Abstract
Introduction: Lymphoma is one of the most common types of cancer in dogs, characterized by the proliferation of lymphoid cells. The treatment of this type of cancer is usually based on drugs with high toxicity, which can cause severe side effects.
Objectives: Therefore, the aim of this study was to measure the levels of advanced oxidation protein products (AOPP), thiobarbituric acid reactive substances (TBARS), and ferric reducing antioxidant power (FRAP) in dogs with multicentric lymphoma before and after chemotherapy.
Methods: For this purpose, serum samples of 25 dogs diagnosed with multicentric lymphoma and 15 healthy dogs were used. The animals were exposed to CHOP chemotherapy (cyclophosphamide, vincristine, doxorubicin, and prednisone) and serum samples were collected 5 weeks after treatment.
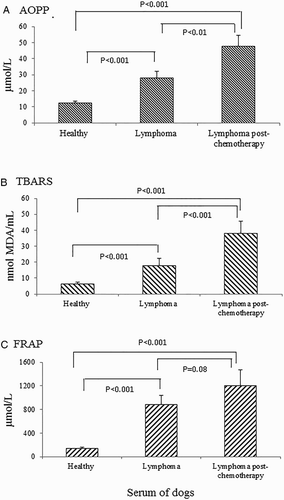
Results: High levels of TBARS, AOPP, and FRAP were observed in sera of dogs with multicentric lymphoma when compared to healthy dogs (P < 0.01), and even higher levels (TBARS and AOPP) were found after chemotherapy i.e. treatment exacerbated the oxidative stress levels. On the other hand, FRAP levels did not differ statistically between animals with lymphoma before and after treatment (P > 0.05). Exacerbated oxidative stress was observed in dogs with multicentric lymphoma Group II (Stage IV-V: involvement of lymph nodes and organs ) compared to those in Group I (Stage I-III: only affected lymph nodes) of the disease, as well as the dogs with clinical signs and T immunophenotype. Another important result was observed after chemotherapy, where FRAP levels were higher in dogs that showed complete disease remission compared to animals with progressive disease.
Conclusions: Therefore, dogs with lymphoma showed protein oxidation and lipid peroxidation, as well as increased total antioxidants before and after chemotherapy compared to the control group.
Keywords:
Introduction
Lymphoma is one of the most common neoplasms diagnosed in dogs and is normally characterized by lymphoid cell proliferation.Citation1 Canine lymphoma represents approximately 8.5–9.0% of all canine tumors and there is no gender dominance. Although this type of neoplasm affects dogs of all ages it is mainly diagnosed in middle-aged or older dogs.Citation2
Multicentric lymphoma is the most common form and accounts for approximately 80% of all canine lymphomas. Clinically it is characterized by anorexia, lethargy, fever, weight loss, and anemia; however, in advanced stages of the disease hepatosplenomegaly and bone marrow infiltration also may be observed.Citation3 Since it is a systemic disease, the treatment recommended is an association of anti-neoplastic drugs. According to the literature, this is considered the treatment of choice for this type of neoplasia due to its relatively high survival rate and therapeutic response.Citation4
Studies have shown alterations in the oxidant/antioxidant balance in dogs with lymphomaCitation5 and mammary carcinoma.Citation6 The imbalance between the concentrations of oxidants and antioxidants characterizes a situation of oxidative stress. Free radicals, when in excess, have detrimental effects, such as the peroxidation of membrane lipids and damage to proteins and enzymes.Citation7 There are some common markers of oxidative stress, such as lipid peroxidation through thiobarbituric acid reactive substances (TBARS) and advanced oxidation protein products levels (AOPP), as well as the estimation of total antioxidant capacity that can be performed with various assays, such as ferric reducing antioxidant power (FRAP).Citation8
In this sense, oxidative stress may play a role in carcinogenesis, as well as in impacting on the morbidity and mortality of dogs with lymphoma.Citation9 However, reactive oxygen species (ROS) also are important for contributing to the efficacy of chemotherapeutic agents and radiation-mediated cytotoxicity.Citation10 Therefore, the purpose of this study was to evaluate biomarkers of oxidative stress in dogs with lymphoma before and after chemotherapy.
