oxidant agent. Noteworthy, GS-Pro signal increases in the nuclear compartment after oxidation, thus resembling the nuclear shift observed during cell proliferation. Therefore, besides to support the concept of redox regulation of the cell cycle, the increase of GS-Pro nuclear staining may also represent a marker in diseases where oxidative stress has been proposed as a pathogenetic cause.Citation38
In conclusion, so far the analysis of intracellular glutathione has traditionally been performed by chemical derivatization techniques, coupled to chromatography and spectrophotometric detection.Citation39,Citation40 These methods, however, fail to reveal any measure of intracellular distribution of the GS-Pro, which, conversely, is of growing importance as a redox biomarker both during constitutive metabolism and under oxidative stress. Recently, confocal microscopy analysis has been carried out for detecting the intracellular distribution of GS-Pro in cardiac myocytes and in neutrophils.Citation41,Citation42
Thus, the ability to visualize the glutathionylated proteins in cultured cells and/or in tissues may provide an additional tool to investigate their ‘abnormal’ compartmentalization and, potentially, to highlight pathways in turns ‘redox-activated’. This may acquire a pathogenic significance particularly in diseases where oxidative stress plays an important role, as for Friedreich's AtaxiaCitation2 and non-alcoholic fatty liver disease.Citation38
Acknowledgement
This work was supported by funds from the Italian Ministry of Health (Ricerca Corrente). The first two authors contributed equally to this work.
References
- Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann NY Acad Sci 2002;973:488–504.
- Pastore A, Tozzi G, Gaeta LM, Bertini E, Serafini V, Di Cesare S, et al. Actin glutathionylation increases in fibroblasts of patients with Friedreich's ataxia: a potential role in the pathogenesis of the disease. J Biol Chem 2003;43:42588–95.
- Cotgreave IA, Gerdes RG. Recent trends in glutathione biochemistry. Glutathione–protein interactions: a molecular link between oxidative stress and cell proliferation? Biochem Biophys Res Commun 1998;242:1–9.
- Klatt P, Lamas S. Regulation of protein function by S-glutathionylation in response to oxidative and nitrosative stress. Eur J Biochem 2000;267:4928–44.
- Fratelli M, Demol H, Puype M, Casagrande S, Eberini L, Salmona M, et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci USA 2002;99:3505–10.
- Giustarini D, Rossi R, Milzani A, Colombo R, Dalle-Donne I. S-glutathionylation: from redox regulation of protein functions to human diseases. J Cell Mol Med 2004;8:201–12.
- Pineda-Molina E, Klatt P, Vazquez J, Marina A, Garcia de Jacoba M, Perez-Sala D, et al. Glutathionylation of the p50 subunit of NF-kB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 2001;40:14134–42.
- Sparaco M, Gaeta LM, Tozzi G, Bertini E, Pastore A, Simonati A, et al. Protein glutathionylation in human central nervous system: potential role in redox regulation of neuronal defense against free radicals. J Neurosci Res 2006;83:256–63.
- Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antiox Redox Signal 2008;10:1941–89.
- Sparaco M, Gaeta LM, Santorelli FM, Passarelli C, Tozzi G, Bertini E, et al. Friedreich's ataxia: oxidative stress and cytoskeletal abnormalities. J Neurol Sci 2009;287:111–8.
- Ravichandran V, Seres T, Moriguchi T, Thomas JA, Johnston RB. S-thiolation of glyceraldehyde-3-phosphate dehydrogenase induced by the phagocytosis-associated respiratory burst in blood monocytes. J Biol Chem 1994;269:25010–5.
- Schuppe-Koisinen I, Moldeus P, Bergmann T, Coatgreave IA. S-thiolation of human endothelial cell glyceraldehyde-3-phosphate dehydrogenase after hydrogen peroxide treatment. Eur J Biochem 1994;221:1033–7.
- Reddy S, Jones AD, Cross CE, Wong PS, Van Der Vliet A. Inactivation of creatine kinase by S-glutathionylation of the active-site cysteine residue. Biochem J 2000;347(Pt 3):821–7.
- Klatt P, Pineda Molina E, Pérez-Sala D, Lamas S. Novel application of S-nitrosoglutathione-Sepharose to identify proteins that are potential targets for S-nitrosoglutathione-induced mixed-disulphide formation. Biochem J 2000;349(Pt 2):567–78.
- Dalle-Donne I, Giustarini D, Colombo R, Milzani A, Rossi R. S-glutathionylation in human platelets by a thiol-disulfide exchange-independent mechanism. Free Radic Biol Med 2005;38:1501–10.
