Abstract
Objectives
Oxidative stress and inflammatory process play an important role in the pathogenesis of Duchenne muscular dystrophy (DMD). We investigated whether deferoxamine (DFX) improves the antioxidant effects of N-acetylcysteine (NAC) on primary cultures of dystrophic muscle cells from mdx mice, the experimental model of DMD.
Methods
Primary cultures of skeletal muscle cells from mdx mice were treated with either NAC (10 mM), DFX (5 mM), or NAC plus DFX for 24 hours. The muscle cells of C57BL/10 mice were used as controls.
Results
Production of hydrogen peroxide (H2O2) and levels of 4-hydroxynonenal (4-HNE), tumor necrosis factor alpha (TNF-α), and nuclear factor kappa-B (NF-κB) were significantly higher in mdx muscle cells than in C57BL/10 muscle cells. Treatment with NAC, DFX, or NAC plus DFX significantly decreased H2O2 production (24, 58, and 72%, respectively), and levels of 4-HNE-protein adducts (62, 33, and 71%, respectively), TNF-α (32, 29, and 31%, respectively), and NF-κB (34, 38, and 52%, respectively) on dystrophic muscle cells.
Discussion
This study demonstrates that mdx muscle cells are able to produce key oxidative stress and inflammatory markers, without the interference of inflammatory cells, and shows that NAC plus DFX reduced the inflammatory and oxidative stress indicators, mainly H2O2 production and NF-κB levels by dystrophic fibers.
Introduction
Duchenne muscular dystrophy (DMD) is a lethal degenerative muscular disorder caused by the absence of dystrophin protein, and affects about 1 in 3500 male births.Citation1 There is evidence that the pathological mechanisms triggered by the loss of dystrophin in DMD include exacerbated inflammatory response and increased oxidative stress.Citation2–Citation6
The dystrophin-deficient muscle fibers of patients with DMD and of the mdx mice (an experimental model of DMD) display elevated levels of oxidative stress markers and lipid peroxidation by-products.Citation6–Citation9 In addition, dystrophin-deficient cells appear to be more susceptible than normal cells to cellular injury when exposed to reactive oxygen species (ROS) in vitro, in particular to hydrogen peroxide (H2O2).Citation6
Previous studies showed that the antioxidant N-acetylcysteine (NAC) provided considerable protection against the ongoing muscle degeneration and the elevated inflammatory processes in mdx mice.Citation7–Citation10 Previous studies have demonstrated that a combination of NAC and deferoxamine (DFX) is an effective treatment for several oxidative stress and inflammatory diseases.Citation11–Citation15 DFX is a potent iron chelator with antioxidant properties under several conditions.Citation16,Citation17 Iron deprivation decreases muscle necrosis in the mdx mice, suggesting that iron-catalyzed free radical reactions have a role in the pathology of dystrophin-deficient muscle.Citation18
We investigated whether DFX synergically improves the antioxidant effects of NAC in dystrophic muscle cells. Using an in vitro primary culture of dystrophin-deficient muscle fibers of the mdx mice, we evaluated the effects of NAC plus DFX on oxidative stress markers, such as H2O2 production and the levels of 4-hydroxynonenal (4-HNE), a major product of lipid peroxidation. Considering that oxidative stress greatly contributes to the inflammatory response in dystrophic muscles,Citation19 we also determined whether NAC plus DFX decreases the levels of nuclear factor kappa-B (NF-κB), a transcription factor that regulates the expression of pro-inflammatory cytokines,Citation20 and tumor necrosis factor alpha (TNF-α), a key cytokine that stimulates the inflammatory cell response in mdx mice.Citation3
Materials and methods
Animals
Both mdx and C57BL/10 (control) mice were obtained from a breeding colony maintained by our institutional animal care facility and were used in all the experiments. The mice were housed according to the institutional guidelines and had access to food and water ad libitum. The animal experiments described here were conducted in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA; process #2128-1) and the guidelines set forth by our institution.