Materials and methods
Animals
Our experimental design included 25 dogs with diagnosis of multicentric lymphoma (Table ). They were from the Service of Veterinary Oncology at the Veterinary Hospital of the Faculty of Agricultural and Veterinary Sciences (FCAV/UNESP), Jaboticabal Campus. After the cytological evaluation (Fig. A) and ultrasound, the diagnosis of lymphoma was confirmed through histopathological (Fig. B and C) and immuno histochemical examinations. Blood samples were collected in order to analyze total erythrocytes, hemoglobin concentration, hematocrit, MCV (Mean corpuscular volume), MCHC (Mean corpuscular hemoglobin concentration), and total leukocytes. Differential examination of leukocytes was performed through blood smears (Table ). All the serum samples were frozen for biochemical analyses as described below.
Figure 1 (A) Cytology of dog diagnosed with multicentric lymphoma. Note the presence of atypical lymphocytes with basophilic cytoplasms, loose chromatin, anisocytosis, apparent nucleoli ‘N’, and cell mitosis ‘M’ (BAR: 20 µm). (B) Photomicrograph of diffuse large canine cell lymphoma (BAR: 25 µm), and (C) a magnified version of image B. Note lymphoid cells with large nuclei (black arrow) and distinct nucleoli. Note the mitotic cells (red arrow) (B and C).
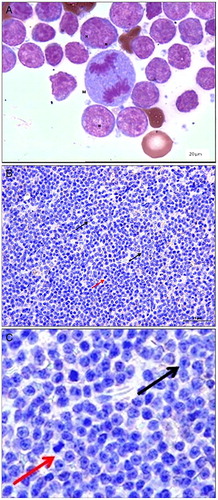
Table 1 Animals used in this study: number, breed, sex, age, and body weight, and histopathological classification of lymphoma
Table 2 Hematological variables of dogs with lymphoma on pre and post CHOP chemotherapy treatment (cyclophosphamide, vincristine, doxorubicin, and prednisone) when compared to healthy dogs
Tissue fragments were fixed in buffered formalin (10%), embedded in paraffin, sectioned at 3 μm, and stained with hematoxylin/eosin. Subsequently, the slides were evaluated according to World Health Organization (WHO) criteria for canine malignant lymphomas.Citation11 In this study, the canine lymphomas were classified as diffuse large B cell (n = 19), lymphoblastic (n = 2), Burkitt's-like (n = 1), follicular (n = 1), lymphocytic (n = 1), or lymphoplasmocytic (n = 1) (Table ).
The clinical stage of each animal was defined by clinical examination, ultrasound, and bone marrow biopsy. Two groups were formed according to the WHO staging of the animals:Citation12 Group I consisted of those dogs that were WHO Stage I–III (n = 9; local, regional, or generalized lymphadenopathy), and Group II contained those cases classified as Stage IV–V (n = 16; liver and/or spleen and/or bone marrow affected by lymphoma, with or without lymphadenopathy). Fourteen dogs were classified as Substage ‘b’, according to the presence of non-specific clinical signs, such as appetite loss, weight loss, vomiting, diarrhea, polyuria, polydipsia, and fever.Citation11 The other animals with lymphoma showed no clinical signs (n = 11).
