Abstract
Objectives
To study the effect of clonidine pre-treatment on hemorrhagic shock (H/S)-induced endotoxemia and oxidative stress (OS) in three vital organs of the rat.
Methods
The study protocol consisted of two arms: one for the measurement of organic hydroperoxide (LOOH) and superoxide radical (O2−·) production in the gut, liver, and lungs (n = 32 rats) and one for the measurement of endotoxin in portal and systemic circulation (n = 32 rats). Four animal groups (sham, clonidine, H/S, and clonidine-H/S group) were used in each arm. Three hours after H/S and concominant blood resuscitation, tissues were collected for LOOHs and O2−· measurement and blood samples were obtained for endotoxin determination.
Results
Clonidine pre-treatment prior to H/S resulted in a significant reduction of LOOHs and O2−· production in all vital organs (P < 0.05–0.001), while additionally, clonidine reduced H/S-induced endotoxemia in portal (P < 0.05) and systemic circulation as well (P < 0.01).
Discussion
Clonidine pre-treatment prevents endotoxemia and OS in the gut, liver, and lungs of rats subjected to severe H/S. The improved intestinal barrier function probably stems from the antioxidant effect of clonidine on the intestinal epithelium, whereas the reduced endotoxemia may contribute to a decreased OS observed in the liver and lungs.
Introduction
Hemorrhagic shock (H/S) is a life-threatening complication,Citation1,Citation2 with complex pathophysiology, especially during reperfusion.Citation3 H/S may lead to the development of systemic inflammatory response syndrome (SIRS), mainly through hyperactivation of phagocytes and excessive production of reactive oxygen species (ROS).Citation4–Citation6 Moreover, ROS production and ischemia during H/S are believed to compromise the integrity of the intestinal barrier, resulting in the translocation of bacteria and endotoxin to remote organs, a process termed as bacterial translocation (BT), that further stimulates SIRS.Citation7,Citation8 Under these conditions, acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS) may develop with a dismal prognosis.Citation9–Citation11
Alpha2-adrenoceptor agonists (e.g. clonidine, dexmedetomidine) have been found to reduce anxiety, induce sedation, produce analgesia, and decrease the anesthetic requirements for induction and maintenance of anesthesia.Citation12,Citation13 Moreover, the perioperative use of this agents in patients undergoing vascular or cardiac surgery has been shown beneficial in terms of reducing the incidence of myocardial ischemia/infarction and mortality.Citation14 Furthermore, a mortality benefit in septic patients sedated with dexmedetomidine has been shown relative to lorazepam.Citation15 For these reasons, their use as adjuncts to anesthesia and as sedative agents in the Intensive Care Unit (ICU) has received much attention.Citation16 Interestingly, concerning the effects of alpha2-adrenoceptor agonists on inflammatory response, it has been shown that, in contrast to other anesthetics, they do not disturb the function of neutrophils,Citation17 while they seem to possess anti-inflammatory properties.Citation18–Citation20 In addition, they have been reported to improve the post-hypoxic function of several organs in animal models and humans.Citation21–Citation24
Based on these observations, the aim of the present experimental study was to test the hypothesis that pre-treatment of rats with clonidine may prevent the H/S-induced oxidative stress (OS) and BT. For this purpose, the superoxide radical (O2−·) and lipid hydroperoxides (LOOHs) production were measured in the liver, gut, and lungs of the animals. Additionally, in the second arm of this study the levels of endotoxin were determined in both the systemic and portal circulation as well.
Materials and methods
Animals
The study was carried out on 64 male Wistar rats weighing 400–450 g. The animals were housed in stainless-steel cages, three rats per cage, in a controlled environment, with a 12-hour light/dark cycle and were allowed free access to standard laboratory food and water. The study protocol was approved by the local Ethics Committee.