Muscle cell cultures
Primary culture of skeletal muscle cells was performed following the method described by Rando and Blau.Citation21 Hind limbs were removed from young mdx and C57BL/10 mice (15 days old) and the quadriceps femoris, tibialis anterior, extensor digitorum longus, gastrocnemius, soleus, and plantaris muscles were used to prepare primary myoblast culture. Muscles were triturated using a pair of scissors and enzymatically digested with collagenase and trypsin solutions at 37°C. The satellite cells (5 × 104 cells/cm2) were plated in 1% MatrigelTM-coated dishes (MatrigelTM - BD Biosciences, Bedford, MA, USA). The myoblasts were cultured in a proliferation and growth medium containing Dulbecco's Modified Eagle Medium (DMEM) with glucose (5.5 mM), l-glutamine (2 mM), fetal bovine serum (10% v/v), horse serum (10% v/v), and penicillin/streptomycin (1% v/v) for 2 days. Myogenesis (myotube differentiation) was induced by the addition of a fusion medium that consisted of DMEM with glucose (5.5 mM), l-glutamine (2 mM), and horse serum (10% v/v). The culture was maintained at 37°C and 5% CO2 and the differentiated muscle cells with contractile properties were observed at 7–8 days of culture in the fusion medium.
Experimental cell groups
Skeletal muscle cell cultures at 7–8 days were used in all experiments and all measurements were obtained from triplicate cultures. The following groups were studied: (1) Ctrl (myotubes from C57BL/10 mice that did not receive any treatment), (2) CtrlN (myotubes treated with 10 mM NAC for 24 hours), (3) CtrlD (myotubes treated with 5 mM DFX for 24 hours), (4) CtrlND (myotubes treated with 10 mM NAC and 5 mM DFX for 24 hours), (5) mdx (myotubes that did not receive any treatment), (6) mdxN (myotubes treated with 10 mM NAC for 24 hours), (7) mdxD (myotubes treated with 5 mM DFX for 24 hours), and (8) mdxND (myotubes treated with 10 mM NAC and 5 mM DFX for 24 hours).
Cell viability
Cell viability was assessed by morphology and by tetrazolium (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT (Sigma) assay (Saint Louis, MO, USA). Skeletal muscle cells were washed in PBS, treated with MTT solution (5 mg/ml, tetrazolium salt), and incubated for 4 hours at 37°C. After 4 hours, the cell supernatants were discarded, MTT crystals were dissolved with acid isopropanol, and the absorbance was measured at 570 nm. All assays were performed in triplicate. Percentage viability was defined as the relative absorbance of treated versus untreated control cells. Plates were analyzed in a multi-mode microplate reader model Synergy H1M (Bio Tek Instruments, Washington, DC, USA) at 570 nm with a 655 nm reference wavelength.
Determination of H2O2 level
Skeletal muscle cells were maintained in phenol red-free culture medium and muscle-derived ROS were determined using a fluorescence assay. The Amplex UltraRed reagent (50 μM) and horseradish peroxidase (0.1 U/ml) were added for 60 minutes (Amplex UltraRed - Invitrogen, Eugene, Oregon, USA). Amplex reacts with H2O2, in the presence of horseradish peroxidase, to produce resorufin, a stable red fluorescent and stable compound. The fluorescence signal of resorufin was determined at 530 (excitation) and 590 nm wavelength (emission). Measurements of ROS were previously calibrated using exogeneous 10 μM H2O2 (positive control). All measurements were performed in phenol red-free culture medium (1 ml), pH 7.4, at 37°C.
