Abstract
Reactive oxygen and nitrogen species (ROS–RNS) and other redox active molecules fulfill key functions in immunity. Beside the initiation of cytocidal reactions within the pathogen defense strategy, redox reactions trigger and shape the immune response and are further involved in termination and initialization of cellular restorative processes. Regulatory mechanisms provided by redox-activated signaling events guarantee the correct spatial and temporal proceeding of immunological processes, and continued imbalances in redox homeostasis lead to crucial failures of control mechanisms, thus promoting the development of pathological conditions. Interferon-gamma is the most potent inducer of ROS–RNS formation in target cells like macrophages. Immune-regulatory pathways such as tryptophan breakdown via indoleamine 2,3-dioxygenase and neopterin production by GTP-cyclohydrolase-I are initiated during T helper cell type 1 (Th1-type) immune response concomitant to the production of ROS–RNS by immunocompetent cells. Therefore, increased neopterin production and tryptophan breakdown is representative of an activated cellular immune system and can be used for the in vivo and in vitro monitoring of oxidative stress. In parallel, the activation of the redox-sensitive transcription factor nuclear factor-kappa B is a central element in immunity leading to cell type and stimulus-specific expression of responsive genes. Furthermore, T cell activation and proliferation are strongly dependent on the redox potential of the extracellular microenvironment. T cell commitment to Th1, Th2, regulatory T cell, and other phenotypes appears to crucially depend on the activation of redox-sensitive signaling cascades, where oxidative conditions support Th1 development while ‘antioxidative’ stress leads to a shift to allergic Th2-type immune responses.
Introduction
The maintenance of redox homeostasis is crucial for appropriate functioning of cellular processes and cell survival. Imbalances between production and removal of reactive oxygen and nitrogen species (ROS–RNS) are critically involved in pathophysiological reactions. The importance of high levels of ROS–RNS produced by the respiratory burst during pathogen defense is well established.Citation1 However, to avoid collateral damage, increased production of reactive molecules has to be regulated spatially as well as temporally otherwise the oxidation of biomolecules leads to toxicity towards self-cellular structures and might finally result in genotoxic damage or cell death. Altered redox balances are often associated with diseases that are characterized by chronic immune activation such as infections, allergies, autoimmune disorders, neurodegenerative diseases, and malignancies.Citation2,Citation3
At lower levels, ROS–RNS and other small redox molecules control many different physiological processes in a dose-dependent manner, such as induction of stress responses, detoxification, immune activation, and systemic signaling, by acting as inducers of redox-sensitive pathways.Citation4 Thus, the redox state is an intrinsic indicator for cellular and systemic homeostasis.
Reactive species and redox active molecules in immune response
Small reactive molecules interfere with immunoregulatory signaling cascades by modifying redox-sensing molecules, enzyme, and transcription factor activities, and/or by depleting the endogenous antioxidant pool. The main reactive species are ROS, such as hydrogen peroxide (H2O2), superoxide anion (O2•−), but also RNS such as peroxynitrite (ONOO−), nitrogen dioxide (NO2•), and nitrogen trioxide, which result from the reaction of nitric oxide (NO•) with ROS.Citation5 More recently, other toxic gases, such as CO and H2S, have been identified as important small molecule immunomodulators.Citation5,Citation6 For an immediate cellular protection against these reactive species, antioxidant molecules are provided as redox buffers in different cellular compartments, among them extensively studied are thiol/disulfide couples glutathione/glutathione disulfide (GSH/GSSG), cysteine/cystine (Cys/CySS), and thioredoxins (Trx).Citation7 Besides acting as monitoring systems for the redox status, these buffer molecules regulate a variety of responsive enzymes and pathways.Citation8
Cellular ROS and RNS production
