Abstract
Fibromyalgia (FM) is characterized by generalized pain and chronic fatigue of unknown etiology. To evaluate the role of oxidative stress in this disorder, we measured plasma levels of ubiquinone-10, ubiquinol-10, free cholesterol (FC), cholesterol esters (CE), and free fatty acids (FFA) in patients with juvenile FM (n = 10) and in healthy control subjects (n = 67). Levels of FC and CE were significantly increased in juvenile FM as compared with controls, suggesting the presence of hypercholesterolemia in this disease. However, plasma level of ubiquinol-10 was significantly decreased and the ratio of ubiquinone-10 to total coenzyme Q10 (%CoQ10) was significantly increased in juvenile FM relative to healthy controls, suggesting that FM is associated with coenzyme Q10 deficiency and increased oxidative stress. Moreover, plasma level of FFA was significantly higher and the content of polyunsaturated fatty acids (PUFA) in total FFA was significantly lower in FM than in controls, suggesting increased tissue oxidative damage in juvenile FM. Interestingly, the content of monoenoic acids, such as oleic and palmitoleic acids, was significantly increased in FM relative to controls, probably to compensate for the loss of PUFA. Next, we examined the effect of ubiquinol-10 supplementation (100 mg/day for 12 weeks) in FM patients. This resulted in an increase in coenzyme Q10 levels and a decrease in %CoQ10. No changes were observed in FFA levels or their composition. However, plasma levels of FC and CE significantly decreased and the ratio of FC to CE also significantly decreased, suggesting that ubiquinol-10 supplementation improved cholesterol metabolism. Ubiquinol-10 supplementation also improved chronic fatigue scores as measured by the Chalder Fatigue Scale.
Introduction
Fibromyalgia (FM) is a chronic disorder characterized by idiopathic chronic widespread non-specific musculoskeletal pain with generalized tender points and allodynia, as well as a heightened and painful response to pressure.Citation1 The syndrome is associated with a constellation of symptoms, including debilitating fatigue, non-refreshing sleep, irritable bowel, and joint stiffness. Children with chronic musculoskeletal pain disorders, including FM, account for up to 25% of new referrals to pediatric rheumatologists in the United States.Citation2 Several factors, such as substance P, serotonin, reactive oxygen species, and genetic factors, are associated with the pathophysiology of FM,Citation3,Citation4 but the etiology of this disorder remains unclear.
Oxidative stress may play a role in the pathophysiology of FM.Citation4,Citation5 In fact, blood mononuclear cells derived from patients with FM have reduced levels of coenzyme Q10, increased formation of mitochondrial superoxide, increased levels of lipid peroxidation, and decreased mitochondrial membrane potential.Citation6,Citation7 This mitochondrial dysfunction was also associated with increased expression of autophagic genes and the elimination of dysfunctional mitochondria with mitophagy.Citation7 These findings suggest that mitochondrial dysfunction may be the origin of oxidative stress in patients with FM.
Coenzyme Q10 is a lipid-soluble substance that functions as an essential cofactor in the mitochondrial respiratory chain. Its reduced form, ubiquinol-10, is an important antioxidant, and the redox balance of coenzyme Q10 is a potential biomarker of systemic oxidative stress.Citation8 Ubiquinol-10 acts as an antioxidant in the mitochondria and in lipid membranes by either directly scavenging free radicals or in conjunction with α-tocopherol.Citation8,Citation9 Coenzyme Q10 is a popular dietary supplement among healthy individuals and those with various ailments, including neurodegenerative and cardiovascular diseases.Citation10,Citation11
The present study was conducted to characterize oxidative stress in juvenile patients with FM and to evaluate the effect of coenzyme Q10 supplementation in a double-blind, placebo-controlled trial.
Subjects
Patients were eligible for inclusion in this study if they met the American College of Rheumatology classification criteria for FM.Citation1 Oral supplementation with coenzyme Q10 or any other medications was not allowed for 3 months prior to the first administration of ubiquinol-10 in this the study. Ten children (two boys and eight girls; age, 14.7 ± 2.9 years) were enrolled. The mean age at onset of FM was 12.0 ± 2.0 years, and the mean duration of disease was 31.4 ± 29.3 months (). The Ethics Committee of Yokohama City University Hospital approved all study protocols (Approval No. B090702013). The parent or legal guardian of each child provided written informed consent, and child assent was obtained when appropriate.
