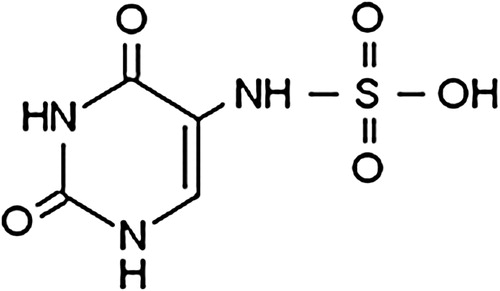
Abstract
Objective
This paper studies the kinetics and mechanism of the sensitized photodegradation of 5-sulfaminouracil (SFU). This compound is a synthetic bacteriostatic belonging to the sulfa drugs, usually detected in surface water and effluent wastewater.
Methods
SFU interacts with electronically excited singlet and triplet states of riboflavin (Rf), 1Rf* and 3Rf*, respectively. The rate constants for these processes were determined in MeOH–H2O by stationary fluorescence spectroscopy (for kQ(1)) and by laser flash photolysis (for kQ(3)).
Results
SFU is photodegraded by visible light irradiation in the presence of the natural sensitizer (Rf). In competitive processes, 3Rf* generates the reactive oxygen species superoxide radical anion (O2•−) and singlet molecular oxygen [O2(1Δg)], which are involved in SFU photodegradation. In aqueous solutions, where SFU adopts different forms depending on the pH of the medium, the participation of O2(1Δg) is predominant. Therefore, the O2(1Δg)-mediated mechanism was evaluated at pHs 7 and 12, employing perinaphthenone as a synthetic photosensitizer.
Discussion
The results indicate that SFU is photodegraded through a relatively efficient process in a neutral environment, whereas it is quickly degraded in alkaline media. This is attributed to the ionization of the sulfamino- group, a substituent in the uracil molecule that exerts an activating power on the molecule. Thus, sensitized photodegradation may be an important tool to reduce the environmental impact of SFU.
Introduction
The importance of uracil derivatives in biological processes, as well as their bioactivity, has been considered by several authors.Citation1–Citation3 In particular, the 5-substituted uracils, such as the 5-halogenated uracils, exhibit antibacterial activity and have been widely used in the treatment of cancer.Citation4
5-Sulfaminouracil (SFU) () belongs to a family of synthetic antibiotics known as sulfonamides or sulfa drugs (SDs). These compounds have played an important role in both human and veterinary medicine.Citation5,Citation6
It is well known that some of these compounds are poorly metabolized and so excreted unchanged, causing their subsequent incorporation into the environment. Therefore, they become potential micropollutants of living organisms in aquatic ecosystems.
SDs have been detected in all kinds of environmental waters,Citation7,Citation8 not only in wastewater effluentsCitation9 but also in surface and groundwater at concentrations up to a few micrograms/liter.Citation10–Citation12
Once antibiotics reach an environmental system, they may undergo different degradation processes, which is very important to limit their presence in the environment. For example, biodegradation and non-biotic elimination processes such as absorption, hydrolysis, oxidation, reduction, and photodegradation have been documented.Citation13 SDs present on surface waters do not absorb visible light. However, there are daylight-absorbing substances called photosensitizers, like riboflavin (Rf), which contribute to the process of photodegradation. Rf is a pigment found in practically all types of natural waters.Citation14
Previous studies have demonstrated that different SDs can be degraded through direct photolysis (by exposure to UV lightCitation15,Citation16) or through indirect photolysis (via sensitized photo-processes). This degradation happens in the presence of either dissolved organic matter or other reactive species that can be formed in natural surface waters.Citation17–Citation19
Regarding the uracil molecule, which is part of the SFU structure, it has been reported by our groupCitation20 that its sensitized photodegradation takes place with the involvement of reactive oxygen species (ROS) such as singlet molecular oxygen [O2(1Δg)] and superoxide radical anion (O2•−), both photogenerated from the natural sensitizer Rf.
We also have demonstrated in previous research work into the interaction of ROS with some SDs derived from sulfanilic acid that sulfisoxasol is efficiently photodegraded by indirect photolysis in the presence of natural and synthetic dyes.Citation21
In this work, we study the kinetics and mechanism of the sensitized photodegradation of SFU under conditions frequently found in nature: in aqueous environments, in the presence of natural photosensitizers, and under visible light illumination. This research is a contribution towards the possible elimination of this bacteriostatic compound from aquatic environments.
Materials and methods
Chemicals
SFU, Rf, D2O (99.9%), superoxide dismutase (SOD) from bovine erythrocytes, l-tryptophan (Trp), and sodium azide (NaN3) were purchased from Sigma Chemical Co. (St Louis, USA), perinaphthenone (PN) was purchased from Aldrich (Steinheim, Germany), and furfuryl alcohol from Riedel deHaën (Buchs, SG Switzerland). Water was triple distilled, and MeOH (high-performance liquid chromatography quality) was purchased from Sintorgan (Buenos Aires, Argentina). In some experiments, water as a solvent was replaced with 1:1 (v/v) MeOH–H2O in order to increase the solubility of SFU. D2O was employed instead of H2O in time-resolved phosphorescence detection (TRPD) experiments to lengthen O2(1Δg) lifetime.Citation22 The pHs/pDs 7 and 12 buffered solutions were prepared according to a reported procedure.Citation23
All experiments were carried out at room temperature and with freshly prepared solutions.
