Abstract
Aim
We have recently demonstrated the quick ex vivo transfer of paraoxonase 1 (PON1) activity from high-density lipoprotein (HDL) to small, dense low-density lipoprotein (sdLDL). We set out to assess whether sdLDL contains active PON1 in vivo.
Methods
We conducted a nested case–control, proof of principle study with the Japanese healthy subjects with normal lipids (n = 23) and age and gender-paired dyslipidemic subjects (n = 17). Lipid panels, lactonase and arylesterase assays, and PON1 zymogram in the LDL and HDL subclasses were assessed.
Results
PON1 specific activity in the high-molecular weight lipoprotein fraction corresponding to LDL migration was found in 48% of normo and in 29% of dyslipidemic Japanese subjects. This band co-localizes with apoB100 and not Lp(a) and displays a lower molecular mass than the bulk of LDL.
Conclusion
We provide evidence, for the first time, that native sdLDL contains up to 4% of the total PON1 activity in the serum of up to 48% of the Japanese subjects. Could the PON1-containing sdLDL represent a set of particles with a defense mechanism from oxidation and therefore its levels actually prove to be atheroprotective? If further studies confirm this contention, a zymogram of PON1 in LDL subclasses could be a functional assay that complements the current methods that only inform on the size and lipid concentration of these particles.
Introduction
Paraoxonase 1 (PON1) is a circulating esterase and lactonase.Citation1–Citation6 In the cardiovascular context, research on PON1 has been focused on its ability to prevent lipid oxidation and limit atherosclerotic lesion development.Citation7–Citation9 Several studies have provided persuasive arguments for a role for PON1 in these processes. PON1 hydrolyzes the lipoprotein-associated peroxides and lactones.Citation1,Citation3 Most PON1 circulates are associated with high-density lipoproteins (HDL); however, small amounts are found in very low-density lipoprotein (VLDL) and in chylomicrons.Citation10,Citation11 It has been suggested that PON1 may use VLDL as a vehicle to get into HDL.Citation11 However, PON1 has not been found in low-density lipoprotein (LDL) in studies using ultracentrifugation (UC) and proteomics.Citation10,Citation12 Since LDL stems from VLDL, these previous results suggest that VLDL-PON1 moves back into HDL as the lipoprotein lipase and the hepatic lipase (HL) metabolize VLDL into LDL. A recent proteomic study found that PON1 is carried by Lp(a) in some subjects with a high level of this lipoprotein.Citation13 Lp(a) contains high levels of oxidized phospholipids and may be their preferential carrier or scavenger, thereby the presence of PON1 may play a physiological role.Citation13 Since Lp(a) is a variant of LDL, this finding indicates that the previously reported absence of PON1 in LDL may need further exploration.
In this regard, in our validation study for a practical method to detect the PON1 activity in HDL subclasses in human serum, we made the unexpected observation that, in some subjects, specific PON1 activity can be revealed at the origin of the run as well as in the particles >12 nm, corresponding to the range of LDL particles diameter, and especially in smaller subspecies.Citation8
Clinical studies suggest that high serum levels of small and dense LDL (sdLDL) are associated with coronary artery disease (CAD) risk.Citation14 Subjects with pattern type B (high sdLDL) had a three-fold higher acute myocardial infarction risk compared to those with pattern type A, and this relationship was independent of the age, sex, and relative weight.Citation15,Citation16 Nevertheless, while the sdLDL cholesterol (sdLDL-C) is closely associated with coronary atherosclerosis, convincing studies showing a reduction in CAD risk by lowering sdLDL-C are not abundant.Citation17 A step beyond a mere assessment of cholesterol content or the total mass of these particles (which is what the current methods report) is to look for the functional differences in these particles. It is believed that PON1 may exert part of its beneficial effects by acting on the macrophage and LDL oxidized lipids.Citation18,Citation19 If the latter are more concentrated in sdLDL, would PON1 transfer from HDL to act on sdLDL oxidized lipids? If this were true, would the particles that putatively contain PON1 be less atherogenic?
In a follow-up recent study, we determined that a short, ex vivo incubation of the serum leads to a quick activation of PON1 associated with the transfers to HDL3c, large HDL, and sdLDL, respectively. The process is blocked by cholesterol ester transfer protein (CETP) and lecithin cholesterol acyl transferase (LCAT inhibitors).Citation20 Having demonstrated that PON1 is transferred to sdLDL in a few hours of incubation ex vivo and that the process is dependent on a lipid transfer, the next logical step was to conduct the present study by using the same methodology to ascertain whether an active PON1 is present in vivo in sdLDL.