The characterization of the predominant lymphoma cell type was investigated through the use of primary antibody markers for T lymphocytes (CD3) and B lymphocytes (CD79α). Immunohistochemistry was performed using the horseradish peroxidase (HRP) and 3,3′ diaminobenzidine tetrachloride (DAB) method. In essence, tissue sections (4 μm) were mounted onto charged slides, kept at 57 °C for 24 hours and, subsequently, deparaffinized with xylene and re-hydrated in decreasing concentrations of ethylic alcohol. Heat antigen recovery with citrate solution (pH 6.0) was performed in a pressure chamber (Pascal®; Dako, Carpinteria, CA, USA) for 30 seconds at 128 °C. After returning to room temperature, the slides were washed in deionized water, incubated twice in 3% hydrogen peroxide (10 V) for 10 minutes (in order to block endogenous peroxidase activity) and rinsed with deionized water for 5 minutes. Blocking of unspecific proteins was carried out using an unspecific reaction blocking solution (protein block serum-free – catalog no X0909 – DAKO Corp.). Slides were incubated with the primary antibody at the standardized dilution (anti-CD3 – clone F.7.2.38 dilution 1:100; anti-CD79α – clone HM57 dilution 1:20; both Dako®) overnight (18 hours) at 4 °C, and washed with TRIS buffered solution (Tris-HCl – 0.05M, NaCl – 0.15M; pH = 7.6). Subsequently, slides were incubated with Envision polymer (Dako, Carpinteria, CA, USA) and washed three times in TRIS buffered solution. Color development was obtained through the use of DAB (Dako Cytomation, Carpinteria, CA, USA) and slides were counterstained with Harris's Haematoxylin. After dehydration through increasing concentrations of alcohol, slides were placed in xylene and mounted with resin and cover slips. The lymph nodes of healthy dogs, as well as samples of lymphoma, were used as negative controls and incubated using Universal Negative Control Mouse antibody (Dako® N1698-1). These lymph nodes were also used as positive controls for the reactions.
It was noted that only four dogs showed multicentric lymphoma with immunophenotype T, and the other 21 dogs showed immunophenotype B. Chemotherapy was unsuccessful for immunophenotype T animals, consisting of dogs who had progressive disease.
The animals diagnosed with multicentric lymphoma were exposed to CHOP chemotherapy treatment (cyclophosphamide (250 mg m2), vincristine (0.75 mg m2), doxorubicin (30 mg m2), and prednisone (fourth dose: 2.0 mg kg−1 first cycle; 1.5 mg kg−1 second cycle; 1.0 mg kg−1 third cycle; and 0.5 mg kg−1 fourth cycle)).Citation13 During this period, the animals were re-evaluated in order to check the post-treatment effects and classified as follows: five dogs had complete remission (disappearance of clinical disease); 13 dogs had partial remission (greater than or equal to 50% of tumor size reduction, without new foci); and seven dogs had progressive disease (increase by at least 50% in tumor size, or appearance of new foci).
Sera samples
Twenty-five serum samples from dogs with lymphoma, and 15 serum samples from healthy (the control group) dogs were used to evaluate AOPP, TBARS, and FRAP levels (Table ). A new blood sampling was performed prior (30 minutes) to the beginning of the fifth cycle of chemotherapy, which corresponded to the 5th week of the protocol. All serum samples were stored at −80 °C.
AOPP and TBARS levels
Protein oxidation was assessed through AOPP measured automatically on sera as previously described by Hanasand et al.Citation14 AOPP results were expressed as µmol L−1.
Lipid peroxidation (TBARS level) was measured in sera by spectrophotometry at 535 nm as described by Jentzsch et al.Citation15 and the results were expressed as nanomoles of malondialdehyde per milliliter of serum.
FRAP levels
Levels of FRAP were measured automatically on sera according to the technique described by Benzie and Strain.Citation16 The measurement of this parameter was performed on the modified Griess method using a Cobas Mira automated analyzer and the results were expressed as µmol L−1.
Statistical analysis
Firstly, the data from the hemogram, TBARS, AOPP, and FRAP were subjected to a normality test and the data that showed abnormal distribution were transformed into logarithms (leucogram and FRAP). Normal and transformed data were subjected to the Student t-test to compare between the three groups (healthy dogs (1), lymphoma (2), and post-treatment lymphoma (3)). The AOPP, TBARS, and FRAP levels were compared by t-test in dogs with lymphoma to clinical stage, presence of clinical signs, immunophenotype, and post-treatment effect. Values with probability (P) less than 5% were considered statistically significantly different.
Results
Hematological results are shown in detail in Table . There was no difference between dogs in the control group compared to those with lymphoma regarding total erythrocytes counts; however, post-treatment the dogs with lymphoma showed lower counts on total erythrocytes, hematocrit, and hemoglobin concentration when compared with the before treatment values and with the control group. We found no differences for MCV and MCHC in all groups. These results indicated that the CHOP treatment caused a normocytic and normochromic anemia in these dogs. Total leukocytes were statistically significantly increased in dogs with lymphoma pre-treatment when compared with the control group; however, this difference disappeared post-treatment. The leukocyte increase was due to the elevation of neutrophils, characterizing a neutrophilic leukocytosis. There was a lymphopenia in treated dogs, since lymphocyte counts were reduced in post-treatment groups when compared to pre-treatment data and with the control group.