Under normal conditions, mitochondria are the main site of intracellular oxygen consumption and the main source of ROS (O2•–) formation.Citation9 Other endogenous sources of ROS include the membrane-bound NADPH-dependent oxidases (NOX), lipoxygenase, cytochrome P-450, and xanthine oxidase.Citation10–Citation12 While in general ROS production occurs as a byproduct of biological reactions, the sole function of the NOX enzyme family is the production of ROS by reducing molecular oxygen to O2•–, and phagocyte NADPH oxidase was the first NOX identified to be responsible for the high amounts of O2•– and H2O2 produced during the respiratory burst reaction.Citation13,Citation14 In the resting cell, the NOX components are separated at the plasma membrane and the cytosol. Upon stimulation, membrane-bound flavocytochrome B, the catalytic core of the respiratory-burst oxidase, assembles with proteins of cytosolic origin (the small G protein Rac, p47phox, and p67phox) that activate the production of O2•−.Citation15
NO• is synthesized during the conversion of l-arginine to l-citrulline by nitric oxide synthase (NOS) and is classically viewed as a regulator of the vasomotor tone. NO• activates several signaling pathways in a dose-dependent manner as it is able to migrate though cell membranes by diffusion.Citation5,Citation16 Although of low reactivity, NO• is a potent antioxidant molecule that can protect from ROS injury.Citation17,Citation18 Low amounts of NO• (nM) are normally produced by the constitutively expressed endothelial and neuronal NOS and are involved in the regulation of physiological processes such as vasodilation and neurotransmission.Citation19 Upon activation of inducible NOS (iNOS) due to inflammatory stimuli such as cytokines, e.g. interferon-gamma (IFNγ) and tumor necrosis factor-alpha, or lipopolysaccharide, NO• levels in the cellular microenvironment can rise to micromolar concentrations.Citation20 These high amounts of NO• may have cytostatic effects on parasitic target cells but also contribute to the pathophysiology of inflammatory diseases and septic shock.Citation21 At sites of inflammation and phagocyte activation where enhanced rates of NO• and O2•– production occur, NO• and O2•– can combine to form ONOO−.Citation22,Citation23
ROS–RNS production for bacterial killing during the respiratory burst is concentrated in the phagosome to minimize collateral damage. The regulation of the phagosomal pH and O2•– production follows distinct schemes in neutrophils, macrophages, and dendritic cells (DC) due to differences in the localization of NOX and the phagosomal NO• concentrations.Citation24 The presence of O2•– and NO• in the phagosome leads to the generation of nitrite and thus to the formation of NO2• and nitrogen trioxide. Also, peroxidase activity may lead to the consumption of NO•, leading to limited NO• bioavailability and the formation of nitrite, which is further oxidized to NO2•.Citation5,Citation23
The diversity of chemical species generated from NO• and ROS–RNS provides a refined pathogen defense strategy that goes far beyond the simple killing of microbes.Citation24,Citation25 The diversity of NOS activities can produce different temporal and concentration profiles of NO• and, depending on the concentration of ROS, redox profiles may change. The ROS–RNS balance is crucial for the regulation of immunological mechanisms and both oxidative and nitrosative stress can interfere with intracellular redox buffer systems, resulting in a temporally decreased antioxidant capability of the affected cells.Citation5
Endogenous antioxidant molecules
Antioxidant molecules provide the cell with redox buffer capacities and have been identified to play an important role in regulating immune responses. Trx, GSH/GSSG, and Cys/CYSS are largely independently regulated in different subcellular compartments.Citation7 Quantitatively, GSH is the major intracellular redox buffer in mammalian cells and GSH/GSSG couples can be found in cytoplasm, nucleus, mitochondria, and other organelles although with different redox potential.Citation7 Upon secretion, the majority of GSH is cleaved into its components and the resulting cysteines fill the extracellular Cys/CySS pool, representing the major thiol/disulfide redox buffer outside the cell.Citation26 Mammalian Trx are selenoproteins that additionally have cytokine-like properties when secreted. In the extracellular space, Trx can act as a chemotactic factor for monocytes, polymorphonuclear leukocytes, and T cells.Citation27