Animal pre-treatment
The study consisted of two arms: one for the measurement of the oxidative load in the organs and one for the measurement of endotoxin. Four animal groups (n = 8 per group) were used for each arm (sham, clonidine, H/S, and clonidine-H/S group). In the sham group rats were subjected merely to the surgical catheter placement and received 1 ml saline (s.c.) twice a day for 2 days and one dose in the morning before the experiment. Rats in the clonidine group were treated with clonidine (150 µg/kg s.c.) twice a day for 2 days and one dose in the morning before the experiment. In the H/S group rats were subjected to H/S as will be described, while in the clonidine-H/S group, rats were pre-treated with clonidine prior to H/S.
Experimental design
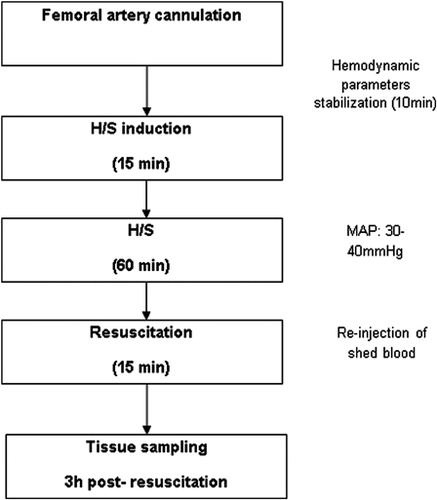
All experiments started approximately at 8 a.m. Rats were fasted overnight before the experiment but allowed water ad libitum. The animals were anesthetized with intraperitoneal injections of midazolam (5 mg/kg) and ketamine (60 mg/kg). With minimal dissection and under sterile conditions, the left femoral artery was isolated and catheterized with a 26 gauge intravenous catheter (Abbott Laboratories, Illinois, USA) which was connected to a pressure transducer for the measurement of mean arterial blood pressure (MAP), which was displayed on a monitor (DINAMAP™ PLUS, CRITIKON; GE Healthcare, Little Chalfont, UK). Upon completion of the catheterization, the cardiovascular parameters were allowed to stabilize for 10 minutes. Blood was then withdrawn until MAP was reduced to 30–40 mmHg within 15 minutes (induction of shock). Thereafter, the MAP was maintained at this level for 60 minutes by withdrawal or reinjection of shed blood that was stored in heparinized syringes at room temperature. Finally, the animals were resuscitated with reinjection of the shed blood over 15 minutes. Upon resuscitation, the intravenous catheter was removed and rats were placed in their cages after recovering from anesthesia ().
Tissue sampling
One hundred and five minutes after resuscitation the rats in the first arm of the experiment, received dihydroethidine (DHE) under light ether anesthesia. Three hours after resuscitation, rats were anesthetized with ketamine and were subjected to midline laparotomy under strict sterile conditions. The left hepatic lobe was then excised, followed by part of the terminal ileum. The left hemidiaphragm was then opened and the left lung was excised. Finally, the rats were sacrificed by exsanguination.
In the second arm of the experiment, three hours after resuscitation, rats were subjected to midline laparotomy under strict sterile conditions and portal vein and the abdominal aorta were punctured and samples of blood were obtained for the estimation of endotoxin.
Reagents
DHE, horseradish peroxidase, DNA type III, bovine serum albumin (BSA, fraction V), butylated hydroxyanisole, xylenol orange, Coomassie Brilliant Blue-G250 (CBB-G250), Triton X-100, and Dowex 50X-8 (mesh 400) were from Sigma, St Louis, MO, USA. Dimethyl sulfoxide (DMSO), acetone, chloroform, acetonitrile, absolute methanol and ethanol, hydrogen peroxide, sodium cyanide, ammonium ferrous sulfate, sorbitol, and trifluoroacetic acid were from Merck, Darmstadt, Germany. Hydrophobic Oasis HLB (Hydrophilic-Lipopholic Balance) 1 cm3 (30 mg) extraction cartridges were from Waters Corp, Milford, MA, USA. All reagents and solvents used were of the highest purity.
