Abstract
Hemorrhagic cystitis (HC) is a major complication after allogeneic stem cell transplantation (allo-SCT) and can be life threatening. To analyze risk factors and prognosis, we retrospectively reviewed 249 cases receiving allo-SCT in our institution. Median age was 47 years (13–72 years). Disease status at SCT was progressive in 73 cases. Conditioning was myeloablative (MAC) in 146 cases. Acute graft-versus-host disease (aGVHD) grade II–IV treated with prednisolone occurred in 82 cases, and cytomegalovirus (CMV) was reactivated in 91 cases. HC was reported in 47 cases at a median of 35 days (7–469 days) after SCT, and 34 (72.3%) cases recovered after a median of 19.5 days (2–252 days). In univariate analysis, the identified risk factors for HC included age over 45 years, progressive disease status, MAC, aGVHD treated with prednisolone, and CMV reactivation. In multivariate analysis, older age, MAC, and CMV remained independent predictors (hazard ratios: 2.35, 3.50, and 2.87). In patients with severe HC, percentage recovery was lower (3 in 13 cases; 23.1%) and the median duration was longer (54 days) than in those with moderate HC (31 in 36 cases; 86.1%, 17 days, P < 0.01). Treatment-related mortality was also higher (59.1%, P = 0.03) and overall survival was poorer (16.7%, P < 0.01) at 1 year after SCT. Prospective studies should be started considering prophylactic antiviral administration in high-risk patients such as those identified in this study.
Introduction
Hemorrhagic cystitis (HC) is a major complication after allogeneic stem cell transplantation (allo-SCT) and is life threatening in severe cases.Citation1,Citation2 Early-onset HC occurring within 2–3 days after SCT is usually due to thrombocytopenia and toxic effects of conditioning regimens containing high-dose cyclophosphamide (CY) or busulfan (BU).Citation3 The use of mesna (2-mercaptoethane sulfonate) and forced hydration can protect against the occurrence of CY-induced early-onset HC. Late-onset HC occurring weeks or months after SCT is caused by graft-versus-host disease (GVHD) and infections with bacteria or viruses such as adenovirus (ADV), BK virus (BKV), and JC virus (JCV).Citation4–Citation7 The manifestations vary from microscopic hematuria with minimal urinary symptoms to severe hemorrhage involving the entire urinary tract, leading to clot formation with obstruction requiring surgical intervention, such as cystoscopic clot evacuation and cauterization. HC is usually self-limited and resolves without other complications, whereas severe HC can be life threatening due to exacerbation of GVHD or opportunistic infections. In this study, we retrospectively analyzed the risk factors for late-onset HC, in addition to the outcomes of patients with this complication, in order to improve the prognosis of those receiving allo-SCT.
Patients and methods
Patients and treatment characteristics ()
We analyzed 249 patients who underwent allo-SCT in our institute from January 1996 through September 2010, excluding 23 patients who received a second allo-SCT. The underlying disease was acute myelogenous leukemia (AML) in 68 patients, myelodysplastic syndrome (MDS) in 46 patients, acute lymphoblastic leukemia (ALL) in 47 patients, malignant lymphoma (ML) in 49 patients, chronic myelogenous leukemia (CML) in 12 patients, multiple myeloma (MM) in 17 patients, and others (aplastic anemia, or pure red cell aplasia) in 10 patients. The median age at SCT was 47 years (range, 13–72 years), and the median follow-up of survivors was 1723 days (range, 61–5274 days) after SCT. Disease status at SCT was controlled (partial or complete remission for AML, ALL, ML, MM; marrow blasts less than 10% in MDS; chronic phase for CML; all non-malignant hematological diseases) in 176 cases, and progressive (those unsatisfying the criteria for controlled disease described above) in 73 cases. Conditioning was myeloablative (MAC) in 146 cases and reduced intensity conditioning (RIC) in 103 cases. One hundred and fifteen patients received transplants from related donors (using bone marrow (BM) in 96 cases, peripheral blood stem cells (PBSC) in 19 cases), and 134 from unrelated donors (BM in 116 cases, cord blood (CB) in 18 cases).