Western blotting
Proteins were extracted in a buffer containing Tris–HCl (100 mM), pH 7.5; EDTA (10 mM), pH 8.0; sodium pyrophosphate (10 mM); sodium fluoride (0.1 mM); sodium orthovanadate (10 mM); PMSF (2 mM); and aprotinin (10 μg/ml). The cell extracts were sonicated for 30 seconds at 4°C. The homogenates were centrifuged at 11 000 g for 20 minutes at 4°C and the supernatants were treated with Triton X-100 (1%) and transferred to a −80°C freezer until they were used for Western blotting analysis. An aliquot from the supernatant was used to determine the total protein content by the Bradford method. Samples of 30 µg of total protein homogenate were loaded onto 6–15% SDS-polyacrylamide gels. Proteins were transferred from the gels to a nitrocellulose membrane using a submersion electrotransfer apparatus (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked for 2 hours at room temperature with 5% skim milk/Tris–HCl buffer saline-Tween buffer (TBST; 10 mM Tris–HCl, pH 8, 150 mM NaCl, and 0.05% Tween 20). The membranes were incubated with the primary antibodies overnight at 4°C, washed in TBST, incubated with the peroxidase-conjugated secondary antibodies for 2 hours at room temperature, and developed using the SuperSignal West Pico Chemiluminescent Substrate kit (Pierce Biotechnology, Rockford, IL, USA). To control protein loading, Western blot transfer, and nonspecific changes in protein levels, the blots were stripped and reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Band intensities were quantified using ImageJ 1.38X (National Institutes of Health, Bethesda, MD, USA) software. The following primary antibodies were used for Western blotting: (1) dystrophin (mouse monoclonal; Vector Laboratories, ON, Canada); (2) NF-κB (goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA); (3) TNF-α (rabbit anti-mouse polyclonal; Chemical, Temecula, CA, USA); (4) 4-HNE (goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA); (5) GAPDH (rabbit polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The secondary antibody used was peroxidase-labeled affinity purified mouse or rabbit IgG antibody (KPL, Gaithersburg, MD, USA).
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical analysis for direct comparison between means of two groups was performed by the Student's t-test. For multiple statistical comparisons between groups, analysis of variance (ANOVA) was used, followed by the Bonferroni test used for multiple statistical comparisons between groups. Values of P ≤ 0.05 were considered statistically significant.
Results
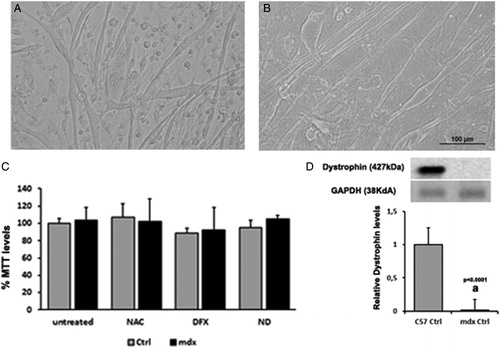
Primary cell cultures from normal (C57BL/10) and dystrophic muscles (mdx) showed the typical progression of proliferation to differentiation and fusion into thick, branching myotubes (A and B). Treatment with NAC, DFX, and NAC plus DFX did not cause any morphological alteration and did not affect cell viability as compared with untreated cultures (C). The presence or absence of dystrophin was ascertained using Western blots (D).
Figure 1. Morphology of normal (A) and dystrophic (B) myotube cultures. In (C), cell viability was assessed by measurement of MTT assay in the muscle cells from C57BL/10 mice (Ctrl) and mdx mice (mdx). Myotubes were treated for 24 hours with N-acetylcysteine (NAC), deferoxamine (DFX), NAC plus DFX (ND), or did not receive any treatment (untreated). In (D), Immunoblot analysis of dystrophin and graph showing protein level in the muscles cells from C57BL/10 mice (Ctrl) and mdx mice (mdx). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. All the experiments were performed in triplicate, and; the relative value of the band intensity was quantified and normalized by the corresponding Ctrl. ‘a’ versus Ctrl. Error bars, SD.
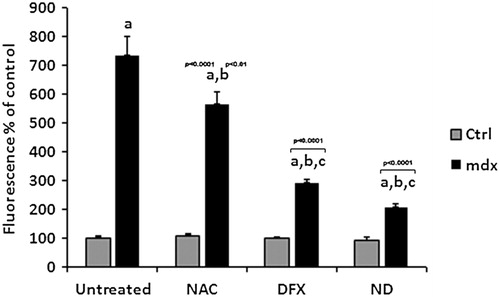
To test whether the treatments affected ROS in dystrophic muscle cells, we analyzed H2O2 production and 4-HNE-protein adducts levels. We initially verified that treatment with NAC, DFX, or NAC plus DFX did not change the H2O2 production and 4-HNE-protein adducts levels in control (C57BL/10) myotubes. Production of H2O2 was significantly higher in mdx myotubes than in control muscle cells (P < 0.05, ). Treatment with NAC, DFX, or NAC plus DFX significantly decreased H2O2 production (by 24, 58, and 72%, respectively) in mdx myotubes ().