Tissue treatment
Organ tissues were homogenized with a glass–glass Potter-Elvehjem homogenizer in 1:1 (for lungs and intestine) or 3:1 (for liver) tissue wet weight:volume ice-cold phosphate buffer (50 mM, pH 7.8, containing 10 mM sodium cyanide as inhibitor of non-specific peroxidases).
Superoxide assay
The method is based on the reaction between O2−· and DHE administered to the rats in vivo.Citation25 Specifically, DHE 8.5 mg/kg dissolved in 40% DMSO to a final volume of 1 ml was administered subcutaneously. This amount was in excess so as to trap efficiently the O2−· formed over experimental incubation periods up to 75 minutes and was assessed by preliminary experiments where tissue samples were incubated with various doses of DHE at several time intervals to establish the following criteria: (a) the minimum dose of DHE above which the rate of formed 2-HO-ethidium remains constant (at the tested time intervals) and (b) the detection of unreacted DHE in the tested rat organs to ensure DHE excess during incubation.
The reaction of O2−· with DHE generates the specific product 2-OH-ethidium, the formation of which is measured and converted to O2−· production rate. 2-OH-ethidium is estimated, after being extracted from the tissue in alkaline acetone, isolated via cation and hydrophobic microcolumn chromatographies, and quantified by the use of its fluorescence properties and its reaction with hydrogen peroxide. Fluorescence measurements were performed in a Shimadzu RF-1501 spectrofluorometer set at 10 nm excitation/emission slit width and high sensitivity.
Lipid peroxidation assay
LOOHs were determined by the ferrous oxidation-xylenol orange (FOX) assay. The photometric assay is based on the reaction of an assay reagent containing Fe(II) with LOOH (Fenton reaction), the subsequent production of Fe(IΙI), its re-reaction with the reagent dye xylenol orange, and the formation of a chromogenic product absorbing at 560 nm.Citation26 Specifically, 0.2 ml homogenate is extracted 2× with equal volume of chloroform:methanol 2:1 and the bottom organic layer is collected (after centrifugation at 15 000 g) and vacuum dried. The lipid pellet is dissolved in 0.22 ml absolute ethanol.
The reagents of the FOX assay were prepared as follows and in the stated mixing order: In 3 ml distilled water 7.6 mg xylenol orange, 1.82 g sorbitol, and 0.14 ml concentrated sulfuric acid were dissolved, and the solution was brought to final 5 ml volume with distilled water. This solution was split into two 2.5 ml portions. One portion (Reagent A) was used for making sample blank and the other was mixed with 3.8 mg ammonium ferrous sulfate (Reagent B) and was used for the quantification of LOOHs.
Two dilutions (25 and 75 µl) of the already-dissolved lipid pellet were mixed with 25 µl of Reagent A (for sample blank) and 25 µl of Reagent B (for LOOH measurement), were brought to final volume 0.5 ml with absolute ethanol, and were incubated for 30 minutes at room temperature. The absorbance of the Reagent B-treated sample was measured against the Reagent A-treated sample blank with a Shimadzu UV-1201 UV-VIS spectrophotometer set at 560 nm, and its absorbance value was converted to cumene hydroperoxide equivalents from a standard curve (0.1–2 µM).
Protein concentration assay
Protein in sample homogenates was determined by a modification of a CBB-based method.Citation27 Specifically, 0.063 ml of various dilutions of the homogenate was mixed with 0.02 ml 0.5% (v/v) Triton X-100 and 0.017 ml 6 N HCl. The mixture was incubated at 100°C for 10 minutes, brought to room temperature, and mixed with 0.9 ml 0.033% (w/v) CBB-G250 stock reagent (made in 0.5 N HCl, stirred for 30 minutes, and filtrated through Whatman #1 filter paper by water pump aspiration, and stored in the dark) and incubated for 5 minutes at room temperature. The absorbance at 620 nm of the mixture was converted to protein mg from a 0–0.05 mg BSA standard curve using a Shimadzu UV-VIS 1201 spectrophotometer.