Materials and methods
Materials
All chemicals were from Sigma-Aldrich (St Louis, MO, USA) unless otherwise indicated.
Study subjects
We conducted a nested case–control, proof of principle study. Healthy subjects with normal lipids (n = 23, 60.7 ± 12 years old, 11 males, 12 females) and age and gender-paired dyslipidemic subjects (hypertriglyceridemia and low HDL cholesterol (HDL-C)), n = 17, 58.1 ± 8.2 years old were recruited at Jichi Medical University, Tochigi, Japan. A fasting blood sample from both sets of subjects was obtained by venipuncture and collected in the evacuated tubes. There was no clinical analytical evidence of renal or liver disease, neoplasia, diabetes, or neurological disorders in these participants.
Fasting and postprandial samples, timecourse
To rule out a possible confounding by triglyceride-rich lipoproteins (TRLs) and/or their remnants (TRL: VLDL and chylomicrons), four healthy volunteers of European descent at Touro University California fasted for 12 hours and the blood samples were drawn at fasting and 1, 2, 3, and 5 hours after an 800 calories meal containing 40% lipids, 40% carbohydrates, and 20% protein.
Fasting venous blood samples (and the appropriate postprandial samples) were obtained from all the participants and the serum was aliquoted immediately and stored at −80°C until measurements were performed. Aliquots were thawed and used only once and then discarded. All assays were performed in one batch on the same day. All the participants provided fully informed consent to participation in the study on the understanding that anonymity of all data is guaranteed. The study was approved by the Institutional Review Boards of the participating sites: Touro, Jichi, and Showa Universities.
General laboratory assessments
Plasma glucose and serum lipids, such as LDL-cholesterol (LDL-C), HDL-C, and triglycerides (TGs), respectively, were measured by using enzymatic methods.
LDL subclasses were analyzed for comparison and control purposes by using the Lipoprint LDL subfraction analysis system from Quantimetrix (Redondo Beach, CA, USA) according to the manufacturer's instructions. LDL subfractions were distributed from LDL1 to LDL7 (Rf 0.32, 0.38, 0.45, 0.51, 0.56, 0.6, and 0.64, respectively). A proportion of large LDL (large LDL%) was defined as the sum of the percentage of LDL1 and LDL2, whereas a proportion of small LDL (small LDL%) was defined as the sum of LDL3–LDL7.
PON1 activity
Serum PON1 arylesterase activity was kinetically measured by using phenylacetate as a substrate at 37°C, and the absorbance changes were recorded at 270 nm in a Versamax Microplate Reader (Molecular Diagnostics, CA, USA), as described previously.Citation6,Citation20
PON1 activity detection in situ
Detection of the PON1 activity in lipoprotein subclasses was performed by employing our published zymogram method.Citation8 LDLs and HDLs were separated in non-denaturing 4–12% gradient gels as well as 3–8% polyacrylamide gel electrophoresis (PAGE) (Novex® 4–12% and 3–8% Tris-Glycine gel, Invitrogen, Carlsbad, CA, USA) as previously described.Citation8 In these electrophoretic conditions, chylomicrons and VLDL do not penetrate the gel, the LDL is found in the upper one-fourth of the running distance, the rest of the gel allows for a separation of different HDL subclasses in the appropriate range of 7–12 nm particle diameter.Citation8 The gels were next equilibrated in 2 mM phenylacetate, 20 mM 4 amino antipyrine (4-AAP), and 2 mM K3Fe(CN)6, respectively, as previously described.Citation8 The relative proportions of PON1 activity in each LDL or HDL subclass were estimated by an optical densitometry analysis employing the Image J software (NIH, Bethesda, MD, USA), using as reference the high-molecular weight calibration kit (Amersham Pharmacia Biotech, Buckimghamshire, UK) that were run in each gel. The relative proportion of PON1 in each lipoprotein subclass is expressed as the percentage of the total PON1 area under the curve.
Reproducibility
For the assessment of the intra- and inter-assay coefficient of variation (CVs), we obtained a pool of serum from 10 volunteers. HDLs were isolated and separated on the basis of their hydrodynamic diameter by non-denaturing electrophoresis in a 4–12% PAGE. PON1 was sequentially stained and the densitometry scans were obtained and analyzed as described above. Under these conditions, eight samples and the corresponding duplicate of the reference globular proteins were run per each electrophoresis gel. The intra-assay CV of the method was 8.9% as assessed after 32 repetitions of the same sample in four independent gels. The mean inter-assay CV was 12.8% as determined by the analysis of the same sample in 10 independent electrophoresis gels.