Methods
SFU stationary photolysis in aqueous solutions with PN or Rf sensitizers was performed in a homemade photolyser for non-monochromatic irradiation (150 W quartz-halogen lamp), using a cut-off filter of 400 nm in order to ensure that the light was only absorbed by sensitizers.
Ground-state absorption spectra were recorded in a Hewlett-Packard 8452A diode array spectrophotometer. Stationary fluorescence experiments were performed with a Shimadzu RF5301-PC spectrofluorimeter at 25°C in air-equilibrated solutions. Excitation and emission wavelengths for Rf were 445 and 515 nm, respectively. The interaction of the excited singlet state, Citation1Rf*, with SFU was evaluated using the Stern–Volmer treatment: Io/I = 1 + KSV[SFU], where KSV (Stern–Volmer constant) can be obtained by determining the static fluorescence intensity of Rf in the presence (I) and in the absence (Io) of SFU.
Laser flash photolysis was used to determine the quenching rate constant, kQ(3), of 3Rf*–SFU interaction (process 7) by means of a previously reported apparatus.Citation24 Nitrogen-saturated aqueous solutions of Rf (0.01 mM) were photolyzed using the same technique. The disappearance of 3Rf*, generated by laser pulse (355 nm), was monitored from the first-order decay of the absorbance at 670 nm, a zone where the interference from other possible species is negligible. The decay of 3Rf* was measured at low Rf concentration and at low laser energy to avoid undesirable effects such as self-quenching and triplet–triplet annihilation. 3Rf* lifetime was evaluated in the presence (τ3) and in the absence (°τ3) of SFU. The kQ(3) was obtained from the Stern–Volmer expression: 1/τ3 = 1/°τ3 + kQ(3) [SFU].
In order to obtain SFU concentrations higher than those allowed by the solubility of SFU in water, a solvent mixture, MeOH–H2O (1:1, v/v), was employed to evaluate the interactions of the bacteriostatic with singlet and triplet excited states of Rf.
TRPD was used to determine the overall quenching rate constant of O2(1Δg) by SFU, kt, in the system described below. Basically, O2(1Δg) phosphorescence at 1270 nm, generated by excitation with the emission (355 nm) from a Nd:YAG laser (Spectron), was detected at right angles using an amplified Judson J16/8Sp Germanium detector, after passing through suitable filters. The detector output was coupled to a digital oscilloscope and to a personal computer for signal processing. To obtain a good signal-to-noise ratio, 16 shots were usually averaged, from which decay times were calculated. Within this context, sensitizer PN displayed an A355 ≅ 0.4.
O2(1Δg) lifetimes were evaluated in the presence (τ) and in the absence (τ°) of the quencher, SFU, and the data were plotted as a function of SFU concentration according to the Stern–Volmer treatment: τ°/τ = 1 + kt τ°[SFU].
The relative rates of oxygen consumption (Vox) upon PN-sensitized irradiation were determined from the initial slopes in graphs that show oxygen consumption vs. irradiation time, employing a specific oxygen electrode (Orion 97-08).
The values of the reactive rate constants (kr) were determined by a comparative method,Citation25 with PN as photosensitizer and Trp as reference. Basically, solutions containing PN (Abs364 = 0.5) and SFU (0.4 mM) or Trp (0.4 mM) were irradiated (150 W quartz-halogen lamp) under identical conditions using a cut-off filter (400 nm) in order to assure that radiation was only absorbed by PN. Consumption of substrate was followed by spectroscopy. Under these conditions, kr values were determined from the expression kr/krR = slope/slopeR, where slope and slopeR are the respective slopes of first-order plots showing absorbance (or fluorescence emission) decrease vs. irradiation time of SFU and Trp, assuming that only the reaction of SFU with O2(1Δg) leads to SFU consumption.
Results
Difference spectra (SFU + Rf) vs. Rf showed no modifications within a concentration range used for the photochemical experiments, indicating that there is no ground-state association between SFU and Rf.
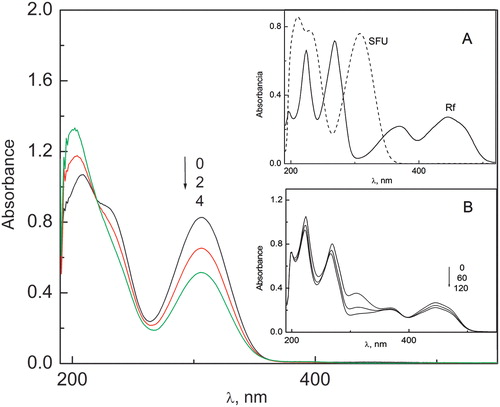
After visible light irradiation of solutions containing SFU 0.3 mM and Rf 0.02 mM under aerobic conditions, changes in the absorption spectra of both compounds were observed ().