Table 1. Patient characteristics
As a healthy control, blood samples from 67 schoolchildren in Shinagawa, Tokyo were obtained (37 boys and 30 girls; age, 11.5 ± 2.0 years). All samples were included since all schoolchildren were diagnosed to be healthy.
Methods
Study design
This study consisted of three sequential double-blind phases: (1) treatment with ubiquinol-10 for 12 weeks, (2) treatment with placebo for 8 weeks, and (3) treatment with ubiquinol-10 for 8 weeks. All patients received either daily oral supplementation of ubiquinol-10 or a placebo (Softgel capsules, 100 mg/day; Kaneka, Osaka, Japan). Plasma levels of ubiquinol-10 (reduced form of coenzyme Q10), ubiquinone-10 (oxidized form of coenzyme Q10), vitamin E (VE), free cholesterol (FC), cholesterol esters (CE), and the percent contents of palmitoleic acid (16:1), oleic acid (18:1), and polyunsaturated fatty acids (PUFA) in total free fatty acids (FFA) (%16:1, %18:1, and %PUFA, respectively) were measured at 0, 2, 4, 8, 12, 16, 20, 24, and 28 weeks after initiation of the study. All data (week 0) were compared with those in age-matched healthy individuals.
Changes in the clinical manifestations of FM were evaluated by assessing subjective pain intensity, quality of life (QOL), and general fatigue using the pain Visual Analog Scale (VAS),Citation12 Pediatric Quality of Life Inventory (PedsQL),Citation13 and the Chalder Fatigue Scale,Citation14,Citation15 respectively. All assessments were completed in the presence of clinical research coordinators. The Chalder Fatigue Scale consists of 14 items that assess symptoms of physical and mental fatigue, such as tiredness, sleepiness, lack of energy, lack of muscle strength, and difficulties with concentration and memory. Each item asks participants to rate the frequency of a symptom by choosing from among ‘less than usual’, ‘no more than usual’, ‘more than usual’, and ‘much more than usual’. Scores ranging from 0 to 3 were given using the Likert scoring system. The total score of the scale was calculated by adding the rating for each item, resulting in a score that ranged from 0 to 42.
Analytical procedures
Plasma levels of VE, ubiquinol-10, ubiquinone-10, FC, and CE were determined as describedCitation16 with modifications. In brief, plasma extracted with a 19-fold volume of 2-propanol was analyzed by HPLC using an analytical column (Type Supelcosil LC-8, 5 µm, 25 cm × 4.6 mm i.d.; Supelco Japan, Tokyo, Japan), a reduction column (Type RC-10-1; Irica, Kyoto, Japan), and an amperometric electrochemical detector (Model Σ985; Irica) with an oxidation potential of +600 mV (vs. Ag/AgCl) on a glassy carbon electrode. The mobile phase consisted of 50 mM sodium perchlorate in methanol/2-propanol (9/1, v/v) delivered at a flow rate of 0.8 ml/minute.
Plasma FFA were derivatized with monodansylcadaverine and then analyzed by HPLC.Citation17 Briefly, plasma samples (50 µl) were mixed with 200 µl of methanol containing 12.5 µM margaric acid (internal standard) and then separated by centrifugation at 13 000 × g for 3 minutes. Samples (50 µl) of supernatants were dried under a stream of nitrogen gas, and then each residue was mixed with diethyl phosphorocyanidate (1 µl) and N,N-dimethylformamide (50 µl) containing monodansylcadaverine (2 mg/ml) and then placed at room temperature in the dark for 20 minutes. A 5-μl sample was injected onto an octadecylsilyl column (3 µm, 3.3 cm × 4.6 mm i.d.; Supelco Japan) and a pKb-100 column (5 µm, 25 cm × 4.6 mm i.d.; Supelco Japan) connected in series. The FFA components were measured by fluorescence detection (Model 821-FP; Japan Spectroscopic, Tokyo, Japan) with excitation at 320 nm and emission at 520 nm. The mobile phase consisted of acetonitrile/methanol/water (17.5/65.0/17.5, v/v/v) delivered at a flow rate of 1.5 ml/minute with the analytical columns maintained at 40°C.