PON1 zymogram in postprandial serum
To rule out a possible confounding by VLDL and chylomicrons, we run the serum from four volunteers at 12 hours fasting and 1, 2, 3, and 5 hours postprandially and processed it as above.
Detection of apoB and Lp(a) apolipoproteins in the same gel after the zymogram
After the PON1 activity detection, the gels were transferred to polyvinylidene difluoride. Apo (lipoprotein) B, and Lp(a) were detected on sequential immunoblots by using anti-ApoB-total (horseradish peroxidase (HRP)) AbCam ab27622, Lp(a) Ab 31675, and anti-ApoA-I (HRP) AbCam ab20784 as previously described.Citation8 The blots are then incubated in ECL PLUS Western blot reagent (Amersham), developed, scanned with a Licor C-Digit chemiluminescence scanner from Licor Biosciences (Lincoln, NE, USA), and processed with Image Studio from the same company.
Isolation of VLDL, LDL HDL2, and HDL3
VLDL, HDL and HDL as well as HDL2, and HDL3 were separated by UC of pooled serum in a Beckman L8–70M ultracentrifuge at 110 000 rpm in 8.4 ml polycarbonate tubes in a 50 Ti rotor as described previously. Briefly, VLDL was floated for 20 hours at density <1.006 mg/dl, then LDL for 24 hours at density <1.063 mg/dl, total HDL (density 1.063–1.21 g/ml) was obtained after 24 hours centrifugation and re-purified at density 1.21 g/ml for another 24 hours centrifugation. HDL2 and HDL3 were further isolated by UC at density 1.12 and 1.21 g/ml, respectively. VLDL, LDL, and HDL fractions were dialyzed against phosphate-buffered saline containing antiproteases and 1 mM ascorbic acid.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) for variables with a normal distribution and as the median and interquartile range for variables with a skewed distribution. The variables with a skewed distribution were log-transformed in the analyses. The difference in parameters was tested by the Student's t-test. Statistical significance was set at P < 0.05. Statistical analysis was performed by using SPSS version 11 software (SPSS Inc., Chicago, IL, USA).
Results
shows the clinical and laboratory data of the Japanese subjects studied. Dyslipidemic subjects have higher levels of TGs, a higher sdLDL, and a lower HDL-C (P < 0.0001).
Table 1. Selected biochemical variables in Japanese subjects with normo or dyslipidemia
PON1 activity is found in the high-molecular weight lipoprotein fraction corresponding to LDL migration
As depicted in A, the zymogram shows that PON1 is found in HDL and a small percentage of the activity is also found at the origin and in the LDL region. In those subjects in whom the band was present, PON1 in LDL amounted to 4 ± 2% of the total. This fraction was found in 48% of the normolipidemic subjects as well as in 29% of the dyslipidemic subjects. Neither the VLDL nor LDL isolated by UC displayed activity (data not shown). Note in B that the range of PON1 activity of HDL isolated by UC is narrower than in native sera, suggesting PON1 is lost from very small and very large HDL by this process. The specificity of PON1 activity was assessed by control experiments as above, in the presence of 1 mM of the PON1 inhibitor 2-hydroxyquinoline or 1 mM ethylenediaminetetraacetic acid both of which abolished all the bands.
Figure 1. PON1 activity in the LDL and HDL subclasses separated by native gradient electrophoresis gels and western blot detection of LDL apolipoproteins after zymogram. Fig. 1A shows the PON1 zymogram of seven representative Japanese male subjects. N stands for normolipidemic and D for dyslipidemic. LDL and HDL were separated by on 4–12% Tris-Glycine gels as described in the Materials and methods section. PON1 activity in lipoprotein subclasses was determined by the enzymatic detection of PON1 hydrolysis of phenylacetate in situ as described in the Materials and methods section. Fig. 1C–E depicts the western blots conducted after the zymogram depicted in Fig. 1A, as described in the Materials and methods section. ApoB, Lp(a), and ApoA-I were detected on sequential immunoblots using HRP-conjugated antibodies as described in the Materials and methods section. Fig. 1F depicts apoA-I detection in the isolated HDL2 and HDL3. Note the presence of PON 1 activity at the origin in all Japanese subjects and a region compatible with LDL migration well within the gel, which in this example is more apparent for normolipidemics than the dyslipidemic subjects. The presence of this band was found in 5 of 17 dyslipidemic subjects and in 11 of 23 normolipidemics subjects. Note in N2 that apoB100 distributes in higher molecular weight fractions and extends to smaller particles. Note that Lp(a) is present essentially at the origin. VLDL (also containing apoB100) and chylomicrons are excluded from 4–12% gels, they can be ruled out as responsible for PON1 activity found in the particles that penetrate the gel and correspond to the LDL range. Data show a typical experiment out of the three.