Figure 2. Quantification of H2O2 production in the muscle cells from C57BL/10 mice (Ctrl) and mdx mice (mdx). Myotubes were treated for 24 hours with N-acetylcysteine (NAC), deferoxamine (DFX), NAC plus DFX (ND), or did not receive any treatment (untreated). All the experiments were performed in triplicate and all values are expressed as mean ± SD. Results are expressed relative to Ctrl. ‘a’ versus Ctrl untreated, Ctrl NAC, Ctrl DFX, and Ctrl ND; ‘b’ versus mdx; ‘c’ versus mdx NAC.
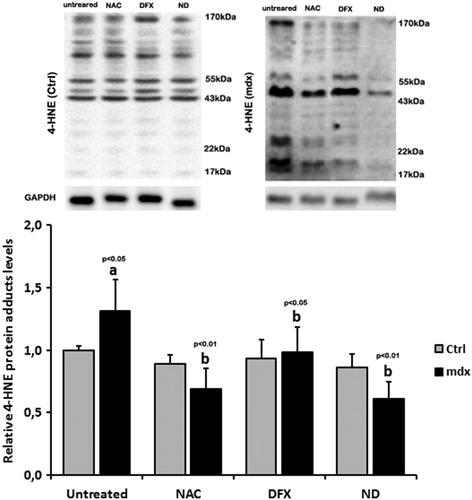
Representative immunoblots and quantification of 4-HNE-protein adducts are shown in . Bands of 4-HNE-protein adducts from 17 to 170 kDa were detected in control and in dystrophic muscle cells. Proteins of approximately 170, 55, 43, 22, and 17 kDa exhibited elevated 4-HNE binding in the dystrophic myotubes compared with control cells. Levels of 4-HNE-protein adducts were significantly reduced by NAC, DFX, and NAC plus DFX (by 62, 33, and 71%, respectively; P < 0.05, ANOVA followed by Bonferroni test) in mdx myotubes compared with untreated dystrophic muscle cells.
Figure 3. Immunoblot analysis shows several bands of 4-HNE-protein adducts, ranging from 17 to 170 kDa. Graphs show protein level in the muscle cells from C57BL/10 mice (Ctrl) and mdx mice (mdx). Myotubes were treated for 24 hours with N-acetylcysteine (NAC), deferoxamine (DFX), NAC plus DFX (ND), or did not receive any treatment (untreated). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. All the experiments were performed in triplicate, and; the relative value of the band intensity was quantified and normalized by the corresponding Ctrl. ‘a’ versus Ctrl untreated, Ctrl NAC, Ctrl DFX, and Ctrl ND; ‘b’ versus mdx. Error bars, SD.
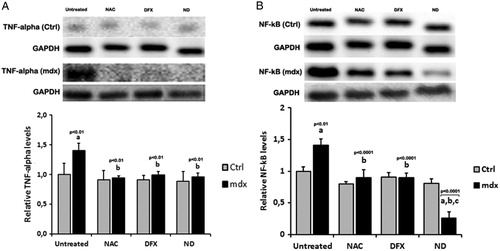
To address whether NAC and DFX attenuated inflammation, we analyzed the levels of TNF-α and NF-κB. In control muscle cells, the treatment with NAC, DFX, or NAC plus DFX did not affect the TNF-α and NF-κB levels. Already, in the dystrophic myotubes, immunoblotting analysis demonstrated a marked increase in TNF-α and NF-κB levels compared with control cells (A and B). In contrast, the treatment with NAC, DFX, and NAC plus DFX reduced the TNF-α levels in dystrophic myotubes (by 32, 29, and 31%, respectively; P < 0.05, ANOVA followed by Bonferroni test; A). Levels of NF-κB were markedly reduced after NAC (34.2%) and DFX (38.3%) treatment (B); NAC plus DFX reduced the NF-κB levels in mdx muscle cells (by 52.2%; B).
Figure 4. Immunoblot analysis showed several bands of TNF-α (A) and NF-κB (B). Graphs show protein level in the muscle cells from C57BL/10 mice (Ctrl) and mdx mice (mdx). Myotubes were treated for 24 hours with N-acetylcysteine (NAC), deferoxamine (DFX), NAC plus DFX (ND), or did not receive any treatment (untreated). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. All the experiments were performed in triplicate, and the relative value of the band intensity was quantified and normalized by the corresponding Ctrl. ‘a’ versus Ctrl untreated, Ctrl NAC, Ctrl DFX, and Ctrl ND; ‘b’ versus mdx; ‘c’ versus mdx NAC and mdx DFX. Error bars, SD.