Statistical analysis
Data were statistically analyzed by Student's t-test. To assess the time course efficacy of ubiquinol-10 supplementation, post-treatment data were assessed using a repeated-measure analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results and discussion
Hypercholesterolemia and coenzyme Q10 deficiency in juvenile FM
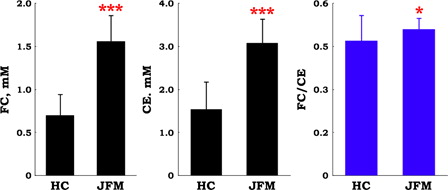
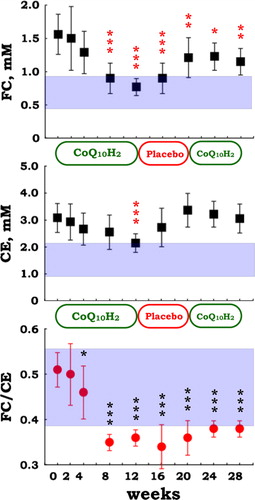
shows baseline plasma levels of FC and CE in juvenile FM before the ubiquinol-10 supplementation. Levels of FC and CE were two-fold greater in patients with juvenile FM than in age-matched healthy controls, suggesting that juvenile FM is associated with hypercholesterolemia. In adult patients with FM, one previous study showed the slight increase in total cholesterol as compared with healthy control subjectsCitation18 while another study showed no change in cholesterol when comparing with controls.Citation19 The ratio of FC to CE is a good indicator of lecithin-cholesterol acyltransferase (LCAT) activity in plasma. There was a small but a significant difference in the FC/CE ratio when comparing juvenile FM patients and healthy control subjects (). This will be discussed later.
Figure 1. Plasma levels of free cholesterol (FC) and cholesterol esters (CE), and the ratio of FC to CE in patients with juvenile fibromyalgia (JFM, n = 10) and in healthy control (HC, n = 67) subjects. Data are means ± SD, *P < 0.05, ***P < 0.001 vs. HC.
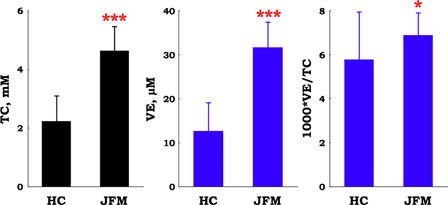
Total cholesterol (FC + CE) level in patients with juvenile FM was two-fold greater than that in healthy control subjects (). Levels of the lipid-soluble antioxidant, VE, were also two-fold higher in patients with juvenile FM than in healthy control subjects (). Therefore, the ratio of VE to TC was nearly identical in the two groups ().
Figure 2. Plasma levels of total cholesterol (TC) and vitamin E (VE), and the ratio of VE to TC in patients with juvenile fibromyalgia (JFM, n = 10) and healthy control (HC, n = 67) subjects. Data are means ± SD, *P < 0.05, ***P < 0.001 vs. HC.
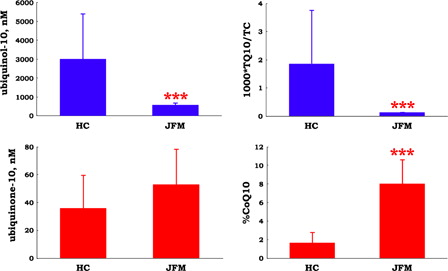
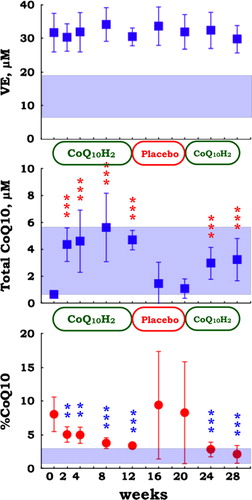
On the other hand, plasma level of ubiquinol-10, another lipid-soluble antioxidant, was significantly lower in patients with juvenile FM when compared with healthy control subjects (). Level of the oxidized form of coenzyme Q10, ubiquinone-10, was similar in the two groups (). The ratio of total coenzyme Q10 (ubiquinol-10 + ubiquinone-10) to total cholesterol was 13-fold higher in healthy control subjects than in juvenile FM patients (). This finding is consistent with coenzyme Q10 deficiency in patients with juvenile FM.
