Abstract
Objectives
Patients with end-stage renal failure (ESRF) treated with erythropoiesis-stimulating agents (ESAs) are often ESA-hyporesponsive associated with free radical production. Hydroxyl free radical converts phenylalanine into ortho-tyrosine, while physiological isomer para-tyrosine is formed enzymatically, mainly in the kidney. Production of ‘para-tyrosine’ is decreased in ESRF and it can be replaced by ortho-tyrosine in proteins. Our aim was to study the role of tyrosines in ESA-responsiveness.
Methods
Four groups of volunteers were involved in our cross-sectional study: healthy volunteers (CONTR; n = 16), patients on hemodialysis without ESA-treatment (non-ESA-HD; n = 8), hemodialyzed patients with ESA-treatment (ESA-HD; n = 40), and patients on continuous peritoneal dialysis (CAPD; n = 21). Plasma ortho-, para-tyrosine, and phenylalanine levels were detected using a high performance liquid chromatography (HPLC)-method. ESA-demand was expressed by ESA-dose, ESA-dose/body weight, and erythropoietin resistance index1 (ERI1, weekly ESA-dose/body weight/hemoglobin).
Results
We found significantly lower para-tyrosine levels in all groups of dialyzed patients when compared with control subjects, while in contrast ortho-tyrosine levels and ortho-tyrosine/para-tyrosine ratio were comparatively significantly higher in dialyzed patients. Among groups of dialyzed patients the ortho-tyrosine level and ortho-tyrosine/para-tyrosine ratio were significantly higher in ESA-HD than in the non-ESA-HD and CAPD groups. There was a correlation between weekly ESA-dose/body weight, ERI1, and ortho-tyrosine/para-tyrosine ratio (r = 0.441, P = 0.001; r = 0.434, P = 0.001, respectively). Our most important finding was that the ortho-tyrosine/para-tyrosine ratio proved to be an independent predictor of ERI1 (β = 0.330, P = 0.016). In these multivariate regression models most of the known predictors of ESA-hyporesponsiveness were included.
Discussion
Our findings may suggest that elevation of the ratio of ortho-tyrosine/para-tyrosine could be responsible for decreased ESA-responsiveness in dialyzed patients.
Introduction
Anemia is a common complication in patients suffering from chronic kidney disease (CKD). Recombinant human erythropoietin (rHuEPO) has been used in CKD since 1989, resulting in the improvement of quality of life. Approximately 10% of the patients on rHuEPO therapy are hyporesponsive, associated with an increased risk of death.Citation1 Any consensus definition is lacking but according to the review by Bamgbolaan evaluation of erythropoiesis-stimulating agent (ESA)-resistance is suggested when the hemoglobin deficit is persistent despite more than 3 months' treatment with ≥400 IU/kg or ≥20 000 IU/week of rHuEPO.Citation2 The European Best Practice Guidelines (EBPG) defines ESA-resistance as an epoetin dose >300 IU/kg/week.Citation3 Kidney Disease Improving Global Outcomes (KDIGO) classifies patients as having initial ESA-hyporesponsiveness if they have no increase in Hb concentration from baseline after the first month of ESA treatment on appropriate weight-based dosing, while subsequent (acquired) ESA-hyporesponsiveness is defined as being when patients after treatment with stable doses of ESA require two increases in ESA doses up to 50% beyond the dose at which they had been stable in an effort to maintain a stable Hb concentration.Citation4
The reasons for ESA-hyporesponsiveness are not yet clearly identified. The roles of iron, folate, cobalamin or carnitine deficiency, bleeding, inflammation, angiotensin converting enzyme (ACE)-inhibitor administration, uremic toxins, insufficient dialysis, hyperparathyroidism, and malignancy are suggested by several studies.Citation3,Citation5,Citation6 Van der Putten et al. have highlighted the role of cytokine-inducible sulfhydryl group containing protein (CIS), which is upregulated by inflammatory cytokines and which binds to the EPO-receptor (EPO-R) and inhibits EPO-dependent cell proliferation. They also evaluated the influence of hematopoetic cell phosphatase in the inhibition of signal transduction of EPO-R.Citation1 In the post hoc study of Rossert et al.Citation6 diabetes mellitus was the primary cause of kidney disease among the non-responder group. These findings are consistent with the observations of Abe et al., who state that insulin resistance is associated with ESA-hyporesponsiveness since the ESA-dose was significantly higher in the group of diabetic patients. According to their investigations, homeostasis model assessment-insulin resistance (HOMA-IR), hemoglobin, IL-6, TNF-alfa, and high molecular weight adiponectin were the independent predictors of the required ESA-dose.Citation7
