Abstract
In this study, we investigate the validity and laboratory utility of flow cytometry when analyzing platelet activation by studying CD41, CD42b, CD62P and CD63. We compare flow cytometry results from citrated whole-blood and finger-prick samples directly after collection and also after storing both a finger-prick and whole-blood sample for 24 hours. Citrated whole-blood and finger-prick samples were taken from three healthy individuals on two occasions, and a total of 60 000 cells were analyzed for each of the four phycoerythrin-labeled monoclonal antibodies. Half of each sample was analyzed immediately after sampling while the other half was kept in the fridge at 6°C for 24 hours before analysis. No significant difference was found between the sampling methods or the period of time before analysis. Results therefore suggest that an appropriately prepared finger-prick sample can be used for platelet function analysis, and samples can be stored for 24 hours in the fridge at 6°C before analysis.
Introduction
Flow cytometry is a powerful technique, and its importance in research and clinical investigation has long been established.Citation1 Markers used in flow cytometry may give us valuable information regarding cellular activity and since 1989 it has been deemed an essential tool for the investigation of platelets.Citation2 Flow cytometry thus provides a numerical technique, which is both objective and quantitative, to assess platelet function.Citation3
Several surface glycoproteins (GPs) are found on the membrane of platelets and flow cytometry has been used to a great extent in the immunophenotyping of these entities. The study of platelet function, physiology, and their interaction with other cells has been advanced by investigating the recognition of these surface GPs by specific monoclonal antibodies (MoAbs). Not only can the GPs on the surface of the platelet membrane be detected by MoAbs, but molecules transferred to the surface from the internal platelet granules can be identified.Citation3 This is of particular interest in the study of platelet activation.
The application of a panel of MoAbs is preferred for flow cytometric analysis of platelets, as different flow cytometric probes reveal different characteristics of platelet function.Citation4 CD41 as well as CD42b are frequently used as platelet identifiers, as they are only present on platelets and not any other circulating blood cell.Citation2,Citation5,Citation6 Alterations in antigenic determinants can also be investigated and MoAbs are used to label epitopes specifically expressed on the platelet membrane. Studies of extracorporeal circulation have mainly used CD62P and CD63 to investigate platelet activation.Citation7 Therefore, flow cytometry can be employed to establish the amount of activated and non-activated platelets.Citation3
Whole blood can be obtained from either blood drawn in blood tubes with added anti-coagulants (e.g. citrate tubes) or by sampling blood from a finger-prick. These methods have been used previously by Wall et al. to investigate platelet dense granule release and uptake. Both whole blood collected in sodium citrate tubes and 20 µl of blood from a ‘fingerstick’, as they referred to it, without added anticoagulant were used. After collection, the samples were immediately diluted with Hanks balanced salt solution and a fluorescent marker, mepacrine, was added.Citation8
In research where a sample population needs to be followed over a prolonged period of time, or over consecutive days, it is not practical to frequently draw blood. It is much more acceptable for the participants to donate a finger-prick sample. The question also arose whether storage times would influence results. Therefore, in this study we investigate the repeatability of flow cytometry results when using blood drawn in citrate tubes and samples from finger-pricks – either used immediately, or after samples were stored for 24 hours.
Materials and methods
Blood collection
Blood was collected from three healthy, control male individuals. Ethical approval was obtained from the University of Pretoria, Human Ethics Committee, and all participants completed informed consent forms. The participants were non-smokers, did not use any chronic medication and did not have a history of thrombotic disease. None of the participants were using aspirin or aspirin analogues. Firstly, 5 ml of blood was drawn into a citrate tube (0.5 ml of sodium citrate (3.8%) for 4.5 ml of blood) for the determination of the platelet gate. Another 5 ml of blood was drawn into a citrate tube for the subsequent experiments. Sodium citrate is an anticoagulant usually used for flow cytometric analysis of whole blood.Citation4,Citation9 A volume of 20 µl of blood was collected from finger-pricks. For each concentration of citrate analyzed, a separate finger-prick was taken to ensure that any possible platelet activation after the first sampling was eliminated. Procedures were done in duplicate.
Sample preparation
Platelet-rich plasma preparation
Whole blood was left to stand for 45 minutes to obtain platelet-rich plasma (PRP) by self-sedimentation. Twenty microliters of PRP was transferred into a 1-ml aliquot of IsoflowTM Sheath Fluid (Beckman Coulter, Krefeld, Germany). IsoflowTM Sheath Fluid, an isotonic fluid used exclusively for flow cytometry, is specifically formulated to ensure low particle and fluorescence backgrounds to guarantee superior signal-to-noise ratio measurements. This mixture of PRP and IsoflowTM was stained with 20 µl (the quantity of product sufficient to stain, as prescribed by Beckman Coulter) of one of the following probes separately: CD41-PE (clone P2), CD42b-PE (clone SZ2), CD62P-PE (clone CLB-Thromb/6), and CD63-PE (clone CLBGran/12).