Figure 2. Spectral changes of SFU (0.3 mM) vs. Rf (0.02 mM) in MeOH–H2O 1:1 (v/v) solutions upon irradiation under aerobic conditions. (A) Absorption spectra of Rf and SFU vs. MeOH:H2O. (B) Spectral changes of SFU (0.3 mM) + Rf (0.02 mM) in MeOH–H2O (1:1, v/v) solutions upon irradiation under aerobic conditions. Numbers above the spectrum indicate irradiation time in minutes.
Oxygen consumption was detected when solutions of MeOH–H2O 1:1 (v/v) containing Rf 0.04 mM and SFU 0.5 mM were irradiated under aerobic conditions. The rate of oxygen consumption clearly diminished in the presence of the enzyme SOD 14 mg/ml (see ). This decrease in the rate of oxygen consumption strongly suggests the involvement of O2•− in the process. SOD is frequently employed as an auxiliary reactive in order to confirm or deny the participation of O2•− in a certain reaction.Citation26,Citation27
Figure 3. Consumed oxygen vs. irradiation time in MeOH–H2O (1:1, v/v) solution. Solutions were irradiated with visible light (>400 nm) under air-saturated conditions in the presence of 0.04 mM Rf: (a) with SFU 0.4 mM and (b) with SFU 0.4 mM plus 14 µg/ml SOD.
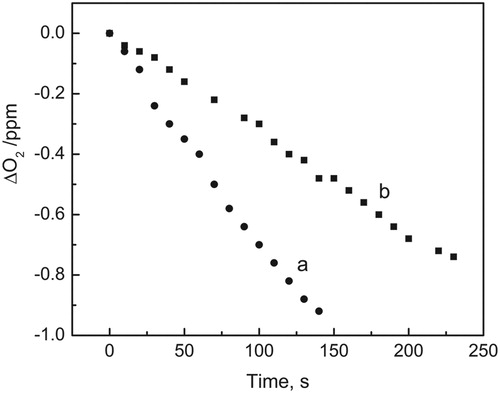
The relative rates of oxygen consumption of SFU under Rf sensitization in the absence and presence of SOD are shown in .
Table 1. Rate constants for the quenching of Citation1Rf*, kQ(1) (M−1 s−1) and 3Rf*, kQ(3) (M−1 s−1) by SFU in MeOH–H2O 1:1 (v/v) and relative rates of oxygen consumption by SFU upon Rf sensitization of solutions in the absence [Vox(Rf)] and in the presence of SOD 14 µg/ml [Vox(Rf + SOD)]
The photolysis rate of solutions containing Rf 0.02 mM, in the absence of oxygen, diminished in the presence of low SFU concentration (0.05 mM). Spectral changes are shown in , suggesting the existence of an interaction of SFU with 3Rf*, the excited triplet state.
Figure 4. Evolution of the 445 nm band of Rf in MeOH–H2O (1:1, v/v) (deareated) upon irradiation with visible light. (a) Rf (0.02 mM) not irradiated, (b) Rf (0.2 mM) + SFU (0.05 mM) irradiated for 6 minutes, (c) Rf (0.02 mM) irradiated for 6 minutes. Inset: Sterrn–Volmer plot for the quenching of Rf triplet state by SFU in argon-saturated solutions of MeOH–H2O 1:1 (v/v).
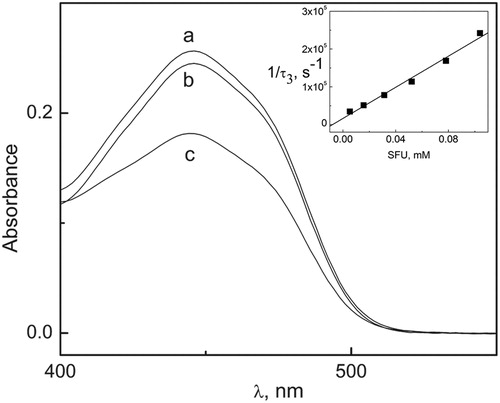
On the basis of the results described above, after visible light irradiation of SFU solutions with sensitizer Rf, three experimental facts can be established: (a) Rf and SFU are degraded; (b) such degradation involves ROS; and (c) SFU interacts with Rf-electronically excited states.
Different processes involved in the generation of ROS can be described as follows:
Rf absorbs light and initially generates the singlet excited state, Citation1Rf* (process 1), which can decay to the ground state (process 2) or which can be quenched by SFU (process 4). Also, through a process of intersystem crossing, Citation1Rf* can generate 3Rf* (process 3), which decays to the ground state or yields products (process 5). 3Rf* can be quenched by ground-state oxygen, O2(3Σg−), dissolved in the medium, generating O2(1Δg) by energy transfer (process 6), or it can be quenched by SFU, yielding the semi-reduced species Rf•− (process 7).
3
4
5
6
7
Rf•− can generate O2•− by electron transfer to O2(3Σg−) (process 8). At physiological pH, Rf•− can be protonated to form the radical RfH• (pK = 8.3).Citation28 The bimolecular decay of RfH• is known to proceed through a disproportionation reaction, yielding Rf and fully reduced Rf (RfH2) (process 9). In the presence of O2(3Σg−), RfH2 is reoxidized, giving rise to radicals RfH2•+ and O2•− and, eventually, Rf and H2O2 (process 10).Citation29,Citation30 The semiquinone radical RfH• has been detected as a product of electron transfer processes to 3Rf* from different electron-donor substrates of environmental and biological importance.Citation28,Citation31,Citation32
The O2•− generated through process (8) or/and (10) can react with SFU yielding oxidized products (process 11).