Several publications have proven that oxidative stress plays a major role in hormone resistances, such as insulin resistance.Citation8,Citation9 Bearing the above-mentioned observations in mind we assumed that oxidative stress could be a common mediator in the development of insulin resistance as well as ESA-resistance. Oxidative stress is defined as the loss of balance between pro- and antioxidant systems. This imbalance results an overproduction of such free radicals as hydroxyl radical (HO•) leading to direct tissue damage or functional impairment. Hydroxyl radical converts l-phenylalanine into ortho-tyrosine, an isomer of the natural amino acid para-tyrosine.Citation10,Citation11 Consequently, elevated levels of ortho-tyrosine detect hydroxyl radical-induced tissue damage. On the other hand, para-tyrosine is formed enzymatically under physiological circumstances in the liver and – to a greater proportion – in the kidney.
It is well known that high levels of oxidative stress are present in patients with end-stage renal failure (ESRF).Citation12–Citation14 There are data proving that inflammation and duration of dialysis treatment are the most important factors in the development of oxidative stress in these patients.Citation14 In our previous studies we have shown that levels of oxidatively modified derivatives of phenylalanine (such as ortho-tyrosine) were significantly elevated among such circumstances when oxidative stress develops in the human body. A higher ortho-tyrosine concentration was measured in cataractous lenses in comparison with that of the control group.Citation15 In another study of our workgroup, plasma and urine levels of para- and ortho-tyrosine were measured in patients with chronic kidney disease (CKD) and/or diabetes (DIAB-CKD/DIAB). Urinary ortho-tyrosine excretion was higher in all groups of patients than in the control group, and higher in DIAB patients and DIAB-CKD patients than in CKD patients.Citation16 Formerly we had also observed that the total urinary albumin/creatinine ratio and urinary non-immunoreactive albumin/creatinine ratio show a significant correlation with the urinary ortho-tyrosine/creatinine ratio in patients with acute ischemic stroke.Citation17 Finally, administration of natural antioxidant resveratrol significantly decreased ortho-tyrosine excretion and improved insulin resistance in patients with type 2 diabetes mellitus.Citation18 Vivekanadan-Giri et al.Citation19 found the ortho-tyrosine level to be elevated in the aortic proteins of diabetic monkeys.
It is known that structural analogs of para-tyrosine, such as 3-iodotyrosine, 3-fluorotyrosine, and 3,4-dihydroxy-l-phenylalanine, are incorporated by post-transcriptional mechanisms into structural or functional proteins of tissues.Citation20 Gurer-Orhan et al.Citation21 have shown that the cytotoxicity of another hydroxyl free radical product of phenylalanine, meta-tyrosine, may be mediated by the concentration-dependent integration of this abnormal amino acid into cellular proteins. Furthermore, both meta-tyrosine and ortho-tyrosine inhibited tumor cell proliferation in different murine models of cancer. It has been shown that the antitumor effects of these two pathological tyrosine isoforms were mediated, in part, by early inhibition of the mitogen-activated protein/extracellular signal-regulated kinase (MAP/ERK) pathway and inactivation of STAT3.Citation22
Intracellular signal transduction of the EPO-receptor (EPO-R) is well described, but complex.Citation23,Citation24 The receptor is activated on eight cytoplasmic tyrosine domains, and continued in the downstream signal of the JAK2/STAT1–5 pathway.Citation23 EPO also stimulates the catalytic activity of several mitogen-activated protein kinases such as extracellular-regulated kinases 1/2 (Erk1/2).Citation24 Tyrosine phosphorylation plays an essential role in both of the above-mentioned signaling pathways.Citation23–Citation25 Moreover, EPO-R activation may cause hydroxyl free radical production through stimulation of the NADPH-oxidase enzyme.Citation26 We hypothesized that altered availability of para- and ortho-tyrosine may be – in part – the cause and mediator of decreased ESA-responsiveness observed in patients on dialysis.