Figure 3. Plasma levels of ubiquinol-10 and ubiquinone-10, and the ratio of total coenzyme Q10 (TQ10) to total cholesterol (TC) and ubiquinone-10 to TQ10 (%CoQ10) in patients with juvenile fibromyalgia (JFM, n = 10) and healthy control (HC, n = 67) subjects. Data are means ± SD, ***P < 0.001 vs. HC.
Oxidative stress in juvenile FM
Oxidative stress is defined as a disturbance in the prooxidant–antioxidant balance in favor of the former. When human plasma was incubated under aerobic conditions at 37°C, ubiquinol-10 levels decreased after the depletion of ascorbate, α-tocopherol level remained stable, and concomitant formation of ubiquinone-10 was observed.Citation7 Ubiquinol-10 is very reactive to oxygen radicals. Therefore, the ratio of ubiquinone-10 to total coenzyme Q10 (%CoQ10) is a useful biomarker of systemic oxidative stress.Citation7 The baseline value of %CoQ10 in juvenile FM patients was significantly higher than that in healthy control subjects (), suggesting that an increased formation of reactive oxygen species in circulating blood in patients with juvenile FM (i.e. increased oxidative stress state).
As discussed above, Cordero et al. reported that FM was associated with coenzyme Q10 deficiency, increased mitochondrial superoxide formation, and increased level of lipid peroxidation as measured by thiobarbituric acid reactive substances formation in blood mononuclear cells.Citation6 On the other hand, another report concluded that there was no increase in oxidative stress in FM, as there was no change in urinary levels of F2 isoprostane (a free radical oxidation product of arachidonic acid (20:4) and one of the most reliable oxidative stress markers) when comparing patients with FM and controls.Citation20 These contradictory results prompted us to further investigate the role of oxidative stress in juvenile FM.
Tissue oxidative damage can be measured by studying plasma FFA and its composition. When tissues are under oxidative stress, plasma FFA are increased; this is because the activity of phospholipases A2 and A1 increases under oxidative stressCitation21–Citation23 and because the FFA produced by this process may enter the bloodstream through leakage or lysis of oxidatively damaged brain cells. If this were indeed the case, we would expect a lower concentration of PUFA, such as linoleic acid (18:2), linolenic acid (18:3), 20:4, and docosahexaenoic acid (22:6) in the plasma, since those substances are highly susceptible to oxidation. A previous study reported that plasma concentrations of PUFA decreased while those of monoenoic acids, such as oleic acid (18:1) and palmitoleic acid (16:1), increased to compensate for the oxidative loss of PUFA under various conditions of oxidative stress.Citation24 Such changes were observed in patients with adult respiratory distress syndrome,Citation25 multiple sclerosis,Citation26 Papillon–Lefevre syndrome,Citation27 and in newborn babies.Citation28 We recently observed that an elevation of plasma FFA levels and the contents of 16:1 and 18:1 in total FFA (%16:1 and %18:1, respectively) was induced by 2-hour occlusion–reperfusion of the middle cerebral artery in rats and that this change was attenuated by the administration of a free radical scavenger drug, edaravone.Citation29
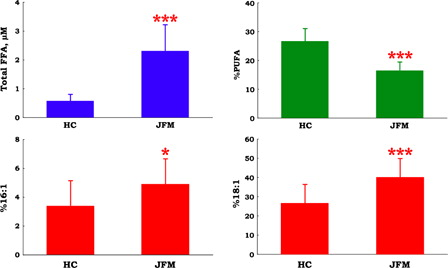
shows that plasma FFA level was four-fold higher in patients with juvenile FM when compared with healthy control subjects, while %PUFA was significantly lower in patients with juvenile FM when compared with healthy control subjects. Moreover, %16:1 and %18:1 were significantly higher in juvenile FM patients than in healthy control subjects (). These data indicate the presence of oxidative tissue damage in patients with juvenile FM. These results may correlate with the presence of lipid droplets and abnormal mitochondria in muscleCitation30 and an increase in the levels of the inflammatory cytokine, interleukin-8, in the serum and cerebrospinal fluidCitation31 from patients with FM.
Figure 4. Plasma level of total free fatty acids (FFA), the ratios of polyunsaturated fatty acids (PUFA) to total FFA (%PUFA), oleic acid to total FFA ratio (%18:1), and palmitoleic acid to total FFA ratio (%16:1) in patients with juvenile fibromyalgia (JFM, n = 10) and healthy control (HC, n = 67) subjects. Data are means ± SD, *P < 0.05, ***P < 0.001 vs. HC.