Table 1. Patient characteristics
Transplantation procedure
Definitions of MAC and RIC were in accordance with those of the RIC regimen workshop.Citation8 MAC was CY (60 mg/kg/day for 2 days) plus fractioned total body irradiation (TBI, 12 Gy fractionated into four sessions) in 92 cases, and intravenous BU (3.2 mg/kg/day for 4 days) plus CY in 54 cases, whereas RIC was fludarabine (Flu, 25 mg/m2/day for 5 days) plus melphalan (Mel, 50 mg/m2/day for 2 days) plus intravenous BU (1.6 mg/kg/day for 2 days) in 57 cases, and Flu plus Mel plus fractioned TBI (4 Gy fractionated into two sessions) in 17 cases. High-dose cytarabine (2 g/m2 in 3 hours for 3 days) was added in some cases with progressive disease. Mesna (400 mg) was administered three times a day to prevent CY-induced HC. Prophylaxis against acute GVHD (aGVHD) was mainly calcineurin inhibitors (cyclosporine A, 110 cases; FK506, 139 cases), but at the discretion of the managing physician, short-term methotrexate (175 cases) and corticosteroid (73 cases) were added. One hundred and thirty-three cases developed aGVHD; 102 were grade II–IV, and 82 of these 102 cases (excluding those only with skin lesions) were treated with 0.5–2.0 mg/kg prednisolone (PSL). Acyclovir (1000 mg) was given prophylaxis orally against herpes simplex virus from 7 days before SCT through 35 days after SCT. Cytomegalovirus (CMV) antigenemia was monitored weekly after engraftment using monoclonal antibody C10/C11 assays, and turned positive (reactivation) in 91 cases.
Definition, grading, and treatment of HC
HC was defined as microscopic or macroscopic hematuria with urinary symptoms occurring at least 7 days after SCT. Urinalysis was carried out daily after SCT. We excluded cases with bacteriuria or early-onset HC (occurring within 7 days from SCT). Each HC case was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03Citation9 (grade 1: microscopic hematuria, minimal increase in frequency, urgency, dysuria, or nocturia, new onset of incontinence; grade 2: moderate hematuria, moderate increase in frequency, urgency, dysuria, nocturia or incontinence, urinary catheter placement or bladder irrigation indicated, limiting instrumental activity of daily living; grade 3: gross hematuria, transfusion, intravenous medications or hospitalization indicated, elective endoscopic, radiologic or operative intervention indicated; Grade 4: life-threatening consequences, urgent radiologic or operative intervention indicated; grade 5: death) and we defined moderate as grade 1 or 2 and severe as grade 3 or more, which required surgical intervention or transfusion. Grading was decided at the worst point during the clinical course. Urine culture and virological analysis (polymerase chain reaction (PCR) of ADV, BKV, and JCV DNA) were carried out in all cases with HC at an onset of hematuria. Viral DNA was extracted using QIAamp® DNA Blood Mini Kit and MinElute Virus Kit (Qiagen, Valencia, CA, USA) from the urine specimens. Using mixed primer, multiplex PCR (GeneAmp® PCR System 9700; Applied Biosystems, Foster City, CA, USA) was carried out, and any virus that proved positive was considered to be the cause of HC. Urine viral load quantitation and PCR of blood were not carried out in all cases. Virological analysis in patients without urinary symptoms was not done. Treatment was mainly hydration and intravenous an antihemorrhagic agents (carbazochrome sodium). In cases with severe symptoms, additional therapies were administered, such as intravenous immunoglobulin (IVIg), antiviral agents (gancyclovir or vidarabine), and surgical intervention (ureteral stenting, continuous irrigation, and cystoscopic clot evacuation). Neither hyperbaric oxygen nor mesenchymal stem cells were administered. Recovery from HC was defined as disappearance of all urinary symptoms and hematuria on urinalysis even after cessation of all HC therapies. Urinary virological analysis was not mandatory for a determination of recovery.