Materials and methods
Subjects
Four groups of volunteers were involved in our cross-sectional study. We selected 69 patients from our dialysis unit (University of Pécs 2nd Department of Medicine and Nephrology Center, Fresenius Medical Care) and 16 healthy volunteers (CONTR, median age 40 years, eight males/eight females, data not shown in the table). Patients with acute infections, malignancy, and active autoimmune disease were excluded from the study. Forty-eight patients were treated with hemodialysis (HD), while 21 with continuous peritoneal dialysis (CAPD). The group was included in the cross-sectional study because they were known to respond well to ESA and have better survival . Out of the hemodialyzed patients, 8 did not receive ESA (non-ESA-HD), while 40 were treated with ESA (ESA-HD). They were administered intravenous darbepoietin-alpha (DA) weekly or twice a month. Patients on CAPD were receiving subcutaneous DA. Hemodialyzed patients had been undergoing regular HD three times a week for 4 hours. In all cases we used an FX 50 or 60 Cor Diax high-flux dialyser with Helixone® membrane (Fresenius Medical Care), and a blood flow rate of 180–400 ml/min. The study was approved by the Ethical Committee of the Medical Faculty of the University of Pécs. All patients had given their informed consent.
Routine analytical procedures
Hemoglobin, hematocrit, red blood cell count, serum sodium, potassium, calcium, phosphate, parathyroid hormone (PTH), iron, transferrin, transferrin saturation, ferritin, creatinine, blood urea nitrogen, high sensitivity C-reactive protein (hsCRP), total protein, albumin, total cholesterol, HDL-cholesterol, and triglyceride levels were determined by standard laboratory techniques. All of the blood samples were drawn just before the actual HD-treatment. ESA-demand was expressed using different ESA indices such as ERI1 = ESA-dose/body weight/hemoglobin; ERI2 = ESA-dose/body weight/hematocrit; ERI3 = ESA-dose/body weight/red blood cell count.
High performance liquid chromatography (HPLC) analysis
Blood samples were obtained by way of vein puncture, in tubes containing EDTA. Plasma was obtained by centrifugation and was stored at −80°C pending further examination. Thereafter 125 µl trichloro-acetic acid (TCA) was added to 500 µl plasma and then samples were incubated on ice for 30 minutes. Subsequently precipitate was separated by centrifugation. The supernatant was filtered by a syringe filter (0.2 µm) before analysis. Finally plasma ortho-, para-tyrosine, and phenyalanine levels were determined using reverse phase-HPLC (C18 silica column, 250 × 4 mm) with fluorescence detection (λEX = 275 nm; λEM = 305 nm for the tyrosines and λEX = 258 nm; λEM = 288 nm for phenylalanine) as described earlier.Citation16 Concentrations were calculated using an external standard.
Investigating ESA-effect on ortho-tyrosine level
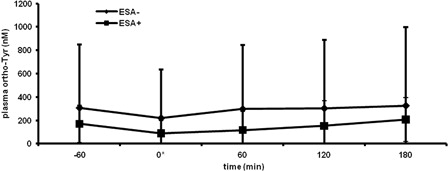
Five patients of the ESA-HD group were included in a longitudinal analysisthat consisted of two sessions. The first session was a HD without DA-treatment (ESA−), while the second session of the same patients was a HD with DA-treatment (ESA+ ; mean 28 µg of DA) at time point ‘0’. Blood samples were obtained five times (60 minutes before the end of HD (−60), at the end of HD (0) and 60, 120 and 180 minutes after the end of HD). The time kinetics of plasma ortho-tyrosine levels at both sessions was examined.