Endotoxin concentration
Endotoxin concentration was determined by the quantitative chromogenic Limulus Amebocyte Lysate test (QCL-1000; BioWhittaker, Walkersville, MD, USA) and expressed in EU/ml. Samples were processed according to the manufacturer's instructions. By this test, it is possible to measure concentrations of endotoxin ≤0.01 EU/ml.
Statistical analysis
All data were analyzed by using one-way analysis of variance, followed by post hoc tests for multiple comparisons (Tukey's method and the Bonferroni's inequality correction). The results are expressed as mean ± standard deviation (SD). Differences were considered significant when P < 0.05.
Results
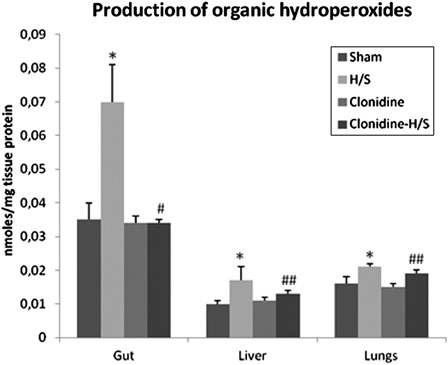
The production of LOOHs in the gut of rats subjected to H/S showed a 100% increase compared to sham (P < 0.001), whereas, in the liver the increase reached the 70% of the baseline value (P < 0.001). The production of LOOHs increased to a lesser extend in the lungs (31.3% compared to sham, P < 0.001). Concerning the effect of clonidine pre-treatment in the production of LOOHs in the gut, a statistically significant reduction (P < 0.001) of 51.4% compared to H/S was observed. Moreover, LOOHs production showed a smaller, but still statistically significant (P < 0.05) reduction in the liver (23.5%) and lungs (9.5%) ().
Figure 2. Production of organic hydroperoxides in the gut, liver, and lungs. Values expressed as mean ± SD. Sham, n = 8: rats were subjected to surgical artery cannulation alone. H/S, n = 8: rats were subjected to H/S. Clonidine, n = 8: rats pre-treated with clonidine were subjected to the surgical artery cannulation. Clonidine-H/S, rats pre-treated with clonidine were subjected to H/S. *P < 0.001 compared to sham, #P < 0.001 compared to H/S, ##P < 0.05 compared to H/S.
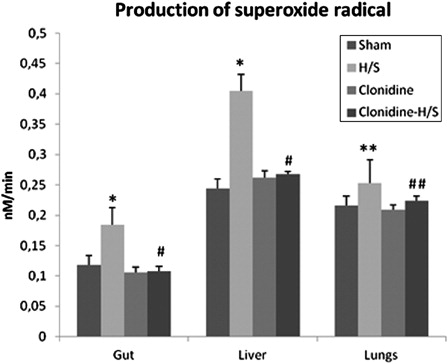
The rate of O2−· production after H/S increased in the gut and the liver of the rats by 55.9 and 65.9%, respectively (P < 0.001), while a less excessive but still statistically significant increase (17.1%, P < 0.05) was observed in the lungs. Rats in the clonidine-H/S group exhibited a statistically significant reduction (P < 0.001) in the production of O2−· in the gut (41.3%) and liver (33.8%), and to a lesser extent in the lungs (11.5%, P < 0.05) compared to H/S group ().
Figure 3. Production of superoxide radical in the gut, liver, and lungs (values expressed as mean ± SD). Sham, n = 8: rats were subjected to surgical artery cannulation alone. H/S, n = 8: rats were subjected to H/S. Clonidine, n = 8: rats pre-treated with clonidine were subjected to the surgical artery cannulation. Clonidine-H/S, rats pre-treated with clonidine were subjected to H/S. *P < 0.001 compared to sham, **P < 0.05 compared to sham, #P < 0.001 compared to H/S, ##P < 0.05 compared to H/S.