Several metabolic routes link the extracellular redox potential to the intracellular buffer system thus paralleling changes that occur in the cellular environment.Citation26 Although the thiol/disulfide buffers share similar functional properties and largely compensate for each other, each system has some unique functions and may support the reduction of some different subsets of proteins.Citation7,Citation27 Beside counteracting the influence of ROS, these redox buffers represent important elements of a variety of signal transduction networks, e.g. in the activation of T cells and their differentiation into effector T cell subsets.Citation28
Key signaling cascades during the Th1 immune response
In a cell-mediated immune response, various T cell subsets are involved, e.g. antigen-specific activated Th1-type cells secrete cytokines and activate T effector cells such as cytotoxic T cells and nonspecific effector cells such as natural killer cells and macrophages. The pro-inflammatory cytokine IFNγ plays a central role in the Th1-type immune response, as it induces a variety of cellular responses.Citation29 IFNγ signaling initiates pathogen and tumor defense mechanisms in target cells and triggers NOX-mediated ROS formation during the respiratory burst reaction.Citation30 Further, several immunoregulatory enzymes such as guanosine triphosphate-cyclohydrolase-I (GTP-CH-I), indoleamine 2,3-dioxygenase (IDO), and iNOS are activated during the immune response (). Pro-inflammatory cytokines, most importantly again IFNγ, as well as lipopolysaccharide-induced Toll-like receptor 4 signaling activate GTP-CH-I and IDO.Citation31–Citation33
Figure 1. During an immune response, the neopterin-producing enzyme GTP-cyclohydrolase-I (GTP-CH-I), the nitric oxide (NO•)-forming nitric oxide synthase (iNOS), and tryptophan-converting enzyme indoleamine 2,3-dioxygenase (IDO) are stimulated. Macrophages (Mφ) and dendritic cells (DC) produce neopterin. Other cells like endothelial cells (EC) and fibroblasts (FB) form tetrahydrobiopterin (BH4), the cofactor of iNOS, thus contributing to the release of NO•. As NO• inhibits the expression and function of IDO, tryptophan levels accumulate.
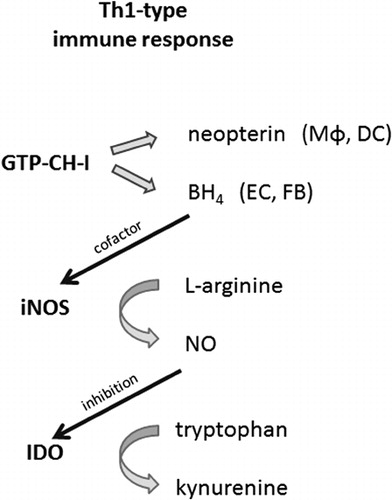
The activation of GTP-CH-I leads to the production of neopterin and 5,6,7,8-tetrahydrobiopterin (BH4), an essential cofactor for several monooxygenases, including iNOS.Citation33 While human fibroblasts or endothelial cells preferentially produce BH4, human and primate monocytes/macrophages are the most relevant source of neopterin and its reduced form 7,8-dihydroneopterin, which are both present at a relatively constant ratio in human serum.Citation3 Both neopterin and 7,8-dihydroneopterin interfere with oxidative signaling pathways, e.g. they are involved in the activation of the redox-sensitive transcription factor nuclear factor kappa B (NF-κB).Citation34 Reduced pteridine derivatives like 7,8-dihydroneopterin are potent antioxidants, while neopterin was found to support the generation of chloride metabolites and to enhance their toxic effects during the respiratory burst reaction in human neutrophils.Citation35,Citation36 Thus, neopterin and 7,8-dihydroneopterin may influence cellular and systemic redox balances.Citation37 High levels of neopterin were found to be associated with increased production of ROS and with low serum concentrations of antioxidants.Citation3,Citation38 Aside from its possible pathophysiologic relevance, neopterin has gained importance in clinical diagnostics as a way to monitor Th1-type responses, which are associated with a variety of disorders.Citation39,Citation40
IDO catalyzes the rate-limiting step in the oxidative degradation of the essential amino acid tryptophan (Trp). The depletion of the essential amino acid Trp produces an antiproliferative environment that counteracts the growth of pathogens or malignant cells and thus represents a primary mechanism of cytotoxicity.Citation41 The ratio of kynurenine to Trp (Kyn/Trp) can be used as an estimate of IDO activity.Citation42 Kyn/Trp has been determined in a variety of patient cohorts and, like increased neopterin, elevated Kyn/Trp has been revealed to be a sensitive indicator for an activated immune system.Citation43