Statistical analysis
Central tendency and width of distribution were expressed using mean ± SD in the cases of normally and, using median(interquartile range), in case of non-normally distributed data. Kolmogorov–Smirnov test was used to test normality. Differences between groups were analyzed using ANOVA with Bonferroni post hoc test (normal distribution) or using Kruskal–Wallis and Mann–Whitney U tests (non-normal distribution). χ2 test was used to compare groups in the case of categoric variables. Correlations were calculated using the Spearman correlation. Linear regression analysis was performed using a stepwise method. P values <0.05 were considered to be statistically significant. All statistical analyses were performed with SPSS 20 software (IBM).
Results
Patient characteristics
Baseline characteristics of the dialyzed patients can be seen in . Among the hemodialyzed patients, diabetic nephropathy (36%) was the leading cause of ESRF. Other causes were benign nephrosclerosis (19%), polycystic kidney disease (13%), primary glomerulopathy (10%), chronic pyelonephritis (10%), and chronic tubulointerstitial nephritis (6%). In three cases (6%) the cause of ESRF remained unknown. Among patients with CAPD, polycystic kidney disease (29%) was most commonly found to be behind ESRF. Further causes were nephrosclerosis (24%), diabetic nephropathy (19%), primary glomerulopathy (14%), and chronic pyelonephritis (9%). In one case (5%) contrast media nephropathy led to ESRF.
Table 1. Clinical parameters of dialysis patients
Levels of plasma para-tyrosine
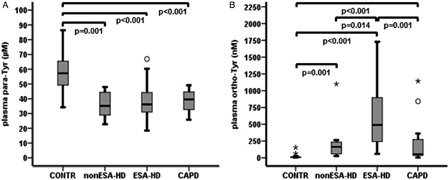
Plasma para-tyrosine levels were significantly lower in all groups of dialyzed patients than in the control subjects (non-ESA-HD, 35.10(17.24) µM, P = 0.001; ESA-HD, 36.25(13.67) µM, P < 0.001; CAPD, 39.53 (13.20) µM, P < 0.001; CONTR, 57.24(18.19) µM), but there was no difference among non-ESA-HD, ESA-HD, and CAPD groups (A).
Levels of plasma ortho-tyrosine and ortho-tyrosine/para-tyrosine ratio
We detected significantly higher plasma ortho-tyrosine levels in groups of dialyzed patients (non-ESA-HD, 162.97(191.24) nM, ESA-HD, 489.92(726.85) nM, CAPD, 54.19(262.91) nM) than in the control subjects (CONTR, 9.74(9.93) nM, all P < 0.001). Plasma ortho-tyrosine levels were significantly lower in non-ESA-HD and CAPD groups than in the ESA-HD group (P = 0.014 and 0.001, respectively), while there was no significant difference between non-ESA-HD and CAPD patients (B).
Using the plasma ortho-tyrosine/para-tyrosine ratio, we found the same pattern as in case of plasma ortho-tyrosine, i.e. significantly higher plasma ortho-tyrosine/para-tyrosine ratio was detected in dialyzed patients (non-ESA-HD, 5.1302(5.2187) nM/μM, ESA-HD, 12.6595(24.2529) nM/μM, CAPD, 1.2364(6.1893) nM/μM) than in the control subjects (CONTR, 0.1684(0.1820) nM/μM, all P < 0.001). Also plasma ortho-tyrosine/para-tyrosine ratios were significantly lower in non-ESA-HD and CAPD groups than in the ESA-HD group (P = 0.018 and P < 0.001, respectively), while we found no significant difference between non-ESA-HD and CAPD patients (data not shown).
Association between ESA-demand and plasma ortho-tyrosine level or ortho-tyrosine/para-tyrosine ratio
There was a significant positive correlation between weekly ESA-dose/body weight and plasma ortho-tyrosine level (r = 0.411; P = 0.002) (A) and a somewhat stronger correlation between weekly ESA-dose/body weight and plasma ortho-tyrosine/para-tyrosine ratio (r = 0.441; P = 0.001) (B). Moreover, ERI1 proved to be in strong correlation with both the plasma ortho-tyrosine level (r = 0.400; P = 0.002) (C) and the plasma ortho-tyrosine/para-tyrosine ratio (r = 0.434; P = 0.001) (D). As the distribution of ortho-tyrosine level and ortho-tyrosine/para-tyrosine ratio was not normal, these variables had been converted to natural logarithm.