8
9
10
11
Interaction between SFU and Rf-electronically excited states
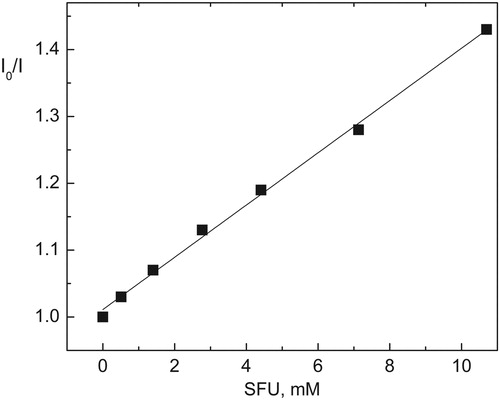
To obtain SFU concentrations higher than those allowed by the solubility of SFU in water, the solvent mixture MeOH–H2O (1:1, v/v) was employed to determine the interactions of the bacteriostatic with singlet and triplet excited states of Rf. Likewise, in order to study the interaction between Citation1Rf* and SFU (process 4), the fluorescence spectra were evaluated. Rf exhibits a fluorescence emission band centered at 515 nm, with a fluorescence quantum yield of 0.25.Citation33 In the presence of SFU concentrations much higher than 1 mM, the stationary Rf-fluorescence emission intensity decreased, but the shape of the emission spectrum remained unchanged, discarding the possible formation of a fluorescent complex. Assuming that the fluorescence inhibition is a consequence of the quenching of 1Rf* by SFU, the bimolecular rate constant, kQ(1), was obtained from KSV, the Stern–Volmer constant for the interaction.
The KSV value, which was determined graphically (), equals the ratio kQ(1)/1kd, where 1kd is the rate constant for 1Rf* deactivation processes towards the ground-state Rf. The value of kQ(1) reported in was calculated according to a literature valueCitation34 5.75 ns for 1/kd.
Figure 5. Stern–Volmer plot for the static fluorescence quenching of Rf (0.01 mM) by SFU in MeOH:H2O (1:1, v/v) solution.
Laser flash photolysis experiments of argon-saturated solutions of Rf in the absence and presence of sub-millimolar SFU concentration display qualitative results similar to those described for the SDs, in previous work by our research group,Citation21 where transient absorption spectra of Rf in the absence and in the presence of SDs were obtained after the laser pulse. The shape of these spectra was in good agreement with that of the semiquinone radical RfH•, previously reported.Citation31,Citation32 These results allow confirmation of the interaction of 3Rf* and SFU. The monoexponential decay curves clearly indicated that 3Rf* lifetime decreased in the presence of SFU. The value of the bimolecular rate constant, kQ(3), was obtained from the classical Stern–Volmer treatment of time-resolved data ( inset, ).
O2(1Δg)-mediated photodegradation of SFU
The species O2(1Δg) produced by process (6) can react with SFU generating oxidation products (process 13), or it can also react with Rf generating other oxidation products (process 14). Also, it can interact physically with SFU (process 12) or it can decay by collision with solvent molecules (process 15).
12
13
14
15
Although our main aim was to study SFU photodegradability at physiological pH values, it is also interesting to investigate the possible influences of pH media on the kinetics of photodegradative processes.
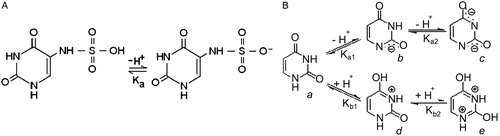
Msagati and NindiCitation35 have reported a pKa value of 11.5 for SFU, which corresponds to hydroxyl ionization in the sulfamic group of the molecule (Scheme A). The kinetic experiments were performed at pHs 7 and 12 where the non-ionized SFU and more than 50% of its ionized form were present, respectively.
In addition, the behavior of the uracil molecule in aqueous solution is described in Scheme B (being the values reportedCitation36,Citation37 for pKa1 = 9.5 and for pKa2 > 13).
Assuming that the presence of the sulfamino- group does not vary the distribution of the species described for uracil substantially, in aqueous reaction media at pH 12 the ionized species b of Scheme B will also be present in the medium.
Initially, Rose Bengal (RB), an efficient generator of O2(1Δg),Citation38 was selected as a sensitizer in order to evaluate the involvement of this oxidative species in SFU degradation. Spectral changes were observed in aqueous solutions with SFU and RB, which may prove the association between the substrate and the xantenic dye in the ground state of both selected pHs. However, since the aim of the current study is not to assess the magnitude of this association, PN was selected as a sensitizer, which generates singlet oxygen species in water with a quantum yield close to unity.Citation39
shows changes in the absorption spectrum upon irradiation of SFU-PN solutions at pH 12. These changes were inhibited by the presence of 1 mM NaN3, a selective quencher of the species O2(1Δg) with reported kt values of 5 × 108–15 × 108 M−1 s−1 in water,Citation22 suggesting singlet oxygen participation. Dark reactions between PN 0.01 mM and SFU 0.1 mM, as well as thermal oxidation by molecular oxygen, were discarded because the spectra of solutions stored in darkness and of air-saturated solutions had not changed during irradiation time.