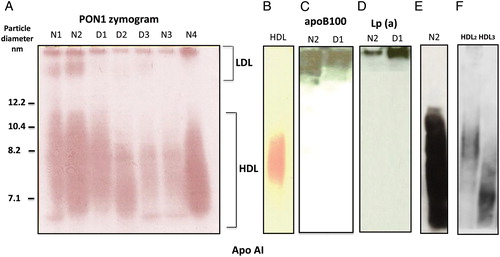
PON1 activity co-localizes with Lp(a) at the origin and with apoB100 (origin and LDL region) but not with apoA
C–E depicts the western blots conducted after the zymogram on the same gel as shown in A. The PON1 activity in LDL of the subjects N2 and D1 and their apoB100 distribution was compared in A and C. Subjects with a higher LDL apoB100 in smaller particles also contain PON1 activity in the same band. Note (D) that Lp(a) is present essentially at the origin and therefore cannot account for the PON1 activity in smaller LDL particles. Note that >12.2 nm band that contains PON1 activity does not contain apoA-I in native sera (E) nor in isolated HDL2 or HDL3 (F).
PON1 activity in the LDL size range is not due to TRL or their remnants
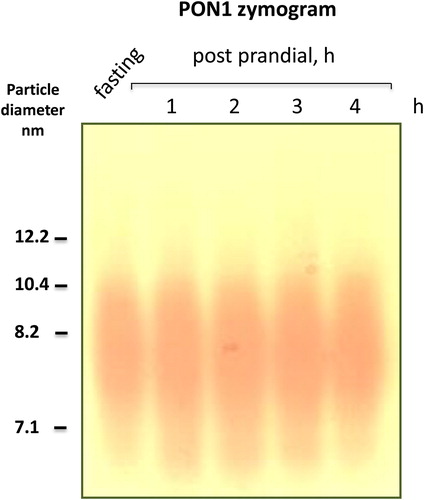
TRLs (chylomicrons and VLDL) do contain up to 1% PON1 activity but they do not penetrate 3–4% gels, they remain at the origin or float during the overnight run (chylomicrons) instead.Citation14,Citation16,Citation21,Citation22 To further confirm with direct evidence that the PON1 activities shown above are not due to VLDL or chylomicron remnants, we performed a postprandial follow-up in four volunteers of European descent after a meal heavy in lipids and carbohydrates. As depicted in in this typical subject, no PON1 activity was detectable in the VLDL-LDL region in spite of the increase in VLDL, chylomicrons, and their remnants typical of the postprandial period: TG levels in this subject were 0.8 mmol/l at fasting and 1.3, 2.2, 1.6, and 1.1 mmol/l at 1, 2, 3, and 5 hours postprandially, respectively. The little PON1 activity in VLDL and chylomicrons found by others in some cases (0.5–1% of the total) is absent here or it falls below the limit of detection of our method. It is certainly not found at the Rf where sdLDL-PON1 activity was found in this and our previous workCitation20 is consistently apparent. A comparison with the prominent band at the origin for the Japanese subjects suggests the possibility of ethnic differences by which the Japanese may have more VLDL-PON1 than the Europeans, a finding worthy of further exploration.
Figure 2. PON1 activity in the LDL size range is not due to TRL or their remnants: a postprandial timecourse study. To further confirm that the PON1 activities in LDL region shown in Fig. 1A–C are not due to VLDL, chylomicrons, or their remnants, we performed a postprandial follow-up in four volunteers of European descent after a meal heavy in lipids and carbohydrates. As depicted in the figure, no PON1 activity was detectable in the VLDL-LDL region in spite of the increase in VLDL, chylomicrons, and their remnants typical of the postprandial period. It is certainly not found at the Rf where the sdLDL-PON1 activity is found. Data show a typical experiment out of three for a total n = 4 for this intervention.