Figure 2. Correlations between [A] plasma ortho-tyrosine or [B] plasma ortho-tyrosine/para-tyrosine and ESA-dose/body weight. Correlations between [C] plasma ortho-tyrosine or [D] plasma ortho-tyrosine/para-tyrosine and ERI1 = ESA-dose/body weight/hemoglobin. ortho-Tyr, ortho-tyrosine; para-Tyr, para-tyrosine.
![Figure 2. Correlations between [A] plasma ortho-tyrosine or [B] plasma ortho-tyrosine/para-tyrosine and ESA-dose/body weight. Correlations between [C] plasma ortho-tyrosine or [D] plasma ortho-tyrosine/para-tyrosine and ERI1 = ESA-dose/body weight/hemoglobin. ortho-Tyr, ortho-tyrosine; para-Tyr, para-tyrosine.](/cms/asset/e7209608-6910-4fad-ae8e-6418b0143c03/yrer_a_11743416_f0002_b.jpg)
We examined the predictors of ESA-demand in a multivariate regression analysis. ESA-demand was expressed in terms of ESA-dose, ESA-dose/body weight, and different ESA-indices such as ESA-dose/body weight/hemoglobin, ESA-dose/body weight/hematocrit or ESA-dose/body weight/red blood cell count (ERI1; ERI2; ERI3, respectively) in different models. We calculated plasma para-tyrosine/phenylalanine, plasma ortho-tyrosine/phenylalanine, and plasma ortho-tyrosine/para-tyrosine ratios. These three different tyrosine values were included separately in models, together with already known predictors of ESA-demand. In the first model serum calcium (se-Ca2+), serum phosphate (se-PO43−), parathormone (PTH), restdiuresis, and serum ferritin; while in the second model age, dialysis duration, high sensitivity C-rective protein (hsCRP), restdiuresis, and serum ferritin were included besides the actual tyrosine value. As a result neither para-tyrosine/phenylalanine nor ortho-tyrosine/phenylalanine ratio proved to be an independent predictor in these two models. In almost all cases restdiuresis was the independent predictor (Tables and ). In contrast, when the ortho-tyrosine/para-tyrosine ratio was included into the model, it proved to be an independent predictor of the ESA-dose and ESA-indices in both models (). Finally, we set a third model where kt/v was also included among age, PTH, hsCRP, serum ferritin, and ortho-tyrosine/para-tyrosine ratio. Also in this case ortho-tyrosine/para-tyrosine ratio proved to be the independent predictor (β = 0.292; P = 0.042) of ESA-dose/body weight (data not shown in the table).
Table 2. Predictors of ESA-demand (plasma para-tyrosine/phenylalanine ratio included)
Table 3. Predictors of ESA-demand (plasma ortho-tyrosine/phenylalanine ratio included)
Table 4. Predictors of ESA-demand (plasma ortho-tyrosine/para-tyrosine ratio included)
ESA-effect on plasma ortho-tyrosine level
A potential explanation for the observed associations shown in the previous part could be a formation of HO• due to ESA-administration. In that regard, larger ESA doses would not be the consequence, but rather the cause of oxidative stress-related amino acid modification. To rule this out, in a longitudinal analysis, time kinetics of plasma ortho-tyrosine levels were compared at two hemodialysis sessions, in the absence of ESA-treatment (ESA−), and in the presence of ESA-therapy (ESA+). As a result, no significant difference was found between the two curves ().
Figure 3. Time kinetics of plasma ortho-tyrosine level when DA is not administered (ESA−) and when administered (ESA+). *, end of HD, at ESA+ the time of DA administration. Pairwise comparisons of ESA− and ESA+ at each time point were not significant. Data represent mean ± SD. ortho-Tyr, ortho-tyrosine.