Figure 6. Time evolution of the absorption spectrum of SFU 0.5 mM upon PN-sensitized irradiation vs. PN in H2O (pH 12).
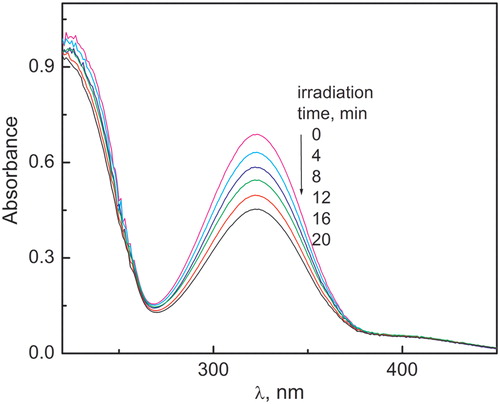
It was observed that oxygen was consumed in SFU-irradiated aqueous solutions with PN at pHs 7 and 12, and it was inhibited by the presence of 1 mM NaN3.
These experiments, in addition to the absence of reactivity in N2 saturated solutions and the inhibition of O2(1Δg) phosphorescence emission at 1270 nm, indicate the participation of O2(1Δg) as a primary oxidizing agent in PN-sensitized photooxidation of SFU aqueous solutions.
The overall rate constant, kt (=kQ + kr) (process 12 plus process 13), was determined by TRPD at pDs 7 and 12. The results are displayed in A and .
Figure 7. (A) Stern–Volmer plots for the quenching of O2(1Δg) phosphorescence by SFU in D2O at (a) pD 7 and (b) pD 12. PN (Abs355 = 0.40) as a sensitizer. (B) First-order plot for the concentration changes of SFU 0.4 mM or Trp 0.4 mM, under irradiation with visible light (cutoff > 400 nm) in the presence of PN (Abs355 = 0.5).
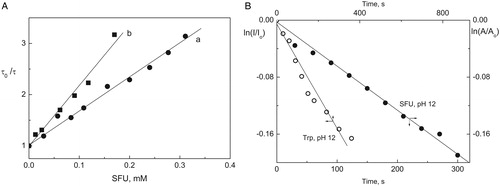
Table 2. Rate constants for the reactive (kr, M−1 s−1) and overall quenching (kt, M−1 s−1) of O2(1Δg) by SFU, ratio kr/kt and relative rates of oxygen consumption (Vox) upon PN-sensitized irradiation of SFU
The reactive rate constant kr was determined using the method described by Scully and HoigneCitation40 by consumption of substrate to pHs 7 and 12. Absorbance changes were measured at low conversions of substrate (<10–15%) in order to avoid eventual interference of photooxidation products absorption in the same spectral zone. Trp was used as reference, where kr is 3.47 × 107 M−1 s−1 at pH 7 and 30.6 × 107 M−1 s−1 at pH 12, values reported by Criado et al.Citation41 Consumption of amino acid and SFU were followed by fluorescence emission and absorbance spectroscopy, respectively, and kr values were obtained from first-order graphs that show SFU and Trp consumption upon sensitized irradiation with PN (B, ).
The quantum efficiency of singlet oxygen-mediated oxidation, φr, is expressed by the following equationCitation42:
16
being kd the rate constant for O2(1Δg) deactivation due to interaction with solvent molecules (2.5 × 105 s−1 in waterCitation43).
Quantum efficiency is difficult to determine due to the fact that the actual concentration of the photooxidizable substrate (represented by SFU in our case) needs to be known beforehand to apply equation (16). Substrate concentration is unknown in many cases, particularly in natural environments. Then a simpler and more useful approach is the evaluation of the kr/kt ratio, which indicates the fraction of overall quenching of O2(1Δg) by the substrate, which effectively leads to a chemical transformation.
The respective kr/kt ratios for SFU at pHs 7 and 12 are included in . For comparison purposes, the values of uracil also have been included. It is noted that this compound exhibits a very limited reactivity at pH 7, with an extremely low rate constant kt for overall interaction and without effective oxidation.
At pH 12, where the main species are ionized SFU forms, not only is a marked increase in the rate constants observed, but it is also noticed that SFU–O2(1Δg) interaction is completely reactive (kr/kt = 1), like that registered for uracil.Citation20
Discussion
Interaction between SFU and Rf-electronically excited states
According to the results of our experiments, both singlet and triplet Rf-electronically excited states are quenched by SFU in aqueous solutions represented by equations (4) and (7), respectively. Although kQ(1) reaches a value close to the diffusional limit, a simple calculation based on the Stern–Volmer treatment indicates that Citation1Rf* is practically unaffected by the presence of SFU under the conditions in which our experiments were performed. Thus, using the value from for kQ(1), 7.4 × 109 M−1 s−1, only 1.4% 1Rf* is quenched in the presence of 0.4 mM SFU, whereas 20 mM SFU is necessary for the quenching of 50% Citation1Rf*, about 70 times the concentration used under stationary photoirradiation conditions.