- Mawatari S, Murakami K. Different types of glutathionylation of hemoglobin can exist in intact erythrocytes. Arch Biochem Biophys 2004;421:108–14.
- Fratelli M, Demol H, Puype M, Casagrande S, Villa P, Eberini L, et al. Identification of proteins undergoing glutathionylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics 2003;3:1154–61.
- Dalle-Donne I, Milzani A, Giustarini D, Di Simplicio P, Colombo R, Rossi R. S-NO-actin: S-nitrosylation kinetics and the effect on isolated vascular smooth muscle. J Muscle Res Cell Motil 2000;21:171–81.
- Dalle-Donne I, Giustarini D, Rossi R, Colombo R, Milzani A. Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Rad Biol Med 2003;34:23–32.
- Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, et al. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem 2001;276:47763–6.
- Wang J, Tekle E, Oubrahim H, Mieyal JJ, Stadtman ER, Chock PB. Stable and controllable RNA interference: investigating and physiological function of glutathionylated actin. Proc Natl Acad Sci USA 2003;100:5103–6.
- Landino LM, Moynihan KL, Todd JV, Kennett KL. Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochem Biophys Res Commun 2004a;314:555–60.
- Landino LM, Robinson SH, Skreslet TE, Cabral DM. Redox modulation of tau and microtubule-associated protein-2 by the glutathione/glutaredoxin reductase system. Biochem Biophys Res Commun 2004b;323:112–7.
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem 2006;52(4):601–23.
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem 2000;267:4904–11.
- Piemonte F, Pastore A, Tozzi G, Tagliacozzi D, Santorelli FM, Carrozzo R, et al. Glutathione in blood of patients with Friedreich's ataxia. Eur J Clin Invest 2001;31:1007–11.
- Markovic J, Borrás C, Ortega A, Sastre J, Viña J, Pallardó FV. Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem 2007;282(28):20416–24.
- Pallardó FV, Markovic J, García JL, Viña J. Role of nuclear glutathione as a key regulator of cell proliferation. Mol Aspects Med 2009;30:77–85.
- Diaz Vivancos P, Wolff T, Markovic J, Pallardó FV, Foyer CH. A nuclear glutathione cycle within the cell cycle. Biochem J 2010;431:169–78.
- Jones DP, Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab 2010;12(2):116–25.
- Pastore A, Piemonte F, Locatelli M, Lo Russo A, Gaeta LM, Tozzi G, et al. Determination of blood total, reduced and oxidized glutathione in pediatric subjects. Clin Chem 2001;47:1467–9.
- Squarzoni S, Sabatelli P, Ognibene A, Toniolo D, Cartegni L, Cobianchi F, et al. Immunocytochemical detection of emerin within the nuclear matrix. Neuromusc Disord 1998;8:338–44.
- Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta 2008;1780:1273–90.
- Dauer WT, Worman HJ. The nuclear envelope as a signalling node in development and disease. Dev Cell 2009;17:626–38.
- Markovic J, García-Gimenez JL, Gimeno A, Viña J, Pallardó FV. Role of glutathione in cell nucleus. Free Radic Res 2010;44(7):721–33.
- Markovic J, Mora NJ, Broseta AM, Gimeno A, de-la-Concepción N, Viña J, et al. The depletion of nuclear glutathione impairs cell proliferation in 3T3 fibroblasts. PLOS One 2009;4(7):1–14.
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience 2001;103(2):373–83.
- Piemonte F, Petrini S, Gaeta LM, Tozzi G, Bertini E, Devito R, et al. Protein glutathionylation increases in the liver of patients with non-alcoholic fatty liver disease. Hepatology 2007;23:457–64.
- Cotgreave IA, Moldeus P. Methodologies for the application of monobromobimane to the simultaneous analysis of soluble and protein thiol components of biological systems. J Biochem Biophys Methods 1986;13:231–49.
- Conlan XA, Stupka N, McDermott GP, Francis PS, Barnett NW. Determination of intracellular glutathione and cysteine using HPLC with a monolithic column after derivatization with monobromobimane. Biomed Chromatogr 2010;24(5):455–7.
- Brennan JP, Miller JIA, Fuller W, Wait R, Begum S, Dunn MJ, et al. The utility of N,N-biotinyl glutathione disulfide in the study of protein S-glutathiolation. Mol Cell Proteomics 2006;5:215–25.
- Lim SY, Raftery MJ, Goyette J, Geczy CL. S-Glutathionylation regulates inflammatory activities of S100A9. J Biol Chem 2010;285(19):14377–88.