Discussion
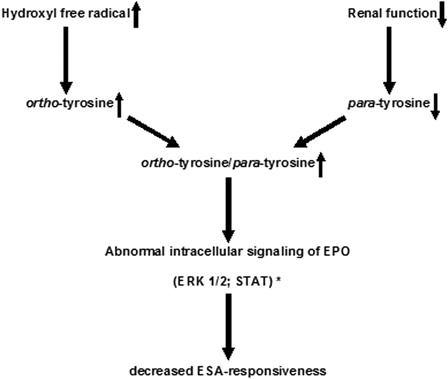
In this study we have highlighted the importance of ortho- and para-tyrosine in decreased ESA-responsiveness. Plasma para-tyrosine levels were significantly lower, while plasma ortho-tyrosine levels and ortho-tyrosine/para-tyrosine ratio were significantly higher in dialyzed patients compared to controls. Furthermore, plasma ortho-tyrosine level and ortho-tyrosine/para-tyrosine ratio were significantly lower in the non-ESA-HD and CAPD groups than in the ESA-HD group. The ortho-tyrosine level and ortho-tyrosine/para-tyrosine ratio showed a significant and positive correlation with weekly ESA-dose/body weight and ERI1. The most important finding is that plasma ortho-tyrosine/para-tyrosine ratio proved to be an independent predictor of ESA-dose, ESA-dose/body weight, and ESA-indices calculated in different ways in such models, where most of the known predictors of ESA-hyporesponsiveness were also included. Neither the plasma ortho-tyrosine/phenylalanine nor the para-tyrosine/phenylalanine ratio were independent predictors of ESA-demand in both models, suggesting that neither an elevation of the ortho-tyrosine level or a decreas in the para-tyrosine level per se is sufficient predictor of the raising ESA-demand, but that combined and inverse alteration of these amino acid isomers is in a strong association with decreased ESA-responsiveness. This may mean that among circumstances when pathological ortho-tyrosine concentration is increased and at the same time physiological para-tyrosine is lowered (e.g. in ESRF), translational integration of ortho-tyrosine instead of para-tyrosine into the signaling proteins could change the intracellular outcome of EPO-receptor activation, causing lowered ESA-responsiveness (). To rule out a confounder effect, finally we provided evidence that ESA-treatment neither increases nor suppresses HO•-production. In a previous study, lipid peroxidation products as malondialdehyde and 4-hydroxynonenal were not elevated in patients with long-term rHuEPO-treatment, leading the authors to conclude that rHuEPO-treatment per se does not inhibit oxidative stress.Citation27
Figure 4. Possible mechanism of development of ESA-hyporesponsiveness in ESRF patients via inhibition of intracellular signaling pathways. * According to the findings of Ruggiero et al.22
In another post hoc study based on CHOIR trial, higher epoetin-alfa doses were associated with increased risks for cardiovascular events, suggesting that epoetin-alfa could have a cardiovascular toxic effect.Citation28 However, our findings raise the possibility of a different mechanism, since an elevated level of hydroxyl radical-derived products may cause decreased responsiveness to ESAs, leading to higher ESA-demand, and that this could also be at the background of higher risk for cardiovascular events. Namely, there is evidence that these products can interfere with the MAP/ERK signaling pathway and that direct inhibition of ERK1/2 can lead to exacerbated cardiomyocyte death and impaired cardiac function.Citation22,Citation29
The association between oxidative stress and ESA-hyporesponsiveness has been investigated in several further studies. Serum and erythrocyte thiobarbituric acid reactive substances (TBARS) and serum superoxide and hydroxyl radical scavenging activities were measured in hemodialysis patients receiving different doses of rHuEPO. Higher TBARS levels were found in erythrocytes of patients receiving high doses of rHuEPO (9000 U/week) as compared with patients with no ESA-treatment, while no significant difference was found in serum TBARS levels. A diminution of serum hydroxyl radical scavenging activity was observed in subjects with high-dose rHuEPO treatment.Citation30 This finding is in agreement with ours, since such a suppressed hydroxyl free radical scavenging activity could lead to elevated hydroxyl radical levels, resulting in higher levels of oxidative derivates of phenylalanine (as ortho-tyrosine).