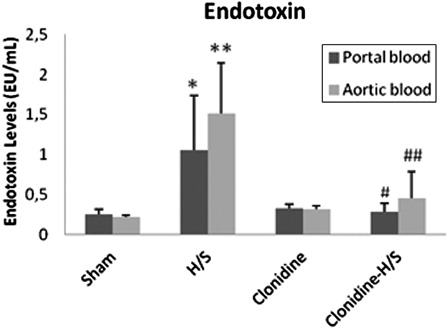
Finally, H/S produced statistically significant increase of endotoxin levels in both portal and systemic circulation (P < 0.05 and P < 0.001, respectively), whereas pre-treatment with clonidine reduced the amount of endotoxin detected both in the portal (P < 0.05) and systemic (P < 0.01) circulation compared to H/S ().
Figure 4. Endotoxin levels in portal and aortic blood samples (values expressed as mean ± SD). Sham, n = 8: rats were subjected to surgical artery cannulation alone. H/S, n = 8: rats were subjected to H/S. Clonidine, n = 8: rats pre-treated with clonidine were subjected to the surgical artery cannulation. Clonidine-H/S, rats pre-treated with clonidine were subjected to H/S. *P < 0.05 compared to sham, **P < 0.001 compared to sham, #P < 0.05 compared to H/S, ##P < 0.01 compared to H/S.
Discussion
This study documents for the first time that clonidine pre-treatment prior to H/S results in a significant reduction of the oxidative load in the gut, liver, and lungs, while additionally prevents endotoxemia in both portal and systemic circulation, probably due to its antioxidant effect on the intestinal epithelium. During H/S, the reduction in blood flow leads to diminished microcirculatory perfusion and regional hypoxia. Upon resuscitation, the formation of ROS at the ischemic region is proposed as a fundamental mechanism of organ damage.Citation28,Citation29 In addition, H/S is followed by activation of the nuclear factor-kappaB,Citation30 which promotes cytokine production and inflammatory responseCitation31 with enhancement of OS.Citation32 Thus, persistently increased OS may further enhance and perpetuate SIRS and vice versa. Moreover, H/S induced gut ischemia could weaken the mucosal barrier and cause BT.Citation33 Although the exact mechanism is not yet known, diminished tissue perfusion and ischemia with increased oxidative load may explain the phenomenon. The excessive presence of endotoxin in the circulation stimulates SIRS, which may produce structural and functional deleterious effects on remote organs resulting in MODS.Citation34
According to our results, acute severe H/S produced – compared to sham – a substantial increase in the production of O2−· and LOOHs in the gut, liver, and the lungs of the rats. These results are in accordance with previous studies.Citation35–Citation37 Importantly, we performed a quantitative and specific measurement of O2−· production in the tested tissues,Citation38 since O2−· formation is the first step in a sequence of reactions leading to the oxidation of biological structures. In addition, we showed that H/S resulted in an increased concentration of endotoxin in the portal and systemic circulation, reflecting obviously the increased BT taking place after H/S. Previous studies have shown that H/S leads to access of gut-derived endotoxin into the systemic circulation predominantly via the portal vein.Citation39,Citation40 It has become clear that the gut mucosa is highly susceptible to ischemia reperfusion injury, and disruption of the intestinal epithelial lining is associated with the translocation of bacteria or bacteria-derived substances from the gut lumen into the bloodstream.Citation41
Interestingly, pre-treatment of the animals with clonidine prevented H/S-induced OS, in terms of retaining O2−· and LOOHs levels in the liver, gut, and lungs to base-line values. In addition, clonidine pre-treatment prevented BT, leading to baseline concentrations of endotoxin in the portal and systemic circulation. The possible mechanism involved in the antioxidant action of clonidine on the tested tissues needs further investigation. However, in vitro experiments revealed a direct antioxidant action of clonidine in terms of inhibition of lipid peroxidation and activation of antioxidant enzymes.Citation42 Moreover, it has been shown that H/S-induced endotoxemia is associated with generation of ROS via a xanthine oxidase-dependent pathway.Citation10 Likewise, the reduced systemic endotoxemia after clonidine pre-treatment may contribute to the decreased OS observed in the liver and lungs. On the other hand, the reduced levels of endotoxin after clonidine pre-treatment may be attributed to the improved intestinal barrier function that stems from the antioxidant effect of clonidine on the intestinal epithelium, resulting in the prevention of BT.