Whole-blood preparation
Experiment 1
From the 5 ml of citrated blood, 20 µl was transferred into 1-ml aliquots of IsoflowTM Sheath Fluid. The 20 µl of blood was chosen as to be able to compare the whole-blood sample to that of the 20 µl of blood collected from the finger-prick. Hundred microliters of the mixture was placed into four flow tubes. The remainder of the blood mixture was placed in the fridge at 6°C. Each tube was stained with the 20 µl of one of the following probes separately: CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE (Beckman Coulter). The flow tubes containing CD62P-PE and CD63-PE were also stained with 20 µl of CD41-FITC.
Experiment 2
Twenty microliters of the 5 ml whole blood drawn in the citrate tube was placed into 1-ml aliquots of sheath fluid. Hundred microliters of the mixture was placed into four separate flow tubes. Each tube was stained with 20 µl of CD41-FITC and 20 µl of one of the following probes separately: CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE (from Beckman Coulter).
Twenty-four hours after sampling, the remaining blood mixture, placed in the fridge at 6°C overnight, was prepared in the same manner as described above.
Experiment 3
From the 5 ml of citrated blood, 20 µl was transferred into 1-ml aliquots of sheath fluid containing various concentrations of thrombin namely (i) 5 µl thrombin, (ii) 10 µl thrombin, and (iii) 20 µl thrombin. This volume of blood (20 µl) was chosen as to be able to compare the whole-blood sample to that of the 20 µl of blood collected from the finger-prick. 1 Hundred microliters of the mixture was placed into four separate flow tubes. Each tube was stained with 20 µl of CD41-FITC and 20 µl of each of the following probes separately: CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE (from Beckman Coulter).
Finger-prick preparation
Experiment 1
For the finger-prick sample collection, three separate finger-pricks were done with a lancet. Twenty microliters of blood was collected from each finger-prick and immediately placed in 1-ml aliquots of sheath fluid containing various volumes of citrate (3.8% sodium citrate), namely (i) no citrate, (ii) 5 µl of citrate, and (iii) 10 µl of citrate. Hundred microliters of the blood, sheath fluid, and citrate combination was transferred to four individual flow tubes from each sample. The remaining blood, sheath fluid, and citrate combination was placed in the fridge at 6°C. Twenty microliters of the following probes were added to each tube separately: CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE (from Beckman Coulter). The flow tubes containing CD62P-PE and CD63-PE were also stained with CD41-FITC.
Experiment 2
The 20 µl samples of blood collected from three separate finger-pricks were immediately placed in 1-ml aliquots of sheath fluid containing various volumes of citrate (3.8% sodium citrate), namely (i) no citrate, (ii) 5 µl of citrate, and (iii) 10 µl of citrate. Hundred microliters of the blood, sheath fluid, and citrate combination was transferred to four individual flow tubes. Each tube was stained with 20 µl of CD41-FITC and 20 µl of one of the following probes: CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE (from Beckman Coulter).
Twenty-four hours after sampling, the remainder of the blood, sheath fluid, and citrate mixture, placed in the fridge at 6°C overnight, was prepared in an identical manner as described above.
Flow cytometric analysis
The flow cytometer was calibrated and standardized before use with fluorochrome-labeled bead (FlowCheckTM Fluorospheres, Beckman Coulter). Samples stained with the different probes, were incubated at room temperature in the dark for 20 minutes before analyzed by a flow cytometer (FC 500, Beckman Coulter). Forward scatter (FS) and 90° side scatter (SS) were displayed on logarithmic scales. Two platelet gates were set. The first gate was set according to the morphological characteristics of platelets in the PRP sample (FS and SS) while the second gate was set according to CD41-FITC fluorescence, a platelet-specific marker (PSM). For the duplicate procedures 60 000 platelets were counted and analyzed per phycoerythrin-labeled monoclonal antibody (including CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE). Instrument configuration and settings are shown in . The fluorescence of the different antibodies was plotted on 256-channel log histograms. The acquired Listmode data were analyzed with CyflogicTM software (CyFlo Ltd, Finland). The results were expressed in arbitrary units as mean channel fluorescence intensity (MCFI).
Table 1. Instrument configuration and settings
Statistical analysis
Results from the flow cytometric analysis were compared by using the paired two-sided Student's t-test. MCFI results are represented as mean ± standard deviation. A P value of ≤ 0.05 was considered significant.