Beside monocytes/macrophages, a variety of other cell types such as endothelial cells, epithelial cells, and fibroblasts exhibit IDO activity. IDO is a haem-containing enzyme that exerts antioxidant activity by scavenging of O2•–, which can be utilized as a substrate for the oxidative metabolism of Trp but also as a cofactor. Reductants such as O2•– bring (Fe3+)-IDO to its active ferrous form.Citation44 NO• and H2O2 inhibit IDO enzyme function and interfere with various steps of Trp catabolism.Citation44
In addition to its importance in protein biosynthesis, Trp is a precursor for the generation of the neurotransmitter 5-hydroxytryptamine (serotonin) by the BH4-dependent Trp 5-hydroxylase, thus providing a rational link to inflammation-induced depression. This could be due to the serotonin deficiency caused by the limited supply of Trp and the oxidation of BH4 in a pro-oxidative environment, and due to the accumulation of neurotoxic Kyn metabolites.Citation45,Citation46
iNOS expression is upregulated in macrophages upon stimulation, but can also be found in a number of cell types including epithelial cells, astrocytes, and endothelial cells.Citation5,Citation21 iNOS activity does not depend on Ca2+/calmodulin and produces high rates of NO• in the presence of l-arginine and NADPH.
The amount of NO• production differs in distinct mammalian species, e.g. NO• is more prominently produced in rodents than in humans and primates. One explanation might be that, in human macrophages, activation of GTP-CH-I during a Th1-type immune response leads to the production of neopterin at the expense of BH4, a cofactor required for normal NOS function.Citation33 This peculiarity of BH4 and neopterin biochemistry might serve as an explanation for why in humans the inflammation is often associated with high blood pressure due to vasoconstriction, rather than vasodilation as is for example the case in coronary artery disease. Interspecies variability in NO• production represents one of the limitations that can be faced when experimental data are extrapolated from murine data to the human situation.
NOS enzymes can produce O2•– in addition to or instead or NO• and this balance is regulated by ROS–RNS in a dose-dependent manner. Oxidant stress decreases NO• generation, this effect being largely mediated via BH4 depletion.Citation22 While ROS induce a reversible inhibition of iNOS, ONOO− promotes an irreversible enzyme inhibition. In the absence of l-arginine or because of BH4 depletion, production of NO• from iNOS becomes uncoupled from the oxidation of NADPH, thus resulting in prominent O2•– generation.Citation47 iNOS uncoupling is further associated with NF-κB activation.
Both oxidative and nitrosative stress are associated with the expression of pro-inflammatory genes and cytokines such as tumor necrosis factor-alpha, interleukin-6 (IL-6), and IL-1beta.Citation48,Citation49 In this context, the most prominent regulatory molecule is the redox-sensitive transcription factor NF-κB, which regulates a variety of genes that control the immune response.Citation50 There are many inducers of NF-κB, including cytokines or reactive small molecules, and, depending on the cell type, the route of induction, the presence of synergistically acting signals, and the cross-talk with other pathways, the expression of selected gene sets is induced.Citation51 Gene induction follows a temporal order, early genes are involved in neutrophil recruitment to the site of inflammation, then genes that are responsible for macrophage and lymphocyte recruitment are expressed to provide a second wave of defenders, and late gene products are involved in restorative processes such as wound healing, thus resolution of inflammation is also a critical aspect of NF-κB control.Citation51,Citation52 NF-κB activity is regulated by ROS–RNS dose-dependently, thereby reactive species might not only interfere directly with the transcription factor or its inhibitor complex, but may react also with the redox-sensitive upstream phosphatases and kinases that are involved in NF-κB regulation.Citation49 Also, the modulation of the cytosolic and nuclear redox status might indirectly interfere with redox-sensitive transcription events.