Table shows the effects of clinical stage, clinical signs, immunophenotype and treatment on TBARS, AOPP, and FRAP. Clinical stage had an influence only on post-treatment TBARS levels, where dogs in Group II showed increased TBARS levels when compared to those in Group I post-treatment. Clinical signs influenced AOPP, TBARS, and FRAP levels before treatment, since the animals with clinical signs had statistically increased levels of AOPP, TBARS, and FRAP when compared with the ones without clinical signs. Immunophenotype influence the levels of TBARS, AOPP, and FRAP. TBARS levels were significantly higher in dogs with type T lymphoma when compared to those with type B. The opposite occurred with FRAP levels, since dogs with type T lymphoma showed statistically lower levels of FRAP when compared with type B. Post-treatment outcomes showed that AOPP progressively increased (with statistical differences) among dogs with complete tumor remission, partial tumor remission and animals with progressive disease. In these groups, TBARS was statistically increased in dogs with progressive disease when compared to dogs with remission; and FRAP showed the opposite pattern, since it was lower in dogs with progressive disease when compared to dogs with complete remission.
Table 3 Effects of disease stage, clinical signs, immunophenotype, and chemotherapy on TBARS, AOPP, and FRAP of dogs with multicentric lymphoma, before and after treatment
AOPP, TBARS, and FRAP levels are shown in Fig. . High levels of TBARS, AOPP, and FRAP were observed in the serum of dogs with multicentric lymphoma when compared to healthy dogs (P < 0.01). In addition, it was possible to observe that after chemotherapy TBARS and AOPP levels were even higher, a sign of exacerbated oxidative stress level. FRAP levels in animals with lymphoma did not differ statistically before and after treatment (P > 0.05).
Discussion
Our findings indicate that canine lymphoma leads to a state of protein oxidation and lipid peroxidation, similar to the findings of other researchers.Citation17,Citation18 In neoplasms, free radicals produced by the tumor cells are responsible for causing oxidative stress, an imbalance between the production of ROS, and antioxidant body defences.Citation19 Our findings are very intriguing since cancer cells are frequently deficient in most crucial antioxidative enzymes, such as catalase, glutathione peroxidase, and superoxide dismutase.Citation20–Citation22 This indicates a high vulnerability of tumor cells to ROS.Citation23,Citation24 High levels of oxidative stress cause cytotoxicity as mentioned above, probably by inhibiting cell proliferation and leading to apoptic/necrotic cell death. However, despite this apparent ‘beneficial’ effect on tumor cells, a high level of ROS continues to induce DNA damage and inflammatory reactions,Citation25 a situation that may explain the increased levels of AOPP.
After CHOP treatment, it was found that chemotherapy exacerbated the oxidative stress i.e. the levels of AOPP and TBARS increased in the serum of dogs treated against lymphoma. Similar results were found by other researchersCitation26 when most patients showed a marked increase in TBARS concentration compared to values measured before therapy. In fact, many conventional anticancer drugs, including vinblastine, doxorubicin, camptothecin, cisplatin, and inostamycin, exhibit antitumor activities via ROS generation.Citation27 Thus, CHOP chemotherapy can also generate oxygen free radicals and lipid peroxidation that are harmful to membrane proteins and DNA.Citation28,Citation29 It is important to highlight that before CHOP treatment, animals with Substage ‘b’ (showing non-specific clinical signs due to the disease) had increased levels of TBARS compared to those with Substage ‘a’. Therefore, chemotherapy for lymphoma leads to increased free radicals, and consequently causes cell damage, which in this study was detected by lipid peroxidation and protein oxidation. Thus, it is suggested that the treatment is a factor aggravating oxidative stress.