Statistical analyses
Risk factors for the development of HC were evaluated using Cox's regression model. The probability of overall survival (OS), treatment-related mortality (TRM), and the cumulative incidence of HC were estimated with the Kaplan–Meier method, and groups were compared using the log-rank test. OS and TRM were calculated using landmark analysis after day 35 (median time-point of HC occurrence) to exclude early deaths from other causes. Statistical calculations regarding the clinical courses of HC were made using the Mann–Whitney test and the χ2 test. All P values were two sided, with P < 0.05 indicating statistical significance. Statistical analyses were performed using SPSS (IBM SPSS Statistics version 19; IBM Corporation, New York, NY, USA).
Results
Incidence of HC
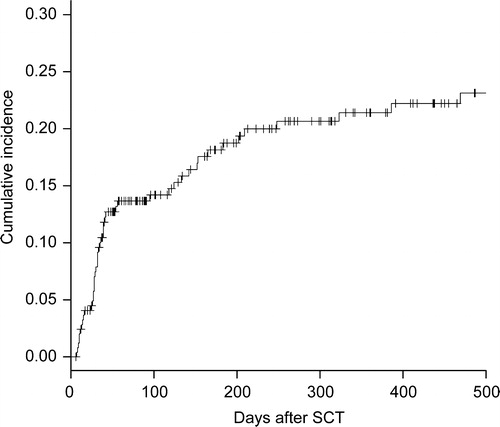
HC was diagnosed in 47 cases at a median of 35 days (range, 7–469 days) after SCT, and the cumulative incidence was 21.4% (95% confidence interval 15.5–26.9%) at 1 year after SCT (). Moderate HC developed in 35 cases (grade 1, 14; grade 2, 21) and severe HC developed in 12 cases (all grade 3). Urine samples of 34 patients (72.4%; 68.6% in moderate cases, 83.3% in severe cases) were positive for at least one virus by PCR: ADV was positive in 20 cases (42.6%; 12 moderate cases, 8 severe cases), BKV was positive in 20 cases (42.6%, 15 moderate cases, 5 severe cases), and JCV was positive in 6 cases (12.8%; 5 in moderate, 1 in severe). In patients with ADV, most cases are caused by ADV type 11 as previously reported.Citation10 PCR was positive for more than one virus in 12 cases. In contrast, in 13 cases (moderate, 5 and severe, 2) PCR was negative for all viral DNA.
Risk factors for HC
Age over 45 years, progressive disease status at SCT, MAC, aGVHD grade II–IV treated with PSL, and CMV reactivation after SCT were identified as risk factors by univariate analysis (). There were no significant relationships between the occurrence of HC and sex, underlying disease, GVHD prophylaxis, or donor source (including related or unrelated, HLA matching, and BM, PBSC, or CB). As for donor source, the percentage of PBSC and CB in our study was too low (19; 7.6% and 18; 7.2%, respectively) to discuss with proper statistical power. In cases with MAC, receiving a BU (non-TBI) regimen was a significant risk factor of HC (P < 0.01, hazard ratio 1.98). In RIC cases, the use of BU versus TBI had no impact on the occurrence of HC (P = 0.84). In multivariate analysis, older age, MAC, and CMV reactivation remained independent predictors. In subgroup analysis of patients with HC, the above five factors plus virology were analyzed in order to determine risk factors for severe HC. MAC (P = 0.06) and ADV by PCR in urine (P = 0.05), and progressive disease (P = 0.07) showed relationships with severe HC. Acute GVHD, BKV, JCV, CMV reactivation, older age were not associated with severe HC ().