In another study the serum level of the hydroxyl free radical-DNA product 8-hydroxy-2′-deoxyguanosine (8-OHdG) was examined in patients on HD with different weekly dosages of rHuEPO. A significantly higher 8-OHdG level was measured in HD patients when compared with that of healthy control subjects. 8-OHdG was positively correlated with patients' age, but not with dialysis duration. Furthermore, 8-OHdG showed a strong positive correlation with the rHuEPO dose and the ratio of the weekly rHuEPO dose/hemoglobin. All of these data are similar to that found in our study and neither their study nor ours proved any correlation with inflammatory or nutritional markers.Citation31
Although there are data proving that ESA-hyporesponsiveness and diabetes commonly appear together and diabetic patients often require a higher ESA-dose, in our study we did not find a significant difference in the frequency of diabetes between non-ESA-HD and ESA-HD groups. Also, no correlation was found between daily insulin doses and ESA-doses or ESA-indices, although these may be due to the relative low number of diabetic cases in our study.
Our findings may suggest that it was not oxidative stress or hydroxyl free radical per se causes that decreased ESA-responsiveness, but that rather the replacement of para-tyrosine with ortho-tyrosine (e.g. in signaling proteins) could be responsible for lowered ESA-responsiveness. In this regard ortho-tyrosine may be a pathogenetic factor: a maker and not only a marker of ESA-hyporesponsiveness.
There are several limitations in our study. The first limitation is the relatively small number of cases under observation. Further large, multicenter and, if possible international studies are needed for significant evaluation of this association. The second limitation is the partially cross-sectional character of our study. Prospective and/or interventional studies are needed to verify the causative association between higher plasma ortho- and lower para-tyrosine levels and decreased ESA-responsiveness.
We believe that the evaluation of the association between plasma ortho-tyrosine/para-tyrosine ratio and ESA-responsiveness may be important since therapeutic interventions decreasing oxidative stress or lowering level of substances produced by oxidative stress (e.g. ortho-tyrosine) and/or supplementation of the physiological para-tyrosine may improve ESA-responsiveness.
Disclaimer statements
Funding
None.
Conflict-of-Interest
None.
Ethics Approval
The study was approved by the Ethical Committee of the Medical Faculty of the University of Pécs.
Acknowledgments
The authors thank Krisztina Szalma and Mrs Ilona Sámikné Varga for excellent assistance.
References
- Van der Putten K, Braam B, Jie KE, Gaillard CA. Mechanisms of disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Nephrol 2008;4:47–57.
- Bamgbola O. Resistance to erythropoietin-stimulating agents: etiology, evaluation, and therapeutic considerations. Pediatr Nephrol 2012;27:195–205.
- Locatelli F, Covic A, Macdougall IC, Wiecek A. ORAMA: a study to investigate EBPG impact on renal anaemia – design and baseline data. J Nephrol 2008;21:592–603.
- KDIGO. KDIGO clinical practice guideline for anemia in chronic kidney disease; 2012. Available at: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO-Anemia%20GL.pdf
- Johnson DW, Pollock CA, Macdougall IC. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology (Carlton) 2007;12:321–30.
- Rossert J, Gassmann-Mayer C, Frei D, McClellan W. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol Dial Transplant 2007;22:794–800.
- Abe M, Okada K, Soma M, Matsumoto K. Relationship between insulin resistance and erythropoietin responsiveness in hemodialysis patients. Clin Nephrol 2011;75:49–58.
- Rudich A, Kozlovsky N, Potashnik R, Bashan N. Oxidant stress reduces insulin responsiveness in 3T3-L1 adipocytes. Am J Physiol 1997;272:935–40.
- Ceriello A. Oxidative stress and glycemic regulation. Metabolism 2000;49:27–9.
- Stadtman ER, Berlett BS. Fenton chemistry – amino acid oxidation. J Biol Chem 1991;266:17201–11.
- Galano A, Cruz-Torres A. OH radical reactions with phenylalanine in free and peptide forms. Org Biomol Chem 2008;6:732–8.
- Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant 2003;18:1272–80.