As three similar participants were chosen, the MCFI observations for the three participants can be regarded as being independent and identically distributed random variables for each of the different blood-preparation techniques. Furthermore, as the MCFI for each participant was calculated as the mean fluorescence of a large sample of platelets (10 000 platelets per individual per sample), the well-known Central Limit Theorem assures us that the Normal distribution is a close approximation for the distribution of the MCFIs. This allows us to make use of the paired two-sided Student's t-test to compare the results from the flow cytometric analysis at a 5% level of significance.
Results
Gating strategies
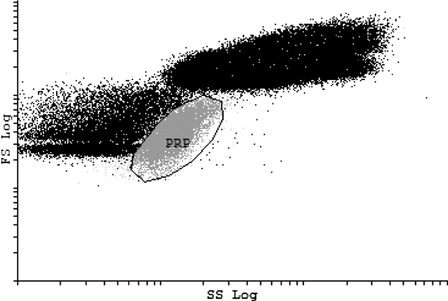
Two platelet gates were set, according to the scatter characteristics and specific platelet marker utilized. The first platelet gate was set according to the FS and SS properties of platelets found in the PRP obtained from self-sedimentation. The PRP contained only platelets and no other blood cells. Therefore, a pure platelet population could be distinguished without additional MoAb staining. represents a subsequent analysis where whole blood (containing all blood cells) was analyzed by the PRP gate.
Figure 1. PRP gate. Platelet gate set according to FS and SS of PRP. This dot plot shows the PRP gate when a whole-blood sample was analyzed. The other populations outside the PRP gate represent the other blood cells found in the whole-blood sample.
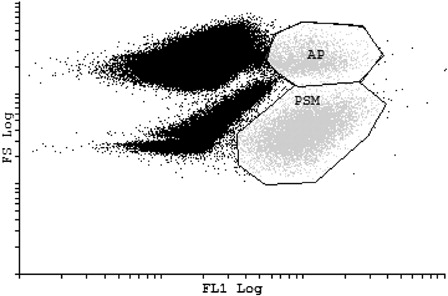
shows the second platelet gate set according to CD41-FITC fluorescence, a PSM on a dot-plot displaying FS on the y-axis and FL1 fluorescence on the x-axis. CD41 is used as a reliable PSM. It enables the differentiation of platelets in a whole-blood sample.Citation2 The CD41-FITC marker was used in conjunction with the mentioned phycoerythrin-labeled monoclonal antibodies (CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE). For the PSM gate, a clear distinction can be made between the unactived platelets (to the lower right of the diagram) and the activated platelets (to the upper right part of the diagram).
Figure 2. PSM gate. Platelet gate set according to FL1 of CD41-FITC PSM. Activated platelets (AP) are also incorporated in the PSM gate. The other populations outside the PRP gate represent the other blood cells found in the whole blood sample.
An overlay of the two platelet gates is shown in . In A it is shown that the PRP gate only corresponds to the non-activated platelets in the population while B shows the activated platelets are incorporated in the PSM gate but not the PRP gate. Therefore, from we can deduce that the gating strategy is very important if all parameters are to be fully evaluated.
Figure 3. Overlay of platelet gates. The PRP sample not containing CD41-FITC was analyzed by use of the PSM gate while the whole-blood sample that did contain CD41-FITC was analyzed by the use of the PRP gate. (A) PRP only corresponds to the non-activated platelets of the PSM-gate. (B) When the activated platelets are represented on the dot plot of the PRP gate, these cells are not incorporated in the PRP gate but are clustered on the upper right part of the dot plot.
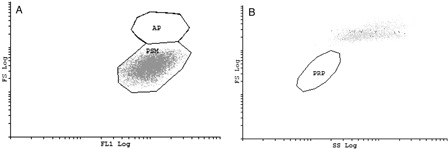
Both gating strategies were used for analysis of the different phycoerythrin-labeled monoclonal antibodies used. The PRP gate showed a significantly lower MCFI value compared with the PSM gate for all instances.
Comparisons
To determine the integrity of the different sampling methods, whole-blood samples were compared with finger-prick samples containing various volumes of citrate immediately after sampling. The volumes of citrated added to the sheath fluid before sampling was (i) no citrate, (ii) 5 µl of citrate, and (iii) 10 µl citrate. The comparison of whole-blood samples with various volumes of thrombin added directly after sampling was done to determine the activation of platelets. Thrombin volumes added to whole-blood samples before analysis was (i) 5 µl, (ii) 10 µl, and (iii) 20 µl. The influence of storage time was compared for whole blood and finger-prick samples analyzed 24 hours after sampling.