Table 2. Risk factors for HC
Table 3. Risk factors for severe HC
Clinical outcomes of HC
Recovery from HC was documented in 72.3% of all HC cases (86.1% moderate cases, 23.1% severe cases, P < 0.01). In cases not showing recovery, death can occur due to relapse or other complications setting in before recovery. The median duration from the onset of HC to recovery was 19.5 days (range, 2–252 days), longer in severe than in moderate HC cases (median, 54 versus 17 days, P < 0.01). During these clinical courses, 21.3% (10 of 47) cases developed the complication of acute renal failure (defined as elevation of serum creatinine by 2.0 folds) due to obstructive uropathy and retrograde inflammation to kidneys, and the percentage was significantly higher (6 of 12; 50.0%) in severe than in moderate HC cases (4 of 35; 11.4%, P < 0.01). Viral infections of other organs (e.g. viral pneumonia or encephalitis) were not documented in patients with HC. In addition to the main treatment of hydration and intravenous hemostasis, IVIg was used in 24 cases, and antiviral agents were administered to 12 (gancyclovir, 10; vidarabine, 2). These therapies did not significantly improve the percentage of recovery; 75.0% versus 70.0% in patients with or without IVIg (P = 0.69), and 66.7% versus 74.3% in patients with or without antiviral agent treatments (P = 0.63). Severe cases required surgical treatment with ureteral stenting (five cases), continuous irrigation (four cases), and cystoscopic clot evacuation (two cases). The clinical courses of severe HC cases were very poor; only three of these severe cases recovered from HC (). The main causes of death were exacerbation of aGVHD and opportunistic infections in addition to relapse of the original disease. TRM at 1 year after the day 35 landmark was 30.3% with HC and 33.0% without HC, showing no significant difference (P = 0.31), whereas it was 59.1% in patients with severe HC, significantly higher than in those with moderate HC (21.5%, P = 0.02) or those without HC (27.9%, P = 0.06) (). The causes of TRM in patients with HC were GVHD in five cases (complicated by hemophagocytic syndrome and diffuse alveolar hemorrhage in two cases) and infection in six cases (including trichosporonosis and legionellosis). OS at 1 year after the day 35 landmark did not differ significantly between cases with and without HC (P = 0.96), but was significantly poorer in cases with severe (16.7%) than in those with moderate HC (67.6%, P < 0.01) and those without HC (61.5%, P < 0.01) ().
Figure 2. TRM (severe hemorrhagic cystitis (severe HC; large dotted line) versus moderate HC (small dotted line) versus without HC (bold line)). TRM at 1 year after day 35 landmark was 30.3% with HC and 33.0% without HC, showing no significant difference (P = 0.31), whereas it was 59.1% in patients with severe HC, significantly higher than in those with moderate HC (21.5%, P = 0.02) or those without HC (27.9%, P = 0.06).
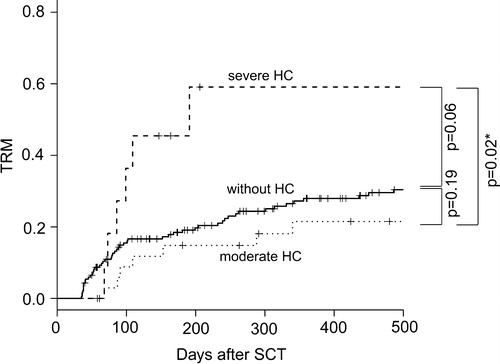
Figure 3. OS (severe hemorrhagic cystitis (severe HC; large dotted line) versus moderate HC (small dotted line) versus without HC (bold line)). OS at 1 year after the day 35 landmark did not differ significantly between cases with and without HC (P = 0.96), but was significantly poorer in cases with severe (16.7%) than in those with moderate HC (67.6%, P < 0.01) and those without HC (61.5%, P < 0.01).
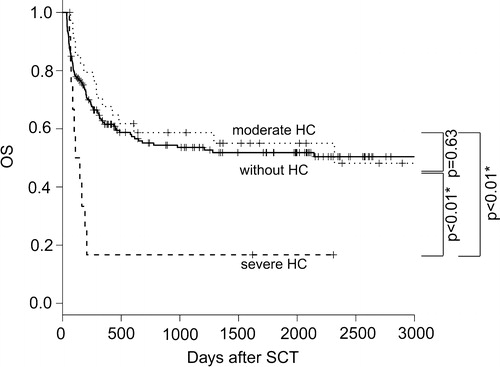
Table 4. Clinical courses of patients with severe HC
Discussion
HC often occurs after allo-SCT. Patients usually recover uneventfully, but in severe cases HC can be life threatening with other complications after allo-SCT.Citation1,Citation2 In order to stop HC progression at an early stage and improve outcomes of allo-SCT, we retrospectively analyzed etiology, risk factors, and the prognosis of patients with HC.