Regarding FRAP, the dogs with multicentric lymphoma showed an increase in total antioxidants. Based on the information mentioned earlier regarding the effect of ROS on tumor cells, an increase in FRAP levels might be a response that protects host cells against exacerbated damage. Our results are in agreement with Vadjdovich et al.Citation5 who estimated the total antioxidant status of dogs with lymphoid tissue lymphoma by FRAP. Interestingly, our results showed that pre-treatment and post-treatment dogs with lymphoma did not show a significant (only a tendency) difference in FRAP levels. Even before treatment, our results showed that animals with clinical signs of disease already had increased levels of FRAP; however, it is important to emphasize that dogs (n = 4) with type T lymphoma showed statistically significantly reduced levels of FRAP, and all of them were dogs with progressive disease. Most studies found that dogs with B-cell lymphoma were more likely to achieve a complete remission (81–84%) than dogs with T-cell lymphoma (50–67%).Citation30–Citation32
The degree of any anemia, leukocytosis, and lymphopenia is tumor prognostic indicators and we observed all of these changes after the chemotherapy protocol. Chemotherapy is one of the most important causes of anemia in cancer patients and the association between dose and duration of chemotherapy with anemia is well known.Citation33 In fact, we noted an anemic status post-treatment without, however, MCV and MCHC changes. Anemia is associated with shorter survival in cancer patients and this association has been established for almost all types of cancer studied.Citation34 Other frequent alterations observed in the hematology of dogs with lymphoma included leukocytosis (40%) and lymphopenia (30%).Citation35 Leukocytosis is often detected in dogs with lymphoma, reflecting the inflammatory condition related to the tumor.Citation36 After chemotherapy, leukocytosis was not observed in treated animals, suggesting the reduction in inflammatory conditions linked to the tumor. Lymphopenia (30%) was a finding in this study after chemotherapy. This usually occurs due to sequestration of lymphocytes because of lymph stasis.Citation37 Lymphocytosis has been observed in 20–22% of dogs with lymphoma,Citation35,Citation38 but in animals with leukemic lymphoma the occurrence increases to 86%.Citation39
After the fourth cycle of chemotherapy, it was possible to verify the absence of clinical signs in all dogs according to anamnesis and clinical examination, although the animals showed an increase in lipid peroxidation levels and protein oxidation. It is worth noting that an increase on these variables is bad for the patient, since they are indicative of cell injury, especially when the total antioxidant levels do not increase proportionally.Citation13,Citation14 However, in the period evaluated, clinically treated dogs showed no side effects, though we cannot rule out a possible negative effect to the patient in the long run if AOPP and TBARS levels remain increased.
Based on the results, we conclude that dogs with lymphoma showed protein oxidation, increased lipid peroxidation, and increased FRAP. It is important to emphasize that exacerbated oxidative stress was observed in dogs with multicentric lymphoma in Stage IV–V (involvement of lymph nodes, visceral organs, and/or bone marrow) compared to those in Stage I–III (involvement of lymph nodes only) of the disease. We also found increased oxidative stress in dogs with Substage ‘b’, and those having the T-immunophenotype form of lymphoma. Another important finding after chemotherapy was that FRAP levels were higher in dogs that showed complete remission of the disease when compared to animals with progressive disease.
Ethical committee
The present study was approved by the Ethics Committee for Use of Animals (CEUA) of Universidade Estadual Paulista (UNESP), Jaboticabal Campus under protocol number 027665/10.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest has no conflict of interest.
Ethics approval Commission of Ethics and Animal Welfare The present study was approved by the Ethics Committee for Use of Animals (CEUA) of Universidade Estadual Paulista (UNESP), Jaboticabal campus the protocol number is 027665/10.
References
- Savage CJ. Lymphoproliferative and myeloproliferative disorders. Vet Clin North Am Equine Pract 1998;14:563–578.