The results from experiments 1 and 2 were pooled and each of the mentioned phycoerythrin-labeled markers were used to analyze specific characteristic of platelets gated by CD41-FITC. For each marker, 10 000 platelets were counted and analyzed per person adding up to a total of 60 000 platelets for the duplicate procedures.
The following results, obtained immediately after sampling, were compared with determine any significant changes. displays results from whole-blood sample containing no thrombin (non-activated whole blood sample) compared with finger-pricks containing various concentrations of citrate. displays results from whole-blood samples containing various concentrations of thrombin.
Table 2. Analysis immediately after sampling: whole-blood sample and finger-pricks containing various concentrations of citrate
Table 3. Analysis immediately after sampling: whole bloodsamples containing various concentrations of thrombin to evaluate activation
shows the results of immediate analysis compared with results of analysis after 24 hours. Whole blood analyzed immediately was compared with the whole-blood sample stored for 24 hours. The finger-prick containing no citrate analyzed immediately was compared with the same sample stored for 24 hours. The same procedures were performed for the other finger-prick samples, containing 5 and 10 µl citrate, respectively, immediately analyzed after collection and the aliquot stored for 24 hours.
Table 4. Analysis 24 hours after sampling
Whole blood vs. finger-prick
There were no significant difference between the whole blood samples and the finger-prick samples containing 10 µl of citrate (P > 0.05). However, significant changes were shown for the other finger-prick samples containing no citrate and 5 µl of citrate separately (P < 0.05).
Both gating strategies were used for analysis of the different phycoerythrin-labeled monoclonal antibodies used to evaluate the different sampling methods. The PRP gate showed a significantly lower MCFI value compared with the PSM gate for all instances.
Non-activated vs. activated
Whole-blood samples were activated by adding different volumes of thrombin to separate samples. The whole-blood sample containing no thrombin (thus, the non-activated sample) was compared with whole-blood samples containing 5, 10, and 20 µl of thrombin. A significant difference was found between the non-activated whole-blood sample and the activated whole-blood samples (P < 0.05). This was also true when the activated whole samples were compared with the finger-prick sample containing 10 µl citrate. The MCFI for all phycoerythrin-labeled monoclonal antibodies (CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE) increased with the increase in thrombin added to the whole-blood samples.
Immediate analysis vs. 24 hours
No significant difference was found for the MCFI of whole blood analyzed immediately compared with whole blood analyzed 24 hours after sampling (P > 0.05). This was also true for the finger-prick samples with 10 µl of citrated added before sampling. The immediate analysis of the finger-prick samples with no citrate and 5 µl of citrate added separately, showed significant differences from the same samples analyzed 24 hours after sampling (P < 0.05).
Discussion
The current investigation confirms that both a whole-blood sample and a finger-prick can be used interchangeably for flow cytometric analysis of platelets. Storage time of up to 24 hours in a fridge at 6°C also does not influence the platelet activation in the blood sample. Aspects such as the strategy employed for gating the sample, the specific flow cytometer instrument used, the preparation of the samples, the specific probes used as well as the storage time will be discussed in greater detail.
Gating strategies
Flow cytometry enables the researcher to detect several parameters from a single sample. Different probes or markers can simultaneously be analyzed by using multicolor flow cytometry.Citation10
The function of ‘gating’ or isolating particular cell clusters in flow cytometry enables classification and investigation of platelets in the mixed populations of cells like found in whole blood.Citation3 Two parameters are employed to facilitate platelet gating namely (1) forward light scatter which arranges cells according to size, and (2) the use of a platelet-specific antibody like CD41 or CD42b.Citation3
Multicolour flow cytometry has the advantage of gathering more information from a single sample therefore minimizing the sample size and decreasing preparation effort. This technique also decreases variation since fewer sample preparation is required and is an effortless method to study platelet population.Citation10
The reliability of the population of platelets in a whole-blood sample is determined by the gating strategy employed.Citation11–Citation13 Van Velzen and colleagues have recently investigated the effect of different platelet-gating strategies. They stated that the changes in scatter characteristics after platelet activation will differ from the morphology of non-activated platelets and therefore a pure platelet population will not be selected. They asserted that utilizing a platelet antigen like CD41 or CD42b, which is commonly present on the platelet membrane, is preferred above the use of the FS/side scatter strategy. By using a PSM not only eliminate coincidence but also excludes complexes platelets form with monocytes and decreases possible contamination.Citation10
This research of van Velzen et al. was done for a fixated whole-blood sample. In this investigation similar results were found for an unfixed whole-blood sample. As the PRP gate only shows the unactived platelets in the absence of CD41-FITC, it is important to firstly stain the sample with CD41-FITC and secondly to set the platelet gate according to the FL1 information obtained from CD41-FITC, i.e. the PSM gate. This indicates the importance of multicolor gating for optimal analysis of platelets.