Discussion
We showed that dystrophic myotubes from mdx mice (15 days old), in comparison with normal myotubes from C57BL/10 mice, at the same age, present increased H2O2 and 4-HNE-protein adducts levels, with significantly higher H2O2 levels than those observed for 4-HNE (600% H2O2 increase versus 31% 4-HNE increase, in comparison with its respective controls). In addition, high levels of NF-κB and TNF-α were detected in mdx myotubes in relation to their controls, with NF-κB showing the highest values (41% TNF-α versus 75% NF-κB, compared with their respective controls). These results reinforce previous studies showing that dystrophic fibers per se can contribute to the production of ROS and inflammatory cytokines,Citation19 and thereby to the progress of necrosis in vivo.
The in vitro treatment of dystrophic fibers with NAC was effective in reducing 4-HNE and TNF-α. This is in agreement with our previous study, in which we demonstrated that in vivo NAC treatment reduces 4-HNE-protein adducts and TNF-α levels in the diaphragm muscle of mdx mice.Citation7 Regarding the 4-HNE-protein adducts, the soluble form of TNF (≈17 kDa),Citation22 the small heat shock protein HspB8 (≈22 kDa),Citation23 actin (≈43 kDa),Citation24 the mitochondrial protein (55 kDa),Citation25 or calcium-binding proteins, such as the calsequestrin-like proteins (170 kDa)Citation26 could be potential proteins modified by 4-HNE and protected by NAC, DFX, and NAC plus DFX treatment. However, further studies are necessary to confirm these proteins as new targets of oxidative stress in dystrophic muscle cells. In this study, we further demonstrated that NAC also reduces the production of H2O2 by dystrophic cells in vitro. Concomitantly, NF-κB is also decreased by NAC. These results are in line with others in vivo studies showing the beneficial effects of NAC on different muscles of the mdx mice.Citation10,Citation27,Citation28 Overall, these results indicate that molecular markers of oxidative stress (mainly represented here by H2O2) and of inflammation (mainly represented here by NF-κB) can be detected in the muscle cell cultures obtained from mdx at an early stage of the disease.
DFX enhances the beneficial effects of NAC. This was mainly observed in relation to the production of H2O2 and NF-κB levels, the two factors that were present in the highest levels in our in vitro dystrophic muscle cell model. Previous in vivo studies have shown that NAC plus DFX display antioxidant activities.Citation11,Citation15 By using an in vitro cell culture of dystrophic fibers, we were better able to evaluate the production of H2O2 by the dystrophin-deficient fibers per se specifically, without the interference of other cells normally present in the whole tissue, such as inflammatory cells, that contribute to H2O2 production. While other in vitro studies have shown that NAC has antioxidant effects,Citation29,Citation30 these studies did not use dystrophic fibers from the mdx mice. Prevention of H2O2 production is an important outcome, because H2O2 in the presence of iron may lead to the formation of highly reactive hydroxyl radicals that can intensify the lipid peroxidation process.Citation31 Although this study does not aim to elucidate the mechanisms through which NAC plus DFX affected ROS formation, it has been suggested that NAC, by increasing glutathione, reduces H2O2 production and, subsequently, DFX reduces the formation of the hydroxyl radicals.Citation32
The action of NAC plus DFX in reducing the levels of NF-κB seen here and previously demonstrated with NAC under different experimental conditionsCitation33,Citation34 may be related to the anti-inflammatory properties of DFXCitation32,Citation35 and/or to the antioxidant effects of NAC and DFX. NF-κB is the major transcription factor involved in mediating the inflammatory response in the dystrophic muscle cellCitation36 and ROS increases the NF-κB pathway.Citation20 Thus, the combination of NAC plus DFX is effective in decreasing the production of key inflammatory mediators by the dystrophic muscle fibers.