Redox regulation of T cell activation and differentiation
The generation of both humoral and cell-mediated responses depends on T helper activation and this process is strongly dependent on the redox potential of the microenvironment.Citation26 Lymphocytes require a reducing milieu for optimal activation, e.g. immunization is associated with a striking increase in free thiols in lymphoid tissue.Citation53,Citation54
The priming of naive T cells involves the specific engagement of the T cell receptor by antigen–MHC class II complex, the interaction between co-stimulatory molecules (CD28 on T cells and CD80/86 on the antigen presenting cells (APCs)), as well as secretion of cytokines.Citation26 Further, a reducing extracellular microenvironment in the immune synapse is needed to facilitate an immune response. Naive T cells are metabolically dependent on APCs as they do not express the cystine transporter xc−, and thus require exogenous thiols for activation and function.Citation53 Upon cross-talk with T cells, APCs stimulate cysteine production and secretion via different metabolic routes and also extracellular Trx1 is augmented.Citation26,Citation53,Citation55
The reducing microenvironment might provide local protection from oxidative stress during T cell activation, but low levels of ROS are essential for the onset of the immune response.Citation26,Citation56 T cell receptor engagement results in enhanced intracellular H2O2 production that is needed for IL-2 and IL-2 receptor alpha chain gene transcription and NF-κB activation, thus expression of inflammatory genes and cytokines.Citation57 Furthermore, when a response to antigen is induced GSH synthesis is stimulated, which serves an important proliferative signal for T cells and further redox-sensitive signaling cascades that promote activation and proliferation, e.g. AP1, cell cycle proteins are activated.Citation58,Citation59
A pro-oxidant environment in the initial phase of an immune response might facilitate priming of T cells, but sustained pro-oxidant conditions would lead to the inhibition of T cell proliferation and finally induce apoptosis.Citation60 Furthermore, redox signaling appears to influence T cell commitment and various T cells have different redox statuses and thus different ROS suceptibility.Citation28
As T cell proliferation also depends on Trp availability, the metabolic control via IDO activation represents a negative feedback loop for immune activation.Citation61 IFNγ-induced IDO signaling is crucial for the generation of regulatory T cells that are involved in the suppression of autoimmune responses and promote tolerance.Citation62,Citation63 The control of Trp metabolism by IDO in mouse DC mediates protection against exaggerated responses through the combined effects of Trp starvation and Kyn metabolites acting via the aryl hydrocarbon receptor of T cells.Citation64 Also arginase secreted by neutrophils during inflammation plays a role in T cell regulation by competing for arginine and thus suppressing T cell proliferation.Citation5,Citation65
Furthermore, T cell commitment to Th1 or Th2 appears to crucially depend on the activation of redox-sensitive signaling cascades, where oxidative conditions support Th1 development while ‘antioxidative’ stress leads to a shift to allergic Th2 responses ().Citation66–Citation68
Figure 2. Redox balance is critically involved in the regulation of T cell activation, proliferation, and in shifting of the T cell phenotype. Stimulation of naive T cells (Th0) by dendritic cells (DC) is followed by thiol induction that is critical for the survival of T cells in an oxidative milieu. A Th1-type immune response is characterized by the secretion of pro-inflammatory cytokines, mainly interferon gamma (IFNγ) and interleukin-2 (IL-2), and leads to the activation of macrophages (Mφ) and cytotoxic T cells (CTL). During a Th2-type immune response, IL-4, -5, -6, and -13 are secreted and B cells are stimulated to produce antibodies. Th1 and Th2-type immune responses are cross-regulated, suppression of Th1 reactions favors Th2 responses and vice versa. Whereas oxidative conditions are involved in Th1 reactions, antioxidative stress preferentially activates Th2-type responses, which may result in ineffective pathogen defense and development of allergies. On the other hand, overwhelming production of ROS–RNS due to a chronic activation of the immune system might lead to severe damage due to the oxidation of biomolecules.