On the other hand, 3Rf* can be quenched by O2(3Σg−) to produce the oxidative species O2(1Δg) (process 6), or by SFU (process 7) to produce other reactive species, such as O2•− via processes (8) and (9)–(10). This sequence has been formerly proposed in the photooxidation of other phenols.Citation31,Citation32 Thus, the species O2•− should be present, together with O2(1Δg), as indicated by the observed effect of SOD on the relative rate of oxygen consumption (). The enzyme SOD catalyzes the dismutation of O2•− according to the process:
17
SOD has been used as a specific quencher of O2•− in a given photooxidation process,Citation26,Citation27 in similar concentrations to those employed in this work.
The high rate constant value for kQ(3) () and the inhibitory effect of SOD in oxygen uptake experiments strongly support the conclusion that O2•− is generated by processes (8) and (10), which are involved in the oxidation of SFU (process 11). The straightforward generation of O2•− by 3Rf* (equation not shown) is not considered, because its quantum yield is very low: 0.009.Citation44
At the same time, processes (8) and (10) regenerate ground-state Rf, a crucial step in living organisms. It is well known that O2•− is a key intermediate in oxygen redox chemistry.Citation45
The Gibbs energy for the electron transfer represented by process (7) can be evaluated from the Rehm–Weller treatmentCitation46:
18
where E(SFU/SFU+) (0.778 V) is the oxidation potential value for SFU,Citation47 E(Rf/Rf−) (−0.80 V) is the redox potential of Rf,ERf* (2.17 eV) is the Rf excited triplet energy, and C (−0.06 V) is the Coulombic energy term.Citation48 Thus, the ΔG° = −0.65 eV deduced from equation (18) indicates that process (7) is thermodynamically feasible. Consequently, species O2•− can be formed from Rf•−, but the kinetic competition between processes (6) and (7) will determine the ratio for these two pathways. Using ∼1 × 109 M−1 s−1 at MeOH–H2O for kET, a literature value,Citation49 2 × 109 M−1 s−1for kQ(3), a value from , and considering equal concentrations of O2(3Σg−) and SFU, both reactive steps occur at similar rates to those seen for other SDs studied in previous work.Citation21 In other words, the species O2(1Δg) and Rf•− seem to be generated to a similar degree through processes (6) and (7), respectively. Therefore, the kinetic balance indicates that, under working conditions, the generation rate of Rf•− (the O2•− precursor species) is similar to the generation rate of O2(1Δg).
O2(1Δg)-mediated photodegradation of SFU
Our results indicate that SFU interacts with O2(1Δg) at pH 7 in its non-ionized form, as well as at pH 12 where the ionized species are present in aqueous solutions. It is interesting to compare the kinetic constants for SFU at pHs 7 and 12 with those of uracil previously published.Citation20 The values of these constants () suggest that the presence of the sulfamino- group as a substituent in the uracil moiety exerts an activating power on its nucleus. This is based on the increase of the constants for the global and for the reactive processes in the interaction with SFU compared to the uracil in alkaline medium. Also, we can observe the high ratio kr/kt for the bacteriostatic in relation with uracil, which does not suffer oxidation at pH 7 (). Therefore, it is clear that the combination of primary amino and sulfonic groups as substituent on the uracilic ring confers high pro-oxidative reactivity to the resulting molecule.
In , it is also important to note that the ratio of relative rates of oxygen consumption Vox(pH 12)/Vox(pH 7) is 1.3, while the ratio of the reactive rate constants kr(pH 12)/kr(pH 7) reached a value close to 5 using the same sensitizer. A possible explanation for such value is that kr was determined following the decrease of SFU concentration during photolysis. Also, oxygen consumption measures the incorporation of oxygen molecules by products. This means that the mole ratio (mol SFU/mol O2(1Δg)) at pH 12 is higher than at pH 7, suggesting that some kind of secondary reaction is involved in the degradation process. Products incorporate less oxygen at pH 12 than at pH 7. Therefore, we can conclude that photolysis products are different depending on the pH present in the medium.
Conclusions
Bacteriostatic SFU is degraded under visible light irradiation in the presence of photosensitizers (PN or Rf) through the reaction of reactive oxygenated species generated in aqueous solutions at different pHs. With PN, photodegradation is mediated totally by O2(1Δg) in a relatively efficient process favored by an alkaline medium. In the presence of the natural sensitizer Rf, SFU reacts with oxidative species O2(1Δg) and O2−, both generated from the interaction of 3Rf* with O2(3Σg−) in competitive processes.
The results indicate that sensitized photodegradation is an efficient pathway to eliminate SFU from natural waters under controlled conditions, especially in an alkaline medium.
Disclaimer statements
Contributors Secretaría de Ciencia y Técnica from Universidad Nacional de la Patagonia San Juan Bosco.
Funding None.
Conflicts of interest None.
Ethics approval This study is not required ethical approval.
Acknowledgments
We thank Secretaría de Ciencia y Técnica from Universidad Nacional de la Patagonia San Juan Bosco (SCyT, UNPSJB) for financial support. We would like to thank the Departamento de Química from Universidad Nacional de Río Cuarto (UNRC) for the use of laboratory equipment, all from Argentina. The authors gratefully acknowledge the permanent scientific assistance of Dr Norman Andino García (from UNRC) during this study. We also wish to thank Cynthia Manzanal and Dr Lucio Ibiricu for their contributions.