In conclusion, this study demonstrates that in vitro dystrophic cells from mdx mice are able to produce key oxidative stress and inflammatory markers, without the interference from inflammatory cells. This suggests that the dystrophic fiber per se, at least in part, can influence the progression of the disease. This highlights the importance of drug therapy that affects the muscle fiber directly, which we were able to address in this in vitro study by showing that NAC plus DFX effectively reduced H2O2 production and NF-κB levels by dystrophic fibers. Overall, considering the well-known side effects of NAC and DFX and their use in other human diseases,Citation37 these results suggest that NAC and DFX might be a suitable complementary treatment for dystrophinopathies and recommend clinical studies of their effectiveness in DMD patients.
Disclaimer statements
Contributors L.H.R.M. conducted the study. L.H.R.M., R.C.B., and D.S.M. analyzed the data and performed the statistical analysis. E.M. participated in the design of the study and coordination. E.M., L.R.S., and M.J.M. helped to draft the manuscript. All authors read and approved the final manuscript.
Funding None.
Conflicts of interest None.
Ethics approval The animal experiments were conducted in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA; process #2128-1) and the guidelines set forth by our institution.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grants 07/50189-1; 11/02474-4; 11/51697-6). M.J.M. is the recipient of a fellowship from Conselho Nacional de Pesquisas (CNPq; grant 301306/2010-9). L.H.R.M. is the recipient of a FAPESP fellowship (grant 10/01087-4), R.C.B. was the recipient of a FAPESP fellowship (grant 10/07775-0), and D.S.M. is the recipient of a CAPES fellowship.
References
- Engel AG,, Yamamoto M, Fischbeck KH. Dystrophinopathies. In: Engel AG, Franzini-Armstrong C, (eds.) Myology. New York: McGraw-Hill Inc; 1994. p. 1133–87.
- Grounds MD, Torrisi J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J 2004;18(6):676–82.
- Hodgetts S, Radley H, Davies M, Grounds MD. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNF alpha function with Etanercept in mdx mice. Neuromuscul Disord 2006;16(9–10):591–602.
- Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 2005;288(2):R345–53.
- Prosser BL, Khairallah RJ, Ziman AP, Ward CW, Lederer WJ. X-ROS signaling in the heart and skeletal muscle: stretch-dependent local ROS regulates [Ca2 + ](i). J Mol Cell Cardiol 2013;58:172–81.
- Rando TA, Disatnik MH, Yu Y, Franco A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscular Disord 1998;8(1):14–21.
- de Senzi Moraes Pinto R, Ferretti R, Moraes LH, Neto HS, Marques MJ, Minatel E. N-acetylcysteine treatment reduces TNF-alpha levels and myonecrosis in diaphragm muscle of mdx mice. Clin Nutr 2013;32(3):472–5.
- Fogagnolo Mauricio A, Minatel E, Santo Neto H, Marques MJ. Effects of fish oil containing eicosapentaenoic acid and docosahexaenoic acid on dystrophic mdx mice. Clin Nutr 2013;32(4):636–42.
- Tonon E, Ferretti R, Shiratori JH, Santo Neto H, Marques MJ, Minatel E. Ascorbic acid protects the diaphragm muscle against myonecrosis in mdx mice. Nutrition 2012;28(6):686–90.
- Terrill JR, Radley-Crabb HG, Grounds MD, Arthur PG. N-Acetylcysteine treatment of dystrophic mdx mice results in protein thiol modifications and inhibition of exercise induced myofibre necrosis. Neuromuscul Disord 2012;22(5):427–34.
- Arent CO, Reus GZ, Abelaira HM, Ribeiro KF, Steckert AV, Mina F, et al. Synergist effects of N-acetylcysteine and deferoxamine treatment on behavioral and oxidative parameters induced by chronic mild stress in rats. Neurochem Int 2012;61(7):1072–80.
- Di-Pietro PB, Dias ML, Scaini G, Burigo M, Constantino L, Machado RA, et al. Inhibition of brain creatine kinase activity after renal ischemia is attenuated by N-acetylcysteine and deferoxamine administration. Neurosci Lett 2008;434(1):139–43.
- Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JMF, Dal-Pizzol F. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 2004;32(2):342–9.
- Ritter C, da Cunha AA, Echer IC, Andrades M, Reinke A, Lucchiari N, et al. Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in the rat. Crit Care Med 2006;34(2):471–7.