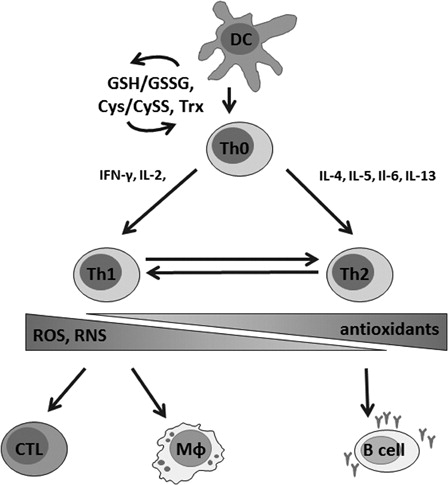
It has been shown in several in vitro studies with PBMC from healthy donors that a variety of antioxidant compounds including vitamins, phytochemicals, preservatives, and colorants were able to suppress mitogen-induced Trp degradation and neopterin production in a dose-dependent manner.Citation69 Also, DCs treated with vitamin C and E become resistant to phenotypic and functional changes following stimulation with pro-inflammatory cytokines, and allogeneic T cells were anergized following exposure to vitamin-treated DCs and secreted higher levels of Th2 cytokines and IL-10 than cells incubated with control DCs.Citation70 Due to the balance of Th1 and Th2 immunity, antioxidants may facilitate a shift towards Th2-type responses by suppression of Th1-type mechanisms.Citation71
NO• represents an additional signal for the induction of the T cell subset response.Citation5 NO• generally decreases T cell responses but low doses of NO• selectively enhance the differentiation of murine and human Th1, but not Th2, cells in vitro.Citation72 This enhancement is achieved by the direct action of NO• on T cells in synergism with IL-12 produced by APCs.Citation72,Citation73
Conclusion
ROS–RNS intermediates serve as an arsenal for pathogen defense but in lower amounts they act as signaling molecules. Small redox molecules are effective immunomodulators that regulate cellular metabolism as well as multiple inflammatory pathways and are crucially involved in restorative and immunosuppressive processes. Effects depend on the cellular context, the location, and duration of exposure. Several immune-associated redox pathways are further involved in the regulation of metabolic functions and thus control the proliferative capacities of immunocompetent cells. Redox imbalances are implicated in the development and progression of a variety of pathological conditions and this is mostly suggested to be mediated though a sustaining pro-oxidant environment. However, oxidative stress is not necessarily an unwanted situation and may also result in beneficial reactions. In this context the consumption of large amounts of ‘health-promoting’ exogenous antioxidants, which will reach relatively high concentrations in the gastrointestinal tract, should be considered with caution, since an exaggerated increase of the antioxidant potential might cause adverse effects due to ‘antioxidative stress’.
References
- Decoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci 2005;62(19–20):2173–93.
- Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem 2006;52(4):601–23.
- Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab 2002;3(2):175–87.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39(1):44–84.
- Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, et al. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol 2011;89(6):873–91.
- Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation – a tale of three gases! Pharmacol Ther 2009;123(3):386–400.
- Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med 2008;44(6):921–37.
- Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal 2005;7(7–8):964–72.
- Le Bras M, Clement MV, Pervaiz S, Brenner C. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol 2005;20(1):205–19.
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal 2008;10(8):1343–74.
- Harrison R. Physiological roles of xanthine oxidoreductase. Drug Metab Rev 2004;36(2):363–75.
- Gottlieb RA. Cytochrome P450: major player in reperfusion injury. Arch Biochem Biophys 2003;420(2):262–7.
- Altenhöfer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, et al. The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 2012;69(14):2327–43.