- Massy ZA, Nguyen-Khoa T. Oxidative stress and chronic renal failure: markers and management. J Nephrol 2002;15:336–41.
- Nguyen-Khoa T, Massy ZA, De Bandt JP, Kebede M, Salama L, Lambrey G, et al. Oxidative stress and haemodialysis: role of inflammation and duration of dialysis treatment. Nephrol Dial Transplant 2001;16:335–40.
- Molnár GA, Nemes V, Bíró Z, Ludány A, Wagner Z, Wittmann I. Accumulation of the hydroxyl free radical markers meta-, ortho-tyrosine and DOPA in cataractous lenses is accompanied by a lower protein and phenylalanine content of the water-soluble phase. Free Radic Res 2005;39:1359–66.
- Molnár GA, Wagner Z, Markó L, Kőszegi T, Mohás M, Kocsis B, et al. Urinary ortho-tyrosine excretion in diabetes mellitus and renal failure: evidence for hydroxyl radical production. Kidney Int 2005;68:2281–7.
- Tóth P, Koller A, Pusch G, Bosnyák E, Szapáry L, Komoly S, et al. Microalbuminuria, indicated by total versus immunoreactive urinary albumin in acute ischemic stroke patients. J Stroke Cerebrovasc Dis 2011;20:510–6.
- Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr 2011;106:383–9.
- Vivekanadan-Giri A, Wang JH, Byun J, Pennathur S. Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage. Rev Endocr Metab Disord 2008;9:275–87.
- Joniau M, Coudijzer K, De Cuyper M. Reaction of alpha-tubulin with iodotyrosines catalyzed by tubulin:tyrosine ligase: carboxy-terminal labeling of tubulin with [125I]monoiodotyrosine. Anal Biochem 1990;184:325–9.
- Gurer-Orhan H, Ercal N, Mare S, Pennathur S, Orhan H, Heinecke JW. Misincorporation of free m-tyrosine into cellular proteins: a potential cytotoxic mechanism for oxidized amino acids. Biochem J 2006;395:277–84.
- Ruggiero RA, Bruzzo J, Chiarella P, di Gianni P, Isturiz MA, Linskens S, et al. Tyrosine isomers mediate the classical phenomenon of concomitant tumor resistance. Cancer Res 2011;71:7113–24.
- Watowich SS. The erythropoietin receptor: molecular structure and hematopoietic signaling pathways. J Investig Med 2011;59:1067–72.
- Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol 2005;15:146–55.
- Ihle JN, Thierfelder W, Teglund S, Stravapodis D, Wang D, Feng J, et al. Signaling by the cytokine receptor superfamily. Ann NY Acad Sci 1998;865:1–9.
- Sharma P, Chakraborty R, Wang L, Tremblay ML, Kawahara T, Lambeth JD, et al. Redox regulation of interleukin-4 signaling. Immunity 2008;29:551–64.
- Sommerburg O, Grune T, Hampl H, Riedel E, van Kuijk FJ, Ehrich JH, et al. Does long-term treatment of renal anaemia with recombinant erythropoietin influence oxidative stress in haemodialyzed patients? Nephrol Dial Transplant 1998;13:2583–7.
- McCullough PA, Barnhart HX, Inrig JK, Reddan D, Sapp S, Patel UD, et al. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol 2013;37:549–58.
- Ruppert C, Deiss K, Herrmann S, Oezkur M, Gorski A, Weidemann F, et al. Interference with ERK(Thr188) phosphorylation impairs pathological but not physiological cardiac hypertrophy. Proc Natl Acad Sci USA 2013;110:7440–5.
- Hirayama A, Nagase S, Gotoh M, Ishizu T, Yoh K, Aoyagi K, et al. Reduced serum hydroxyl radical scavenging activity in erythropoietin therapy resistant renal anemia. Free Radic Res 2002;36:1155–61.
- Kato A, Odamaki M, Hishida A. Blood 8-hydroxy-2'-deoxyguanosine is associated with erythropoietin resistance in haemodialysis patients. Nephrol Dial Transplant 2003;18:931–6.