Several studies have been carried about HC after allo-SCT, but sample sizes were almost all less than 150. As far as we know, the largest study from the United KingdomCitation11 consists of 462 cases of allo-SCT (13.6% of them complicated HC). This study, however, included 101 pediatric cases, and values as of incidence and risk factors were calculated all together. Older age is one of risk factors shown in our study, and HC in adult patients should be discussed separately. The second largest study was one from Hong KongCitation12 including 165 cases (HC occurred in 27 of them). This study discusses the risk factor and duration of HC, but does not clearly discuss the impact on OS. HC is often life threatening, so this discussion is necessary. In these regards, the sample size (now the second largest study) and the discussion range of our study is large enough to be referred in the field of HC after allo-SCT.
The cumulative incidence of HC at 1 year after SCT was 21.4% in this study. The incidences reported in other studies ranged from 10 to 60%. This difference is due to different definitions, underlying diseases, conditioning regimens, and other complications.Citation13–Citation15 Other parameters differed minimally among studies; HC occurred about 4–5 weeks after SCT, and the median duration was about 3 weeks.Citation3,Citation15 These periods may be related to the pathogenesis of HC development; chemotherapy and irradiation in the conditioning regimen damage uroepithelial cells, with viral replication leading to viruria, and allo-immunity against these uroepithelial cells after engraftment causes hematuria. This process is revealed by cystoscopic examination as focal or diffuse hemorrhagic and inflammatory changes of the urinary bladder mucosa.Citation16
Many risk factors were identified in previous studies: age, MAC, BU-based conditioning, matched unrelated donor and unrelated CB (compared with matched related donor), BM (compared with PBSC), CMV infection, delay of platelet engraftment, BKV DNA by PCR in urine, and aGVHD.Citation2,Citation3,Citation7,Citation15,Citation17–Citation20 These risk factors are strongly related to immunosuppressive status, increasing the risk of opportunistic bacterial, fungal, and viral infections, and also reactivation of latent viruses leading to HC.Citation3,Citation4,Citation21,Citation22 Our study found progressive disease status at SCT to be a new risk factor for HC. This might be due to the tendency for a higher total chemotherapy dose and poorer general status in these patients. The percentage of cases without remission at the point of allo-SCT is a little higher in our institute (69.5%) than in others, but actually many patients gather eager for allo-SCT as final therapeutic option. BK viruria has been shown to be the risk of HC,Citation23 but virological analysis before allo-SCT or in patients without urinary symptoms was not done in our study. In subgroup analysis, the severity of HC was related to MAC, ADV in urine, and progressive disease. MAC damages uroepithelial cells more severely than RIC, and ADV viruria reflects replication of ADV leading to strong allo-immunity and severe HC.