- Greenlee PG, Filippa DA, Quimby FW, Patnaik AK, Calvano SE, Matus RE et al. Lymphomas in dogs: a morphologic, immunologic, and clinical study. Cancer 1990;66(3):480–90. doi: 10.1002/1097-0142(19900801)66:3<480::AID-CNCR2820660314>3.0.CO;2-X
- Ettinger SN. Principles of treatment for canine lymphoma. Clin Techniq Small Anim Pract 2003;18(1):92–102. doi: 10.1053/svms.2003.36622
- Vail DM, Young KM. Hematopoietic tumors. In: Small Animal Clinical Oncology. 4th ed. (Withrow SJ, and Vail DM, eds.), WB Saunders, Philadelphia; 2007. pp. 699–784.
- Vajdovich P, Kriska T, Mezes M, Szabó PR, Balogh N, Bánfi A et al. Redox status of dogs with non-hodgkin lymphomas. An ESR Study. Cancer Lett 2005;224(2):339–46. doi: 10.1016/j.canlet.2004.11.037
- Szczubial M, Kankofer M, Lopuszynski W, Dąbrowski R, Lipko J. Oxidative stress parameters in bitches with mammary gland tumours. J Vet Med Series A 2004;51(7–8):336–40. doi: 10.1111/j.1439-0442.2004.00647.x
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biol. Med. 2001;30:1191–212. doi: 10.1016/S0891-5849(01)00480-4
- Winter JL, Barber LG, Freeman L, Griessmayr PC, Milbury PE, Blumberg JB. Antioxidant status and biomarkers of oxidative stress in dogs with lymphoma. J Vet Intern Med. 2009;23(2):311–16. doi: 10.1111/j.1939-1676.2009.0273.x
- Shacter E, Williams JA, Hinson RM, Sentürker S, Lee Y. Oxidative stress interferes with cancer chemotherapy: inhibition of lymphoma cell apoptosis and phagocytosis. Blood 2000;96(2):307–13.
- Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer 2000;37(1):1–18. doi: 10.1207/S15327914NC3701_1
- Valli VE, San Myint M, Barthel A, Bienzle D, Caswell J, Colbatzky F, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol 2011;48(1):198–211. doi: 10.1177/0300985810379428
- Marconato L. The staging and treatment of multicentric high-grade lymphoma in dogs: a review of recent developments and future prospects. Vet J 2011;188(1):34–8. doi: 10.1016/j.tvjl.2010.04.027
- Morrison WB. Chemotherapy options protocols for Lymphoma. In: Lymphoma in dogs and cats. 4th ed. Jackson Wyoming: Teton NewMedia, Texas; 2005. pp. 54–71.
- Hanasan M, Omdal R, Norheim KB, Goransson LG, Brede C. Improved detection of advanced oxidation protein products in plasma. Clin Chim Acta 2012;413(9–10):901–6. doi: 10.1016/j.cca.2012.01.038
- Jentzsch AM, Bachmann H, Fürst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radical Bio Med 1996;20(2):251–6. doi: 10.1016/0891-5849(95)02043-8
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: the FRAP assay. Anal Biochem 1996;239(1):70–76. doi: 10.1006/abio.1996.0292
- Lucas SRR, Gimero MG, Mori CS, Wisthl VABF. Evaluation of oxidant/antioxidant status in dogs with multicentric lymphoma. J Vet Intern Med 2008;22(6):772.
- Winter JL, Barber LG, Freeman L, Griessmayr PC, Milbury PE, Blumberg JB. Antioxidant status and biomarkers of oxidative stress in dogs with lymphoma. J Vet Intern Med 2009;23(2):311–6. doi: 10.1111/j.1939-1676.2009.0273.x
- Klauring JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Ann Rev Pharmacol 2003;44(2):239–67.
- Yamanaka N, Deamer D. Superoxide dismutase activity in WI-38 cell cultures: effect of age, trypsinization and SV-40 transformation. Physiol Chem Phys 1974;6(1):95–106.
- Sato K, Ito K, Kohara H, Yamaguchi Y, Adachi K, Endo H. Negative regulation of catalase gene expression in hepatoma cells. Mol Cell Biol 1992;12:2525–33. doi: 10.1128/MCB.12.6.2525
- Hasegawa Y, Takano T, Miyauchi A, Matsuzuka F, Yoshida H, Kuma K, et al. Decreased expression of glutathione peroxidase mRNA in thyroid anaplastic carcinoma. Cancer Lett 2002;182(1):69–74. doi: 10.1016/S0304-3835(02)00069-1
- Sawa T, Wu J, Akaike T, Maeda H. Tumor-targeting chemotherapy by a xanthine oxidase–polymer conjugate that generates oxygen-free radicals in tumor tissue. Cancer Res 2000;60(4):666–71.