- Rossi F, Zatti M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. NADH and NADPH oxidation by the granules of resting and phagocytizing cells. Experientia 1964;20(1):21–3.
- Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 2002;59(9):1428–59.
- Ho JJ, Man HS, Marsden PA. Nitric oxide signaling in hypoxia. J Mol Med (Berl) 2012;90(3):217–31.
- Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 1998;25(4–5):434–56.
- Wink DA, Vodovotz Y, Grisham MB, DeGraff W, Cook JC, Pacelli R, et al. Antioxidant effects of nitric oxide. Methods Enzymol 1999;301:413–24.
- Mariotto S, Menegazzi M, Suzuki H. Biochemical aspects of nitric oxide. Curr Pharm Des 2004;10(14):1627–45.
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 2008;45(1):18–31.
- Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 2012;33(7):829–37.
- Sun J, Druhan LJ, Zweier JL. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch Biochem Biophys 2010;494(2):130–7.
- Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem 2000;275(48):37524–32.
- Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev 2007;219:143–56.
- Akaike T, Maeda H. Nitric oxide and virus infection. Immunology 2000;101(3):300–8.
- Yan Z, Garg SK, Kipnis J, Banerjee R. Extracellular redox modulation by regulatory T cells. Nat Chem Biol 2009;5(10):721–3.
- Lillig CH, Holmgren A. Thioredoxin and related molecules – from biology to health and disease. Antioxid Redox Signal 2007;9(1):25–47.
- Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 2012, DOI:10.1089/ars.2011.4073.
- Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine 2010;50(1):1–14.
- Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 1983;158(3):670–89.
- Werner ER, Bitterlich G, Fuchs D, Hausen A, Reibnegger G, Szabo G, et al. Human macrophages degrade tryptophan upon induction by interferon-gamma. Life Sci 1987;41(3):273–80.
- Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Tumour necrosis factor-alpha and lipopolysaccharide enhance interferon-induced tryptophan degradation and pteridine synthesis in human cells. Biol Chem Hoppe Seyler 1989;370(9):1063–9.
- Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Yim JJ, et al. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem 1990;265(6):3189–92.
- Hoffmann G, Schobersberger W, Frede S, Pelzer L, Fandrey J, Wachter H, et al. Neopterin activates transcription factor nuclear factor-kappa B in vascular smooth muscle cells. FEBS Lett 1996;391(1–2):181–4.
- Weiss G, Fuchs D, Hausen A, Reibnegger G, Werner ER, Werner-Felmayer G, et al. Neopterin modulates toxicity mediated by reactive oxygen and chloride species. FEBS Lett 1993;321(1):89–92.
- Razumovitch JA, Semenkova GN, Fuchs D, Cherenkevich SN. Influence of neopterin on the generation of reactive oxygen species in human neutrophils. FEBS Lett 2003;549(1–3):83–6.
- Fuchs D, Baier-Bitterlich G, Wede I, Wachter H. Reactive oxygen and apoptosis. In: , Scandalios JG (ed.) Oxidative stress and the molecular biology of antioxidant defences. Cold Spring Habor, New York: Cold Spring Habor Laboratory Press; 1997. p. 139–67.
- Murr C, Fuith LC, Widner B, Wirleitner B, Baier-Bitterlich G, Fuchs D. Increased neopterin concentrations in patients with cancer: indicator of oxidative stress? Anticancer Res 1999;19(3A):1721–8.
- Fuchs D, Möller AA, Reibnegger G, Werner ER, Werner-Felmayer G, Dierich MP, et al. Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett 1991;28(3):207–11.
- Sucher R, Schroecksnadel K, Weiss G, Margreiter R, Fuchs D, Brandacher G. Neopterin, a prognostic marker in human malignancies. Cancer Lett 2010;287(1):13–22.
- Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991;5(11):2516–22.
- Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem 1997;43(12):2424–6.
- Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 2006;364(1–2):82–90.
- Thomas SR, Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep 1999;4(5):199–220.
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression – what is the link? Brain Behav Immun 2002;16(5):590–5.