The virological analysis results in this study are of clinical interest. The virology data differ among studies; reports from western countries show lower incidences of ADV but higher BKV positive rates,Citation24 whereas in Japan, including this study, the ADV-positive rate is higher,Citation25 though in pediatric field, BKV is dominant even in JapanCitation26 as well as in western countries.Citation27 This dominance of ADV in adult cases may be due to racial differences in latent viral infection, drug metabolism, or immune responses. We must pay special attention to ADV infection after allo-SCT, causing invasive infections of the lungs, kidneys, and liver, in addition to HC, leading to multiple organ failure, requiring prompt administration of strong antiviral agents.Citation28 In some cases with ADV-related HC, we used gancyclovir or vidarabine, such that no cases developed disseminated ADV infection, though the effect on HC was limited. Viruses causing HC (ADV, BKV, and JCV) are sensitive to cidofovir, and this agent has shown promising clinical results including with prophylactic administration.Citation16,Citation29 In Japan, however, cidofovir has not yet been approved. To decrease the mortality associated with HC, prompt approval is mandatory. Another point of discussion in virology is whether detection of viral DNA in urine by PCR is always associated with HC. These viruses can be reactivated after allo-SCT due to immunodeficiency, and can be excreted into the urine even in cases without HC after allo-SCT. BKV is, for example, reportedly identified in 29.3% of patients with HC, but also in 11% of urine samples obtained from patients without HC.Citation18 We, however, analyzed only patients with urinary symptomatic HC. In such an analysis, it is reasonable to consider a virus detected in urine by PCR to be the causal pathogen of HC, as in other studies.Citation30
Prognosis is another clinical interest in this study. Other studies have reported patients with HC to have a poor prognosis. The OS and TRM of patients with all grades of HC were equivalent to those without HC, whereas severe HC significantly raised TRM and shortened OS. This means that prevention of severe HC is the key strategy to improving the outcomes of allo-SCT. In cases with HC, aGVHD and infection were the main causes of death. HC caused by viral reactivation often limits the use of strong immunosuppressive therapies for GVHD, which results in exacerbation of GVHD. In some cases, viral infection itself directly activates severe GVHD. Severe HC requiring surgical intervention negatively affects performance status and leads to other infectious diseases due to worsening immunosuppressive status.Citation18
In summary, we identified five risk factors for HC in this analysis of pre-transplant status, transplantation procedures, and complications after SCT. Risk factors for severe HC were also analyzed, and MAC, ADV, and progressive disease showed relationships with the severe form of this disease. The prognosis of HC patients with all grades was also the same as those of patients without HC, whereas that of severe HC was significantly poorer, mainly due to GVHD and opportunistic infections. No successful methods of preventing HC have yet been established. Prospective studies considering, for example, prophylactic antiviral administration in the high-risk patients identified in this study, are needed.
Authorship
Yasuyuki Arai performed the research and wrote the paper. Takeshi Maeda and Yasunori Ueda revised the paper critically. Yasuyuki Arai, Takeshi Maeda, Hiroyuki Sugiura, Hiroyuki Matsui, Tomoyasu Jo, Tomoaki Ueda, Kazuya Okada, Takehito Tawaka, Tatsuhito Onishi, and Chisato Mizutani cared the patients and contributed to the acquisition of data.
References
- Yang CC, Hurd DD, Case LD, Assimos DG. Hemorrhagic cystitis in bone marrow transplantation. Urology. 1994;44:322–8.
- Seber A, Shu XO, Defor T, Sencer S, Ramsay N. Risk factors for severe hemorrhagic cystitis following BMT. Bone Marrow Transplant. 1999;23:35–40.
- Hassan Z, Remberger M, Svenberg P, Elbander M, Omazic B, Mattsson J, et al. Hemorrhagic cystitis: a retrospective single-center survey. Clin Transplant. 2007;21:659–67.
- Ost L, Lonnqvist B, Eriksson L, Ljungman P, Ringden O.. Hemorrhagic cystitis – a manifestation of graft versus host disease? Bone Marrow Transplant. 1987;2:19–25.
- Miyamura K, Takeyama K, Kojima S, Minami S, Matsuyama K, Morishima Y, et al. Hemorrhagic cystitis associated with urinary excretion of adenovirus type 11 following allogeneic bone marrow transplantation. Bone Marrow Transplant. 1989;4:533–5.
- La Rosa AM, Champlin RE, Mirza N, Gajewski J, Giralt S, Rolston KV, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–6.
- Leung AY, Suen CK, Lie AK, Liang RH, Yuen KY, Kwong YL. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood. 2001;98:1971–8.
- Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9.
- Common Terminology Criteria for Adverse Events v4.03. National Cancer Institute, 2010.
- Tomonari A, Takahashi S, Ooi J, Fukuno K, Takasugi K, Tsukada N, et al. Hemorrhagic cystitis in adults after unrelated cord blood transplantation: a single-institution experience in Japan. Int J Hematol. 2006;84:268–71.
- Anoop P, Shaw BE, Riley U, Ethell ME, Taj M, Lancaster DL, et al. Clinical profile and outcome of urotheliotropic viral haemorrhagic cystitis following haematopoietic stem cell transplantation: a 7-year tertiary centre analysis. Hematology. 2011;16:213–20.