References
- Portalone G, Bencivenni L, Colapietro M, Pieretti A, Ramondo F. The effect of hydrogen bonding on the structures of uracil and some methyl derivatives studied by experiment and theory. Acta Chem Scand 1999;53:57–68.
- Gupta N, Price PM, Aboagye EO. PET for in vivo pharmacokinetic and pharmacodynamic measurements. Eur J Cancer 2002;38:2094–107.
- Chomicz L, Zdrowowicz M, Kasprzykowski F, Rak J, Buonaugurio A, Wang Y, et al. How to find out whether a 5-substituted uracil could be a potential DNA radiosensitizer. J Phys Chem Lett 2013;4:2853–7.
- Alcolea Palafox M, Tardajosa G, Guerrero-Martíneza A, Vatsb JK, Hubert J, Rastogib VK. Relationships observed in the structure and spectra of uracil and its 5- substituted derivatives. Spectrochim Acta A 2010;75:1261–9.
- Shao B, Dong D, Wu Y, Hu J, Meng J, Tu W, et al. Simultaneous determination of 17-sulfonamide residues in porcine meat, kidney and liver by solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal Chim Acta 2005;546:174–81.
- Garcia-Galan MJ, Diaz-Cruz MS, Barcelo D. Determination of 19 sulfonamides in environmental water samples by automated on-line solid-phase extraction-liquid chromatography-tandem mass spectrometry. Talanta 2010;81:355–66.
- Diaz-Cruz MS, Garcia-Galan MJ, Barcelo D. Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry. J Chromatogr A 2008;1193:50–9.
- Gobel A, McArdell CS, Suter MJF, Giger W. Trace determination of macrolide and sulfonamide antimicrobials, a human sulfonamide metabolite, and trimethoprim in wastewater using liquid chromatography coupled to electrospray tandem mass spectrometry. Anal Chem 2004;76:4756–64.
- Castiglione S, Bagnati R, Calamari D, Fanelli R, Zuccato E. A multiresidue analytical method using solid-phase extraction and high-pressure liquid chromatography tandem mass spectrometry to measure pharmaceuticals of different therapeutic classes in urban wastewaters. J Chromatogr A 2005;1092:206–15.
- Batt AL, Snow DD, Aga DS. Occurrence of sulfonamide antimicrobials in private water wells in Washington County, Idaho, USA. Chemosphere 2006;64:1963–71.
- Sacher F, Lange FT, Brauch HJ, Blankenhorn I. Pharmaceuticals in groundwaters: analytical methods and results of a monitoring program in Baden-Wurttemberg, Germany. J Chomatogr A 2001;938:199–210.
- Tamtam F, Mercier F, Le Bot B, Eurin J, Tuc Dinh Q, Clement M, et al. Occurrence and fate of antibiotics in the Seine River in various hydrological conditions. Sci Total Environ 2008;393:84–95.
- Kummerer K. Antibiotics in the aquatic environment. Chemosphere 2009;75:417–34.
- Chacon JN, McLearie J, Sinchair RS. Singlet oxygen yields and radical contribution in the dye-sensitized photooxidation in methanol of esters of polyunsaturated fatty acids (oleic, linoleic, linolenic and arachidonic). Photochem Photobiol 1988;47:647–56.
- Trovo AG, Nogueira RFP, Aguera A, Sirtori C, Fernandez-Alba AR. Photodegradation of sulfamethoxazole in various aqueous media: persistence, toxicity and photoproducts assessment. Chemosphere 2009;77:1292–8.
- Lester Y, Avisar D, Mamane H. Photodegradation of the antibiotic sulphamethoxazole in water with UV/H2O2 advanced oxidation process. Environ Technol 2010;31:175–83.
- Guerard JJ, Chin YP, Mash H, Hadad CM. Photochemical fate of sulfadimethoxine in aquaculture waters. Environ Sci Technol 2009;43:8587–92.
- Calisto V, Domingues MRM, Esteves VI. Photodegradation of psychiatric pharmaceuticals in aquatic environments-kinetics and photodegradation products. Water Res 2011;45:6097–106.
- Homem V, Santos L. Degradation and removal methods of antibiotics from aqueous matrices – a review. J Environ Manage 2011;92:2304–47.
- Haggi E, Blasich N, Díaz J, Diaz M, Massad WA, Amat-Guerri F, et al. Kinetics and mechanism of the sensitized photodegradation of uracil-modeling the fate of related herbicides in aqueous environments. Photochem Photobiol 2007;85:520–5.
- Díaz M, Luiz M, Bertolotti S, Miskoski S, García NA. Scavenging of photogenerated singlet molecular oxygen and superoxide radical anion by sulpha drugs. Kinetics and mechanism. Can J Chem 2004;82:1752–9.
- Wilkinson F, Helman WP, Ross AB. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J Phys Chem Ref Data 1995;24:663–1921.
- Weast RC, Astle HJ editors. Handbook of chemistry and physics. 62nd ed. Boca Raton, FL: CRC Press; 1981.
- Criado S, Pajares A, Gianotti J, Stettler G, Escalada JP, Bertolotti S, et al. Kinetic study of the riboflavin-sensitised photooxygenation of two hydroxyquinolines of biological interest. J Photochem Photobiol B 2003;71:19–25.