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012;37(1):137–62.
- Pall ML. Nitric oxide synthase partial uncoupling as a key switching mechanism for the NO/ONOO− cycle. Med Hypotheses 2007;69(4):821–5.
- Turpaev K, Glatigny A, Bignon J, Delacroix H, Drapier JC. Variation in gene expression profiles of human monocytic U937 cells exposed to various fluxes of nitric oxide. Free Radic Biol Med 2010;48(2):298–305.
- Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med 1997;22(6):1115–26.
- Smale ST. Hierarchies of NF-κB target-gene regulation. Nat Immunol 2011;12(8):689–94.
- Baltimore D. NF-κB is 25. Nat Immunol 2011;12(8):683–5.
- Hao S, Baltimore D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol 2009;10(3):281–8.
- Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci USA 2002;99(3):1491–6.
- Castellani P, Angelini G, Delfino L, Matucci A, Rubartelli A. The thiol redox state of lymphoid organs is modified by immunization: role of different immune cell populations. Eur J Immunol 2008;38(9):2419–25.
- Ishii T, Sugita Y, Bannai S. Regulation of glutathione levels in mouse spleen lymphocytes by transport of cysteine. J Cell Physiol 1987;133(2):330–6.
- Matsue H, Edelbaum D, Shalhevet D, Mizumoto N, Yang C, Mummert ME, et al. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol 2003;171(6):3010–8.
- Los M, Schenk H, Hexel K, Baeuerle PA, Dröge W, Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J 1995;14(15):3731–40.
- Secrist JP, Burns LA, Karnitz L, Koretzky GA, Abraham RT. Stimulatory effects of the protein tyrosine phosphatase inhibitor, pervanadate, on T-cell activation events. J Biol Chem 1993;268(8):5886–93.
- Suthanthiran M, Anderson ME, Sharma VK, Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci USA 1990;87(9):3343–7.
- Thorén FB, Betten A, Romero AI, Hellstrand K. Cutting edge: antioxidative properties of myeloid dendritic cells: protection of T cells and NK cells from oxygen radical-induced inactivation and apoptosis. J Immunol 2007;179(1):21–5.
- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2012;S1471–4906(12):00176–7 [Epub ahead of print].
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8(7):523–32.
- Sucher R, Fischler K, Oberhuber R, Kronberger I, Margreiter C, Ollinger R, et al. DO and regulatory T cell support are critical for cytotoxic T lymphocyte-associated Ag-4 Ig-mediated long-term solid organ allograft survival. J Immunol 2012;188(1):37–46.
- Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol 2012;42(8):1932–7.
- Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol 2009;158(3):638–51.
- Poljsak B, Milisav I. The neglected significance of ‘antioxidative stress’. Oxid Med Cell Longev 2012;(2012):480895.
- Hasan AA, Ghaemmaghami AM, Fairclough L, Robins A, Sewell HF, Shakib F. Allergen-driven suppression of thiol production by human dendritic cells and the effect of thiols on T cell function. Immunobiology 2009;214(1):2–16.
- Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA 1998;95(6):3071–6.
- Jenny M, Klieber M, Zaknun D, Schroecksnadel S, Kurz K, Ledochowski M, et al. In vitro testing for anti-inflammatory properties of compounds employing peripheral blood mononuclear cells freshly isolated from healthy donors. Inflamm Res 2011;60(2):127–35.
- Tan PH, Sagoo P, Chan C, Yates JB, Campbell J, Beutelspacher SC, et al. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J Immunol 2005;174(12):7633–44.
- Zaknun D, Schroecksnadel S, Kurz K, Fuchs D. Potential role of antioxidant food supplements, preservatives and colorants in the pathogenesis of allergy and asthma. Int Arch Allergy Immunol 2012;157(2):113–24.
- Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis 2006;65( Suppl 3)
- Niedbala W, Wei XQ, Piedrafita D, Xu D, Liew FY. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur J Immunol 1999;29(8):2498–505.