- Leung AY, Mak R, Lie AK, Yuen KY, Cheng VC, Liang R, et al. Clinicopathological features and risk factors of clinically overt haemorrhagic cystitis complicating bone marrow transplantation. Bone Marrow Transplant. 2002;29:509–13.
- Atkinson K, Biggs JC, Golovsky D, Concannon A, Dodds A, Downs K, et al. Bladder irrigation does not prevent haemorrhagic cystitis in bone marrow transplant recipients. Bone Marrow Transplant. 1991;7:351–4.
- Tsuboi K, Kishi K, Ohmachi K, Yasuda Y, Shimizu T, Inoue H, et al. Multivariate analysis of risk factors for hemorrhagic cystitis after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:903–7.
- El-Zimaity M, Saliba R, Chan K, Shahjahan M, Carrasco A, Khorshid O, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation: donor type matters. Blood. 2004;103:4674–80.
- Leung AY, Yuen KY, Kwong YL. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplant. 2005;36:929–37.
- Ringden O, Ruutu T, Remberger M, Nikoskelainen J, Volin L, Vindelov L, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83:2723–30.
- Akiyama H, Kurosu T, Sakashita C, Inoue T, Mori S, Ohashi K, et al. Adenovirus is a key pathogen in hemorrhagic cystitis associated with bone marrow transplantation. Clin Infect Dis. 2001;32:1325–30.
- Giraud G, Priftakis P, Bogdanovic G, Remberger M, Dubrulle M, Hau A, et al. BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts. Bone Marrow Transplant. 2008;41:737–42.
- Silva Lde P, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–90.
- Paulin T, Ringden O, Lonnqvist B. Faster immunological recovery after bone marrow transplantation in patients without cytomegalovirus infection. Transplantation. 1985;39:377–84.
- Paulin T, Ringden O, Nilsson B, Lonnqvist B, Gahrton G. Variables predicting bacterial and fungal infections after allogeneic marrow engraftment. Transplantation. 1987;43:393–8.
- Bogdanovic G, Priftakis P, Giraud G, Dalianis T. A related donor and reduced intensity conditioning reduces the risk of development of BK virus-positive haemorrhagic cystitis in allogeneic haematopoetic stem cell-transplanted patients. Anticancer Res. 2006;26:1311–8.
- Azzi A, Fanci R, Bosi A, Ciappi S, Zakrzewska K, de Santis R, et al. Monitoring of polyomavirus BK viruria in bone marrow transplantation patients by DNA hybridization assay and by polymerase chain reaction: an approach to assess the relationship between BK viruria and hemorrhagic cystitis. Bone Marrow Transplant. 1994;14:235–40.
- Asano Y, Kanda Y, Ogawa N, Sakata-Yanagimoto M, Nakagawa M, Kawazu M, et al. Male predominance among Japanese adult patients with late-onset hemorrhagic cystitis after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:1175–9.
- Hatakeyama N, Suzuki N, Yamamoto M, Kuroiwa Y, Hori T, Mizue N, et al. Detection of BK virus and adenovirus in the urine from children after allogeneic stem cell transplantation. Pediatr Infect Dis J. 2006;25:84–5.
- Cesaro S, Facchin C, Tridello G, Messina C, Calore E, Biasolo MA, et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:363–70.
- Shields AF, Hackman RC, Fife KH, Corey L, Meyers JD. Adenovirus infections in patients undergoing bone-marrow transplantation. The New England journal of medicine. 1985;312:529–33.
- Bordigoni P, Carret AS, Venard V, Witz F, Le Faou A. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2001;32:1290–7.
- Gaziev J, Paba P, Miano R, Germani S, Sodani P, Bove P, et al. Late-onset hemorrhagic cystitis in children after hematopoietic stem cell transplantation for thalassemia and sickle cell anemia: a prospective evaluation of polyoma (BK) virus infection and treatment with cidofovir. Biol Blood Marrow Transplant. 2010;16:662–71.