Effect of ubiquinol-10 supplementation
The fact that patients with juvenile FM are coenzyme Q10-deficient led us to conduct a study of ubiquinol-10 supplementation in juvenile FM patients. Although plasma levels of VE remained unchanged, total levels of coenzyme Q10 significantly increased after ubiquinol-10 supplementation. Then, after switching to placebo, total levels of coenzyme Q10 returned to baseline. Subsequent reinitiation of ubiquinol-10 supplementation resulted in an increase in total levels of coenzyme Q10 (). Similarly, values of %CoQ10 significantly decreased after ubiquinol-10 supplementation, returned to baseline during the placebo period, and then decreased again with the second ubiquinol-10 supplementation period (). This suggests that oxidative stress in patients with juvenile FM can be ameliorated by ubiquinol-10 supplementation.
Figure 5. Plasma level of vitamin E (VE), total coenzyme Q10 (ubiquinol-10 + ubiquinone-10), and the ratio of ubiquinone-10 to total coenzyme Q10 (%CoQ10) in patients with juvenile FM (n = 10) during supplementation with reduced coenzyme Q10 (100 mg/day for 12 weeks, 0 mg/day for 8 weeks, and 100 mg/day for 8 weeks). Data are means ± SD, **P < 0.01, ***P < 0.001 vs. baseline value at week 0. Mesh shows the means ± SD values in healthy control (n = 67) subjects.
While ubiquinol-10 supplementation did not change the level of tissue oxidative damage, as indicated by the lack of change in FFA level, %16:1, and %18:1 (), hypercholesterolemia was attenuated (). In particular, the FC/CE ratio was significantly reduced after ubiquinol-10 supplementation (), suggesting that plasma LCAT activity was enhanced. LCAT catalyzes the transesterification of fatty acid at the 2 position of phosphatidylcholine (PC) to FC to yield CE and lyso-PC. LCAT is secreted from the liver, and its activity is often reduced in patients with liver disease, such as hepatitis, cirrhosis, and hepatoma.Citation32,Citation33 It is noteworthy that ubiquinol-10 supplementation improved the FC/CE ratio in patients with juvenile FM, which suggests that ubiquinol-10 supplementation may help improve liver function.
Figure 6. Plasma levels of total free fatty acids (FFA), and the ratios of palmitoleic acid and oleic acid to total FFA (%16:1 and %18:1, respectively) in patients with juvenile FM (n = 10) during supplementation with reduced coenzyme Q10 (100 mg/day for 12 weeks, 0 mg/day for 8 weeks, and 100 mg/day for 8 weeks). Data are means ± SD. Mesh shows the mean ± SD values in healthy control (n = 67) subjects.
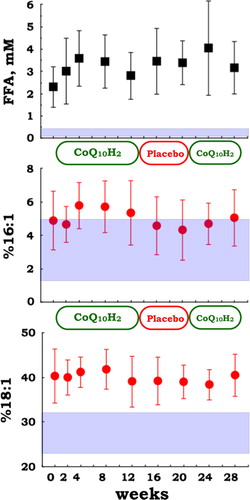
Figure 7. Plasma levels of free cholesterol (FC) and cholesterol esters (CE), and the ratio of FC to CE in patients with juvenile FM (n = 10) during supplementation with reduced coenzyme Q10 (100 mg/day for 12 weeks, 0 mg/day for 8 weeks, and 100 mg/day for 8 weeks). Data are means ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 vs. baseline value at week 0. Mesh shows the mean ± SD values in healthy control (n = 67) subjects.
Effect of ubiquinol-10 supplementation on QOL
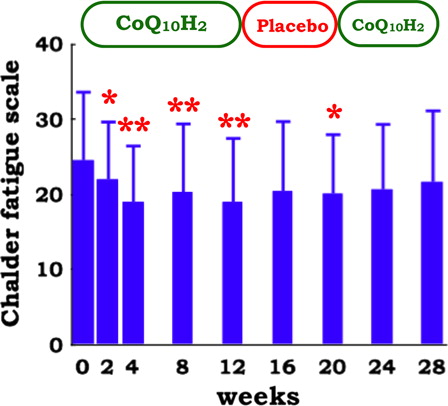
The effect of ubiquinol-10 supplementation on QOL was assessed. Both subjective pain intensity (assessed using the 100-mm VAS) and health-related QOL (evaluated by self- and proxy-rating) did not change throughout the three study phases (data not shown). On the other hand, symptoms of chronic fatigue (as measured by the Chalder Fatigue Scale) significantly decreased in response to ubiquinol-10 supplementation (). This was also confirmed by repeated-measure ANOVA (P = 0.041).