- Fang J, Sawa T, Akaike T, Maeda H. Tumor-targeted delivery of polyethylene glycol conjugated D-amino acid oxidase for antitumor therapy via enzymatic generation of hydrogen peroxide. Cancer Res 2002;62(11):3138–43.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. USA: Oxford University Press; 1999.
- Look MP, Musch E. Lipid peroxides in the polychemotherapy of cancer patients. Chemother 1994;40(1):8–15. doi: 10.1159/000239163
- Simizu S, Takada M, Umezawa K, Imoto M. Requirement of caspase-3 (-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem 1998;273:26900–7. doi: 10.1074/jbc.273.41.26900
- Moore AS, Cotter SM, Frimberger AE, Wood CA, Rand WM, L'Heureux DA. Comparison of doxorubicin and COP for maintenance of remission in cats with lymphoma. J Vet Intern Med 1996;10(3):372–5. doi: 10.1111/j.1939-1676.1996.tb02083.x
- Dias MA, Santana AE, Sobreira MRF, Filho EC. Study of an animal model of blood hypoplasia induced by the antineoplastic agent doxorubicin (Adriblastina). Ars Vet 2003;19(2):246–53.
- Ruslander DA, Gebhard DH, Tompkins MB, Grindem CB, Page RL. Immunophenotypic characterization of canine lymphoproliferative disorders. In Vivo 1997;11:169–72.
- Williams LE, Johnson JL, Hauck ML, Ruslander DM, Price GS, Thrall DE. Chemotherapy followed by half-body radiation therapy for canine lymphoma. J Vet Intern Med 2004;18:703–9. doi: 10.1111/j.1939-1676.2004.tb02609.x
- Kiupel M, Teske E, Bostock D. Prognostic factors for treated canine malignant lymphoma. Vet Pathol 1999;36:292–300. doi: 10.1354/vp.36-4-292
- Coiffier B, Guastalla JP, Pujade-Lauraine E, Bastit P. Anemia study group anemia study group. predicting cancer-associated anaemia in patients receiving non-platinum chemotherapy: results of a retrospective survey. Eur J Cancer 2001;37:1617–23. doi: 10.1016/S0959-8049(01)00169-1
- Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001;91:2214–21. doi: 10.1002/1097-0142(20010615)91:12<2214::AID-CNCR1251>3.0.CO;2-P
- NeuwaldI EB, Teixeira LV, Conrado FO, da Silva MOD, Hlavac NRC, González FHD. Epidemiological, clinical and immunohistochemical aspects of canine lymphoma in the region of Porto Alegre, Brazil. Pesq Vet Bras 2014;34(4):349–54. doi: 10.1590/S0100-736X2014000400009
- Cápua MLB, Coleta FED, Canesin APMN, Godoy AV, Calazans SG, Miotto MR, et al. Linfoma canino: clínica, hematologia e tratamento com o protocolo Madison-Wiscosin. Cienc Rural 2011;41(7):1245–51. doi: 10.1590/S0103-84782011005000090
- Latimer KS, Prasse KS. Leukocytes. In: Latimer KS, Mahaffey EA, Prasse KW, (eds), Veterinary Laboratory Medicine: clinical pathology. 4th ed. Iowa: Iowa State Press. 2003; pp. 46–79.
- Cardoso MJL, Machado LHA, Moutinho FQ, Padovani CR. Linfoma canino: achados clínico-patológicos. Arch Vet Sci 2004;9(2):25–9.
- Tasca S, Carli E, Caldin M, Menegazzo L, Furlanello T, Gallego LS. Hematologic abnormalities and flow cytometric immunophenotyping results in dogs with hematopoietic neoplasia: 210 cases (2002–2006). Vet Clin Pathol 2009;38(1):2–12. doi: 10.1111/j.1939-165X.2008.00099.x