- Tratnyek PG, Hoigné J. Oxidation of substituted phenols in the environment: a QSAR analysis of rate constants for reaction with singlet oxygen. Environ Sci Technol 1991;25:1596–604.
- Baxer RM, Carey JH. Evidence for photochemical generation of superoxide ion in humic waters. Nature 1983;306:575–6.
- Zang LY, Misra HP. Superoxide radical production during the autoxidation of 1-methyl-4-phenyl-2,3-dihydroxypyridinium perchlorate. J Biol Chem 1992;267:17547–52
- Lu CY, Lin WZ, Wang WF, Han ZH, Yao SD, Lin NY. Riboflavin (VB2) photosensitized oxidation of 2′-deoxyguanosine-5′-monophosphate (dGMP) in aqueous solution. A transient intermediates study. Phys Chem Chem Phys 2000;2:329–34.
- Lu C, Bucher G, Sander W. Photoinduced interactions between oxidized and reduced lipoic acid and riboflavin (vitamin B2). Chem Phys Chem 2004;5:47–56.
- Lu CY, Lin WZ, Wang WF, Han ZH, Lin Z, Han H, et al. Generation and photosensitization properties of the oxidized radicals of riboflavin: a laser flash photolysis study. J Photochem Photobiol B 1999;52:111–6.
- Massad WA, Bertolotti S, Garcia NA. Kinetics and mechanism of the vitamin B2-sensitized photooxidation of isoproterenol. Photochem Photobiol 2004;79:428–33.
- Haggi E, Bertolotti S, García NA. Modeling the environmental degradation of water contaminants. Kinetics and mechanism of the riboflavin-sensitised-photooxidation of phenolic compounds. Chemosphere 2004;55:1501–7.
- Heelis PF. The photophysical and photochemical properties of flavins (isoalloxazines). Chem Soc Rev 1982;11:15–39.
- Bertolotti SG, Previtali CM, Rufs AM, Encinas MV. Riboflavin triethanolamine as photoinitiator system of vinyl polymerization. A mechanistic study by laser flash photolysis. Macromolecules 1999;32:2920–4.
- Msagati TAM, Nindi MM. Multiresidue determination of sulfonamides in a variety of biological matrices by supported liquid membrane with high pressure liquid chromatography-electrospray mass spectrometry detection. Talanta 2004;64:87–100.
- Brown DJ. Pyrimidines and their benzo derivatives. In: Katritzky AR, Rees CW, Boulton AJ, McKillop A, (eds.) Comprehensive heterocyclic chemistry. Oxford: Pergamon Press; 1984. vol. 3, p. 57–155.
- Lide DR editor. Handbook of chemistry and physics. 87th ed. Boca Raton, FL: CRC Press; 2006. p. 75.
- Amat-Gerri F, López-González MMC, Matínez-Urtilla R, Sastre R. Singlet oxygen photogeneration by ionized and un-ionized derivatives of Rose Bengal and Eosin Y in diluted solutions. J Photochem Photobiol A Chem 1990;53:199–210.
- Nonell S, Gonzalez M, Trull FR. 1H-phenalen-1-one-2-sulfonic acid: an extremely efficient singlet molecular oxygen sensitizer for aqueous media. Afinidad 1993;448:445–50.
- Scully FE, Hoigné J. Rate constants for reactions of singlet oxygen with phenols and other compound in water. Chemosphere 1987;16:681–94.
- Criado S, Bertolotti S, García NA. Kinetic aspects of the rose bengal-sensitized photo-oxygenation of tryptophan alkyl esters. Ground state and photopromoted dye-tryptophan derivative interactions. J Photochem Photobiol B 1996;34:79–86.
- García NA. Singlet molecular oxygen-mediated photodegradation of aquatic phenolic pollutants. A kinetic and mechanistic overview. J Photochem Photobiol B 1994;22:185–96.
- Rodgers MAJ, Snowden PT. Lifetime of O2(1Δg) in liquid water as determined by time-resolved infrared luminescence measurements. J Am Chem Soc 1982;104:5541–3.
- Heelis PF. The photochemistry of flavins. In: Muller F, (ed.) Chemistry and biochemistry of flavoenzymes. Boca Raton: CRC Press; 1991. vol. 1, p. 171–93.
- Kanofsky JR. Singlet oxygen production from the reactions of superoxide ion in aprotic solvents: implications for hydrophobic biochemistry. Free Radic Res Commun 1991;12–13(Pt 1):87–92.
- Rehm D, Weller A. Kinetics of fluorescent quenching by electron transfer and H-atom transfer. Isr J Chem 1970;8:259–71
- Msagati TAM, Ngila JC. Voltammetric detection of sulfonamides at a poly(3-methylthiophene) electrode. Talanta 2002;58:605–10.
- Porcal G, Bertolotti SG, Previtali CM, Encinas MV. Electron transfer quenching of singlet and triplet excited states of flavins and lumichrome by aromatic and aliphatic electron donors. Phys Chem Phys 2003;5:4123–8.
- Murov SL. Handbook of photochemistry. New York: M. Dekker; 1973.