Discussion
We provide evidence for the in vivo presence of active PON1 in sdLDL particles. This finding may have interesting ramifications in our understanding of HDL and LDL biology and a putative interest as a future development for a functional test to characterize sdLDL.
During the past decade, PON1 has been proven to be an important contributor to the antioxidant activity of HDL as well as an independent negative risk factor in the epidemiological studies. PON1 is versatile and plays a role in several pathways, many of which are protective against atherothrombosis:Citation5,Citation9,Citation23–Citation30
PON1 preferably binds to HDL-phospholipids through its retained N-terminal hydrophobic region, and it is stabilized by HDL-associated apoA-I.Citation11 A very small fraction has been demonstrated in VLDL and chylomicrons but not in LDL.Citation10 However, we have recently demonstrated an ex vivo transfer of PON1 to sdLDL and its inhibition by LCAT and CETP inhibitors.Citation20 Moreover, a recent proteomic study found PON1 in Lp(a) in the subjects with a high level of this particle.Citation13 Based on our recent ex vivo findings and on the fact that Lp(a) is a modified LDL particle, we decided to re-explore the issue of the apparent absence of PON1 in LDL. We set out to study the PON1 activity in the native lipoproteins separated by a non-denaturing gradient gel electrophoresis (GGE). LDL is heterogeneous, and the most pro-atherogenic fraction, the sdLDL bears several differences with the bulk of LDL that predominates in most people.Citation31,Citation32
These particles could benefit from PON1 actions to detoxify the oxidized lipids. However, relatively little attention has been paid to the mechanisms by which these activities are exerted. In the light of these arguments, we set out to demonstrate specifically whether the sdLDL contains active PON1 in vivo. The migration region for sdLDL in GGE is well documented since the pioneer study by Krauss in 1982, and has been since confirmed in multiple studies employing GGE together with an array of complementary approaches.Citation14,Citation16,Citation20,Citation21
Therefore, by employing the well-documented GGE protocolsCitation14,Citation16,Citation20,Citation21 paired with our PON1 zymogram method,Citation8 we provide several lines of evidence that show that sdLDL (the smaller-sized fraction of apoB-containing lipoproteins in GGE) contains the active PON1 in some individuals.
The arguments that support this contention are several:
| 1. | We found the PON1 activity in the high-molecular weight lipoprotein fraction corresponding to LDL migration. | ||||
| 2. | This band co-localizes with apoB100 and not with apoA-I. It displays a lower molecular mass than the bulk of LDL in the control subjects. | ||||
| 3. | The activity is specific (not an unrelated esterase) as it is abolished by the PON1 inhibitors. | ||||
| 4. | Some Japanese subjects with either a very high or a very low Lp(a) equally had PON1 activity in the sdLDL range. This further supports the fact that PON1 activity found in this migration range is due to sdLDL. | ||||
| 5. | VLDL (also containing apoB100) and chylomicrons are excluded from 4 to 12% gels, they can be in principle ruled out as responsible for PON1 activity found at the LDL range. | ||||
| 6. | To strengthen the argument above, a postprandial experiment shows neither a band at the sdLDL range nor at the origin. The previous studies reported a less than 1% PON1 activity in VLDL and chylomicrons; we could not substantiate this here, most likely due to the limit of detection of our method. | ||||
PON1 peptides have been found in proteomic studies on the modified LDL, Lp(a).Citation13 Our data suggest that Lp(a) also displays the PON1 activity, however, PON1 in the larger LDL or VLDL cannot be distinguished from PON1 in Lp(a) with absolute certainty by using this approach as these particles are found at the origin of the run.
Taken together, our data support the contention that in some subjects sdLDL particles carry PON1 in active form in vivo, or readily exchange PON1 with HDL. In this cohort, this occurs in both the normo and dyslipidemic subjects.
The reason why PON1 has not previously been found in LDL may lie in the fact that usually these studies are conducted on LDL prepared from the pooled control human subjects, where the proportion of sdLDL is less than 20%. The activity we detect in situ, in subjects that have sdLDL PON1, amounts to 2–4% of the total PON1, therefore dilution can easily explain this discrepancy. Moreover, the procedure of UC is known to disrupt the lipoprotein structure. The associated peptides not tightly bound can be stripped off to the lipoprotein-free fraction of the plasma.Citation11,Citation12 This is very significant for HDL as apparent by comparing A with B. In this regard, the advantage of our approach is that it allows us to measure the enzymatic activities in situ in the native lipoprotein.