In conclusion, our study suggests that pre-treatment with clonidine prevents endotoxemia and OS in the gut, liver, and lungs of rats subjected to severe H/S. Although laboratory results on animals should not be easily extrapolated to humans, we believe that clonidine merit consideration as adjunctive sedative agent of choice in patients susceptible to develop H/S, such as critically ill ICU patients undergoing surgery where blood loss is anticipated. Pre-treatment of these patients with clonidine before the establishment of H/S may prevent complications, such as ARDS and MODS, improving likewise their outcome. The reduction in cardiac output and mean arterial pressure produced by clonidine might be a reasonable objection against its use in these patients. However, the drug has been already shown to be safe when used in the perioperative setting in patients undergoing vascular or cardiac surgeryCitation43 that are associated with blood loss.
References
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 2006;60 (6 Suppl.):S3–11.
- Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg 2010;34:158–63.
- Rushing GD, Britt LD. Reperfusion injury after hemorrhage: a collective review. Ann Surg 2008;247:929–37.
- Welbourn CR, Goldman G, Paterson IS, Valeri CR, Shepro D, Hechtman HB. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg 1991;78:651–5.
- Lozano Sanchez FS, Gonzalez-Sarmiento R. Systemic inflammatory response, bacterial translocation and nitric oxide donors. Inflamm Allergy Drug Targets 2007;6:139–41.
- Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001;94:1133–8.
- Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, et al. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol 2005;11:5485–91.
- Alexander JW, Boyce ST, Babcock GF, Gianotti L, Peck MD, Dunn DL, et al. The process of microbial translocation. Ann Surg 1990;212:496–510.
- Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci 2006;11:520–8.
- Deitch EA, Bridges W, Baker J, Ma JW, Ma L, Grisham MB, et al. Hemorrhagic shock-induced bacterial translocation is reduced by xanthine oxidase inhibition or inactivation. Surgery 1988;104:191–8.
- Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock 2001;15:1–10.
- Filos KS, Goudas LC, Patroni O, Polyzou V. Intrathecal clonidine as a sole analgesic for pain relief after cesarean section. Anesthesiology 1992;77:267–74.
- Filos KS, Patroni O, Goudas LC, Bosas O, Kassaras A, Gartaganis S. A dose-response study of orally administered clonidine as premedication in the elderly: evaluating hemodynamic safety. Anesth Analg 1993;77:1185–92.
- Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta-analysis. Am J Med 2003;114(9):742–52.
- Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care 2010;14(2):R38.
- Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol 2011;28:3–6.
- Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, et al. The effects of clonidine and dexmedetomidine on human neutrophil functions. Anesth Analg 1999;88:452–8.
- Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med 2004;32:1322–6.
- Memis D, Hekimoglu S, Vatan I, Yandim T, Yuksel M, Sut N. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth 2007;98:550–2.
- Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth 2001;86:650–6.
- Dean JM, George S, Naylor AS, Mallard C, Gunn AJ, Bennet L. Partial neuroprotection with low-dose infusion of the alpha2-adrenergic receptor agonist clonidine after severe hypoxia in preterm fetal sheep. Neuropharmacology 2008;55:166–74.