Figure 8. Chalder Fatigue Scale in patients with juvenile FM (n = 10) during supplementation with reduced coenzyme Q10 (100 mg/day for 12 weeks, 0 mg/day for 8 weeks, and 100 mg/day for 8 weeks). Data are means ± SD, *P < 0.05, **P < 0.01 vs. baseline value at week 0. Repeated-measure ANOVA indicates a significant time course effect of 12 weeks supplementation of reduced coenzyme Q10 (P = 0.041).
It is noteworthy that in five adult patients with FM, ubiquinone-10 supplementation (300 mg/day for 9 months) results in clinical improvement such as tender points, VAS, FM impact questionnaire score, and headache impact test score.Citation34 This results were confirmed by a further study consisting of 20 adult patients with FM who took 300 mg ubiquinone-10/day for 3 months.Citation35 The reason as to why the effect of ubiquinol-10 supplementation was limited in this juvenile FM study is not clear. Age difference may be critical but dose difference should be examined. We, therefore, planned to investigate the effect of higher doses of ubiquinol-10 in future studies.
In conclusion, we found that patients with juvenile FM are hypercholesterolemic and coenzyme Q10-deficient. Increased oxidative stress in the patients was suggested by a significant increase in %CoQ10, FFA level, %16:1, and %18:1, and a significant decrease in %PUFA when compared with healthy control subjects. Pain and tissue oxidative damage, indicated by FFA, %18:1, and %16:1, did not change, but general fatigue and hypercholesterolemia was attenuated in response to ubiquinol-10 supplementation.
Acknowledgements
We thank Kaneka Corporation for supplying capsules with and without ubiquinol-10.
References
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL. The American College of Rheumatology 1990 Criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–72.
- Anthony KK, Schanberg LE. Juvenile primary fibromyalgia syndrome. Curr Rheumatol Res 2001;3:165–71.
- Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clin Proc 2011;86:907–11.
- Ozgocmen S, Ozyurt H, Sogut S, Akyol O. Current concepts in the pathophysiology of fibromyalgia: the potential role of oxidative stress and nitric oxide. Rheumatol Int 2006;26:585–97.
- Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol 2007;83:84–92.
- Cordero MD, Moreno-Fernandez AM, deMiguel M, Bonal P, Campa F, Jimenez-Jimenez LM, et al. Coenzyme Q10 distribution in blood is altered in patients with fibromyalgia. Clin Biochem 2009;42:732–5.
- Cordero MD, De Miguel M, Moreno Fernandez AM, Carmona Lopez IM, Garrido Maraver J, Cotan D, et al. Mitochondrial dysfunction and mitophagy activation in blood mononuclear cells of fibromyalgia patients: Implication in the pathogenesis of the disease. Arthritis Res Ther 2010;12:R17.
- Yamamoto Y. Coenzyme Q10 as a front-line antioxidant against oxidative stress. J Clin Biochem Nutr 2005;36:29–35.
- Lass A, Sohal RS. Effect of coenzyme Q10 and α-tocopherol content of mitochondria on the production of superoxide anion radicals. FASEB J 2000;14:87–94.
- Jones K, Hughes K, Mischley L, McKenna DJ. Coenzyme Q10: efficacy, safety, and use. Alternat Ther 2002;8:42–55.
- Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat. 2009;5:597–610.
- Marques AP, Assumpção A, Matsutani LA, Pereira CA, Lage L. Pain in fibromyalgia and discrimination power of the instruments: Visual Analog Scale, Dolorimetry and the McGill Pain Questionnaire. Acta Rheumatol Port 2008;33:345–51.
- Kobayashi K, Kamibeppu K. Measuring quality of life in Japanese children: development of the Japanese version of PedsQL. Pediatr Int 2010;52:80–8.