Our finding may add a new layer in our understanding of sdLDL role in the context of cardiovascular disease. Together with the increase in large buoyant LDL, the predominance of sdLDL has been accepted as a risk factor for cardiovascular events by the National Cholesterol Education Program Adult Treatment Panel III.Citation16,Citation17 The modulation of LDL particle size by hypolipidemic agents reduces the cardiovascular disease (CVD) risk in some but not all the studies.Citation16,Citation17 Could this be due to a structural and/or a functional heterogeneity? Some evidence for sdLDL structural heterogeneity is starting to surface, namely on apoC-III content.Citation33 The functional studies, however, have mainly focused on the HDL heterogeneity. Our data suggest that in many individuals sdLDL has functional differences from larger LDL in at least the presence of active PON1, an enzymatic activity related to the oxidative stress and atherogenesis. These observations warrant further confirmation by proteomic analysis of the cut-out gel bands, since labeled anti-PON1 antibodies are not commercially available. The normolipidemic subjects had more prevalence of sdLDL with PON1 activity. However, due to the small sample size in this study, we cannot ascertain whether this will hold for a larger population.
What would be the origin of PON1 in sdLDL and why is it present in some subjects but not in others? At this time we can only speculate, as this has not been addressed by this proof of principle study. We may argue that PON1 in sdLDL could stem from a larger VLDL1 which yields the sdLDL. These particles, would keep their PON1 complement when preferentially metabolized by HDL, and that would not be the case for smaller VLDL, which yield a large and buoyant LDL. Alternatively, sdLDL may be a better acceptor for PON1 from HDL than the buoyant LDL. In this regard, the transfer of PON1 from HDL to sdLDL occurs very fast when the serum is incubated ex vivo as we have recently shown.Citation20 Further studies are warranted to address these hypotheses.
A limitation of the study is the small sample size that precludes us from assessing other covariates and determine with certainty the actual prevalence of the presence of PON1 in sdLDL in the population. However, the aim of this study was to prove that active PON1 is found in sdLDL in vivo and not to ascertain the prevalence. We believe that the arguments gathered in our previous ex vivo study together with those in this work are strong indicators of the validity of our findings that should foster confirmation by proteomics as well as larger studies of prevalence, physiological significance, and potential diagnostic and or prognostic value.
Conclusion
We provide evidence, for the first time, that native sdLDL contains up to 4% of the total PON1 activity in serum of up to 48% of a small cohort of the Japanese subjects. This proof of principle study may pave the way for future work aiming at confirming these findings in larger populations, other ethnicities, and establishing correlations with other parameters such as HDL function. Could the PON1-containing sdLDL represent a set of particles with a defense mechanism from oxidation and therefore its levels actually prove to be atheroprotective? If further studies confirm this contention, a zymogram of PON1 in LDL subclasses could be a functional assay that complements the current methods that only inform on the size and lipid concentration of these particles.
Acknowledgments
We are grateful to Dr Teresita Menini for critical reading of the manuscript, to Ms Mallory Davis for expert editorial and technical help, and Dr S. Ogtulakk for valuable insights. This work was funded in part by Touro University California.
References
- Aviram M, Billecke S, Sorenson R, Bisgaier C, Newton R, Rosenblat M, et al. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylsterase/paraoxonase activities: selective action of human paraoxonase allozymes Q and R. Arterioscler Thromb Vasc Biol 1998;18:1617–24.
- Costa LG, Cole TB, Furlong CE. Paraoxonase (PON1): from toxicology to cardiovascular medicine. Acta Biomed 2005;76:50–7.
- Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res 2005;46:1239–47.
- Fuhrman B, Aviram M. Preservation of paraoxonase activity by wine flavonoids: possible role inprotection of LDL from lipid peroxidation. Ann N Y Acad Sci 2002;957:321–4.
- Furlong CE, Cole TB, Jarvik GP, Costa LG. Pharmacogenomic considerations of the paraoxonase polymorphisms. Pharmacogenomics 2002;3:341–8.
- Mackness B, Davies GK, Turkie W, Lee E, Roberts DH, Hill E, et al. Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol 2001;21:1451–7.
- Camps J, Mackness M, Mackness B, Marsillach J, Joven J. Serum paraoxonase-1 activity and genetic polymorphisms: common errors in measurement and interpretation of results. Clin Chem Lab Med 2010;48:893–4.