- Guo H, Takahashi S, Cho S, Hara T, Tomiyasu S, Sumikawa K. The effects of dexmedetomidine on left ventricular function during hypoxia and reoxygenation in isolated rat hearts. Anesth Analg 2005;100:629–35.
- Laudenbach V, Mantz J, Lagercrantz H, Desmonts JM, Evrard P, Gressens P. Effects of alpha(2)-adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedetomidine. Anesthesiology 2002;96:134–41.
- Zhang Y. Clonidine preconditioning decreases infarct size and improves neurological outcome from transient forebrain ischemia in the rat. Neuroscience 2004;125:625–31.
- Georgiou CD, Papapostolou I, Patsoukis N, Tsegenidis T, Sideris T. An ultrasensitive fluorescent assay for the in vivo quantification of superoxide radical in organisms. Anal Biochem 2005;347:144–51.
- Wolff SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol 1994;233:182–9.
- Patsoukis N, Georgiou CD. Determination of the thiol redox state of organisms: new oxidative stress indicators. Anal Bioanal Chem 2004;378:1783–92.
- Itoh M, Guth PH. Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology 1985;88:1162–7.
- Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. Critical role of oxygen radicals in the initiation of hepatic depression after trauma hemorrhage. J Trauma 2000;49:879–85.
- Adcock IM, Brown CR, Kwon O, Barnes PJ. Oxidative stress induces NF kappa B DNA binding and inducible NOS mRNA in human epithelial cells. Biochem Biophys Res Commun 1994;199:1518–24.
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997;336:1066–71.
- Robinson MK, Rounds JD, Hong RW, Jacobs DO, Wilmore DW. Glutathione deficiency increases organ dysfunction after hemorrhagic shock. Surgery 1992;112:140–7.
- Swank GM, Lu Q, Xu DZ, Michalsky M, Deitch EA. Effect of acute-phase and heat-shock stress on apoptosis in intestinal epithelial cells (Caco-2). Crit Care Med 1998;26:1213–7.
- Keel M, Trentz O. Pathophysiology of polytrauma. Injury 2005;36:691–709.
- Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol 1991;260:G355–62.
- Liaudet L, Soriano FG, Szabo E, Virag L, Mabley JG, Salzman AL, et al. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc Natl Acad Sci USA 2000;97:10203–8.
- Menezes J, Hierholzer C, Watkins SC, Lyons V, Peitzman AB, Billiar TR, et al. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. Am J Physiol 1999;277(1 Pt 1):G144–51.
- Panteli ES, Fligou F, Papamichail C, Papapostolou I, Zervoudakis G, Georgiou CD, et al. Quantification of superoxide radical production in 4 vital organs of rats subjected to hemorrhagic shock. Am J Emerg Med 2012;30:476–80.
- Rush BF, Sori AJ, Murphy TF, Smith S, Flanagan JJ, Machiedo GW. Endotoxemia and bacteremia during hemorrhagic shock. The link between trauma and sepsis? Ann Surg 1988;207:549–54.
- Jiang J, Bahrami S, Leichtfried G, Redl H, Ohlinger W, Schlag G. Kinetics of endotoxin and tumor necrosis factor appearance in portal and systemic circulation after hemorrhagic shock in rats. Ann Surg 1995;221:100–6.
- Gullo A, Berlot G. Ingredients of organ dysfunction or failure. World J Surg 1996;20:430–6.
- Karmen NB. Oxidative modification of erythrocyte membranes in the acute stage of severe craniocerebral trauma and its correction with clonidine. Bull Exp Biol Med 2003;136(4):362–5.
- Nishina K, Mikawa K, Uesugi T, Obara H, Maekawa M, Kamae I, et al. Efficacy of clonidine for prevention of perioperative myocardial ischemia: a critical appraisal and meta-analysis of the literature. Anesthesiology 2002;96(2):323–9.