- Tanaka M, Fukuda S, Mizuno K, Imai-Matsumura K, Jodoi T, Kawatani J, et al. Reliability and validity of the Japanese version of the Chalder Fatigue Scale among youth in Japan. Psychol Rep 2008;103:682–90.
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D. Development of a fatigue scale. J Psychosom Res 1993;37:147–53.
- Yamamoto Y, Ames BN. Detection of lipid hydroperoxides and hydrogen peroxide at picomole levels by an HPLC and isoluminol chemiluminescence assay. Free Radic Biol Med 1987;3:359–61.
- Yamamoto Y, Nagata Y, Katsurada M, Sato S, Ohori Y. Changes in rat plasma free fatty acids composition under oxidative stress induced by carbon tetrachloride: Decrease of polyunsaturated fatty acids and increase of palmitoleic acid. Redox Report 1996;2:121–5.
- Gurer G, Sendur OF, Ay C. Serum lipid profile in fibromyalgia women. Clin Rheumatol. 2006;25:300–3.
- Ozgocmen S, Ardicoglu O. Lipid profile in patients with fibromyalgia and myofascial pain syndromes. Yonsei Med J 2000;41:541–5.
- Chung CP, Titova D, Oeser A, Randels M, Avalos I, Milne GL, et al. Oxidative stress in fibromyalgia and its relationship to symptoms. Clin Rheumatol 2009;28:435–8.
- Yasuda M, Fujita T. Effect of lipid peroxidation on phospholipase A2 activity of rat liver mitochondria. Jpn J Pharmacol 1977;27:429–35.
- Weglicki WB, Dickens BF, Mak IT. Enhanced lysosomal phospholipid degradation and lysophospholipid production due to free radicals. Biochem Biophys Res Commun 1984;124:229–35.
- Beckman JK, Borowitz SM, Burr IM. The role of phospholipase A activity in rat liver microsomal lipid peroxidation. J Biol Chem 1987;262:1479–81.
- Gutteridge JMC, Quinlan GJ, Yamamoto Y. Are fatty acid patterns characteristic of essential fatty acid deficiency indicative of oxidative stress? Free Radic Res 1998;28:109–14.
- Quinlan GJ, Lamb NJ, Evans TW, Gutteridge JMC. Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Critical Care Med 1996;24:241–6.
- Love WC, Cashell A, Reynolds M, Callaghan N. Linoleate and fatty-acid patterns of serum lipids in multiple sclerosis and other diseases. Br Med J 1974;6:18–21.
- Battino M, Ferreiro MS, Quiles JL, Bompadre S, Leone L, Bullon P. Alterations in the oxidation products, antioxidant markers, antioxidant capacity and lipid patterns in plasma of patients affected by Papillon-Lefevre syndrome. Free Radic Res 2003;37:603–9.
- Hara K, Yamashita S, Fujisawa A, Ogawa T, Yamamoto Y. Oxidative stress in newborn infants with and without asphyxia as measured by plasma antioxidants and free fatty acids. Biochem Biophys Res Commun 1999;257:244–8.
- Yamamoto Y, Yanagisawa M, Tak NW, Watanabe K, Takahashi C, Fujisawa A, et al. Repeated edaravone treatment reduces oxidative cell damage in rat brain induced by middle cerebral artery occlusion. Redox Report 2009;14:251–8.
- Pongratz DE, Späth M. Morphologic aspects of fibromyalgia. Z Rheumatol 1998;57:47–51.
- Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E. Evidence of central inflammation in fibromyalgia – increased cerebrospinal fluid interleukin-8 levels. J Neuroimmunol 2012;242:33–8.
- Florén CH, Chen CH, Franzén J, Albers JJ. Lecithin: cholesterol acyltransferase in liver disease. Scand J Clin Lab Invest 1987;47:613–7.
- Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, Yoshikawa T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem Biophys Res Commun 1998;247:166–70.
- Cordero MD, Alcocer-Gómez E, de Miguel M, Cano-García FJ, Luque CM, Fernández-Riejo P, et al. Coenzyme Q(10): a novel therapeutic approach for Fibromyalgia? Case series with 5 patients. Mitochondrion 2011;11:623–5.
- Cordero MD, Cano-García FJ, Alcocer-Gómez E, De Miguel M, Sánchez-Alcázar JA. Oxidative stress correlates with headache symptoms in fibromyalgia: coenzyme Q10 effect on clinical improvement. PLoS One 2012;7:e35677.