- Gugliucci A, Caccavello R, Kotani K, Sakane N, Kimura S. Enzymatic assessment of paraoxonase1 activity on HDL subclasses: a practical zymogram method to assess HDL function. Clin Chim Acta 2013;415:162–8.
- Gugliucci A, Kotani K, Kimura S. Paraoxonase 1 in chronic kidney failure. J Lipids 2012;2012:726048.
- Fuhrman B, Volkova N, Aviram M. Paraoxonase 1 (PON1) is present in postprandial chylomicrons. Atherosclerosis 2005;180:55–61.
- James RW, Deakin SP. The importance of high-density lipoproteins for paraoxonase-1 secretion,stability, and activity. Free Radic Biol Med 2004;37:1986–94.
- Hoofnagle AN, Heinecke JW. Lipoproteomics: using mass spectrometry-based proteomics to explore the assembly, structure, and function of lipoproteins. J Lipid Res 2009;50:1967–75.
- von Zychlinski A, Kleffmann T, Williams MJ, McCormick SP. Proteomics of lipoprotein(a) identifies a protein complement associated with response to wounding. J Proteomics 2011;74:2881–91.
- Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res 1982;23:97–104.
- Krauss RM. Triglycerides and atherogenic lipoproteins: rationale for lipid management. Am J Med 1998;105:58S–62S.
- Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol 2010;21:305–11.
- Hirayama S, Miida T. Small dense LDL: an emerging risk factor for cardiovascular disease. Clin Chim Acta 2012;414:215–24.
- Aharoni S, Aviram M, Fuhrman B. Paraoxonase 1 (PON1) reduces macrophage inflammatory responses. Atherosclerosis 2013;228:353–61.
- Aviram M, Fuhrman B. LDL oxidation by arterial wall macrophages depends on the oxidative status in the lipoprotein and in the cells: role of prooxidants vs. antioxidants. Mol Cell Biochem 1998;188:149–59.
- Gugliucci A. Activation of paraoxonase 1 is associated with HDL remodeling ex vivo. Clin Chimica Acta 2014;429:38–45
- Alabakovska SB, Labudovic DD, Tosheska KN, Spiroski MZ, Todorova BB. Low density lipoprotein subclass distribution in children with diabetes mellitus. Bratisl Lek Listy 2008;109:155–9.
- Alabakovska SB, Todorova BB, Labudovic DD, Tosheska KN. Gradient gel electrophoretic separation of LDL and HDL subclasses on BioRad Mini Protean II and size phenotyping in healthy Macedonians. Clin Chim Acta 2002;317:119–23.
- Silliman K, Shore V, Forte TM. Hypertriglyceridemia during late pregnancy is associated with the formation of small dense low-density lipoproteins and the presence of large buoyant high-densitylipoproteins. Metabolism 1994;43:1035–41.
- Aviram M, Vaya J. Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr Opin Lipidol 2013;24:339–44
- Draganov DI. Lactonases with organophosphatase activity: structural and evolutionary perspectives. Chem Biol Interact 2010;187:370–2.
- Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol 2001;21:473–80.
- Furlong CE, Suzuki SM, Stevens RC, Marsillach J, Richter RJ, Jarvik GP, et al. Human PON1, a biomarker of risk of disease and exposure. Chem Biol Interactions 2010;187:355–61.
- Jakubowski H. The role of paraoxonase 1 in the detoxification of homocysteine thiolactone. Advances in Experimental Medicine and Biology 2010;660:113–27.
- La Du BN, Aviram M, Billecke S, Navab M, Primo-Parmo S, Sorenson RC, et al. On thephysiological role(s) of the paraoxonases. Chemico-biological Interact 1999;119–120:379–88.
- Mackness B, Mackness M. Anti-inflammatory properties of paraoxonase-1 in atherosclerosis. Adv Exp Med Biol 2010;660:143–51.
- Mackness MI, Mackness B, Durrington PN. Paraoxonase and coronary heart disease. Atheroscler Suppl 2002;3:49–55.
- Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988;260:1917–21.
- Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, et al. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem 2008;54:1307–16.
- Faghihnia N, Mangravite LM, Chiu S, Bergeron N, Krauss RM. Effects of dietary saturated fat on LDL subclasses and apolipoprotein CIII in men. Eur J Clin Nutr 2012;66:1229–33.