- Valvassori SS, Petronilho FC, Reus GZ, Steckert AV, Oliveira VBM, Boeck CR, et al. Effect of N-acetylcysteine and/or deferoxamine on oxidative stress and hyperactivity in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry 2008;32(4):1064–8.
- Halliwell B. Use of desferrioxamine as a ‘probe‘ for iron-dependent formation of hydroxyl radicals. Evidence for a direct reaction between desferal and the superoxide radical. Biochem Pharmacol 1985;34(2):229–33.
- Halliwell B. Protection against tissue-damage in vivo by desferrioxamine – what is its mechanism of action. Free Radical Bio Med 1989;7(6):645–51.
- Bornman L, Rossouw H, Gericke GS, Polla BS. Effects of iron deprivation on the pathology and stress protein expression in murine X-linked muscular dystrophy. Biochem Pharmacol 1998;56(6):751–7.
- Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol 2006;33(7):657–62.
- Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J 2003;17(3):386–96.
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 1994;125(6):1275–87.
- Bani C, Lagrota-Candido J, Pinheiro DF, Leite PE, Salimena MC, Henriques-Pons A, et al. Pattern of metalloprotease activity and myofiber regeneration in skeletal muscles of mdx mice. Muscle Nerve 2008;37(5):583–92.
- Bartelt-Kirbach B, Golenhofen N. Reaction of small heat-shock proteins to different kinds of cellular stress in cultured rat hippocampal neurons. Cell Stress Chaperones 2014;19(1):145–53.
- Aldini G, Dalle-Donne I, Vistoli G, Maffei Facino R, Carini M. Covalent modification of actin by 4-hydroxy-trans-2-nonenal (HNE): LC-ESI-MS/MS evidence for Cys374 Michael adduction. J Mass Spectrom 2005;40(7):946–54.
- Patel VB, Spencer CH, Young TA, Lively MO, Cunningham CC. Effects of 4-hydroxynonenal on mitochondrial 3-hydroxy-3-methylglutaryl (HMG-CoA) synthase. Free Radic Biol Med 2007;43(11):1499–507.
- Culligan K, Banville N, Dowling P, Ohlendieck K. Drastic reduction of calsequestrin-like proteins and impaired calcium binding in dystrophic mdx muscle. J Appl Physiol 2002;92(2):435–45.
- Whitehead NP, Pham C, Gervasio OL, Allen DG. N-acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol 2008;586(7):2003–14.
- Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol-Heart Circ Physiol 2007;293(3):H1969–77.
- Dycus DL, Au AY, Grzanna MW, Wardlaw JL, Frondoza CG. Modulation of inflammation and oxidative stress in canine chondrocytes. Am J Vet Res 2013;74(7):983–9.
- Mata M, Morcillo E, Gimeno C, Cortijo J. N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV). Biochem Pharmacol 2011;82(5):548–55.
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B 2007;39(1):44–84.
- Teixeira KC, Soares FS, Rocha LG, Silveira PC, Silva LA, Valenca SS, et al. Attenuation of bleomycin-induced lung injury and oxidative stress by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther 2008;21(2):309–16.
- Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol 1996;157(4):1630–7.
- Palacio JR, Markert UR, Martinez P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm Res 2011;60(7):695–704.
- Vlahakos D, Arkadopoulos N, Kostopanagiotou G, Siasiakou S, Kaklamanis L, Degiannis D, et al. Deferoxamine attenuates lipid peroxidation, blocks interleukin-6 production, ameliorates sepsis inflammatory response syndrome, and confers renoprotection after acute hepatic ischemia in pigs. Artif Organs 2012;36(4):400–8.
- Messina S, Bitto A, Aguennouz M, Minutoli L, Monici MC, Altavilla D, et al. Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp Neurol 2006;198(1):234–41.
- Fraga CM, Tomasi CD, Biff D, Topanotti MFL, Felisberto F, Vuolo F, et al. The effects of N-acetylcysteine and deferoxamine on plasma cytokine and oxidative damage parameters in critically ill patients with prolonged hypotension: a randomized controlled trial. J Clin Pharmacol 2012;52(9):1365–72.