Instrumentation
Earlier flow cytometric analysis has been performed on FACScan cytometer (Becton Dickinson, San Jose, CA, USA). Platelet microparticlesCitation14 and magnetic labeling of plateletsCitation15 have been studied on the FC500 (Beckman Coulter). Erythrocytes,Citation16 dysfunctional T regulatory cells,Citation17 and stem cellsCitation18 among others have also been analyzed on the FC500 (Beckman Coulter). This is the first study conducted on the FC500 (Beckman Coulter) to investigate platelets in an unfixated whole-blood and finger-prick samples as well as the effect of storage time on the platelets.
Blood preparation for flow cytometry
Whole blood was drawn in citrate tubes (0.5 ml of sodium citrate (3.8%) for 4.5 ml of blood). For the finger-pricks, citrate had to be added to the sheath fluid to prevent coagulation of the blood sample. WeilCitation19 stated that if blood is mixed with a sodium citrate solution in appropriate portions, the blood will not clot since the calcium salts are not accessible for coagulation. WeilCitation19 added that blood mixed with sodium citrate can be kept in the fridge at 6°C for several days, and only slight changes in cell structure can be observed after 1 week of storage.Citation19 The results indicate that a volume of 10 µl of citrate added to the sheath fluid aliquot is adequate to prevent coagulation of a finger-prick sample.
Typically, platelets have been studied after preparing PRP or washing the platelets.Citation20,Citation21 These separation procedures lead to in vitro activation of platelets due to the formation of artifacts. The pioneer use of whole blood for flow cytometric studies by Shattil et al.Citation11 was just the beginning for important improvements for the relevance of flow cytometry for clinical application.Citation4 The flow cytometric analysis of whole blood holds many advantages for the study of platelet activation. This method has been used for over 20 years to investigate platelet function. Ault et al.Citation22 used the MoAbs specific for GPIb (CD42b) and GMP140 (CD62P) to measure platelet aggregation and release reaction in a whole-blood sample. Activation-dependent variations in multiple surface receptors can be determined in a whole-blood sample. In vitro platelet activation is also prevented, as there is minimal manipulation of the samples and it will decrease the possible loss of platelet subpopulations.Citation4
Probes
A Pubmed search revealed few references in recent literature that discuss the use of CD41-PE, CD42b-PE, CD62P-PE, CD63-PE, and CD41-FITC in whole-blood and finger-prick samples as well as the particular storage methods employed in this study. The current literature showed that flow cytometry has been employed to study platelet function testing in apheresis products, where all the above-mentioned probes were used in conjunction with secondary antibodies.Citation7 Other studies include flow cytometric analysis of platelet count,Citation23 platelet reactivity,Citation24 platelet function in children,Citation25 and the importance of the sampling site in patient about to undergo surgery;Citation26 these studies used only one or two of the above-mentioned probes. All the mentioned studies were performed on a FACScan cytometer (Becton Dickinson).
The most recent investigation of platelet activation was done by van Velzen et al. in 2012. They incorporated all four the mentioned antibodies; however, the conjugated fluorochromes differed from the probes used in this study and the samples were fixated before analysis.Citation10
Sampling method
No significant difference was found between the whole-blood sample and the finger-pricks containing 10 µl of citrate. There was a significant difference between the whole-blood sample and the finger-pricks containing no citrate and with 5 µl of citrate added. This indicated that the method of sampling, both the whole blood drawn in the citrate tube as well as the finger-prick samples, was adequate. It also shows that 10 µl of citrate is the preferred concentration to be added to the sheath fluid before adding blood from a finger-prick. The whole-blood sample and each of the finger-prick samples were done on separate days. This was done to eliminate the possible effect of platelet activation after the first sample.
Activation of platelets
The activated whole-blood samples showed significantly higher MCFI values when compared with the non-activated whole-blood sample and the finger-prick sample containing 10 µl of citrate. We can therefore deduce that the whole-blood sampling containing no thrombin, as well as the finger-prick with 10 µl of citrate added, did not contain an activated platelet population.
Once receptor activation occurs, the internal platelet granules are secreted and the cytoskeleton will be rearranged leading to signal transduction.Citation27,Citation28
Upon activation, platelets exhibit elevated levels of specific activation markers on the platelet surface for example CD62PCitation29 and CD63.Citation30 Both these markers are dominant immunologic indicator of platelet activation.Citation30,Citation31
CD62P, also referred to as P-selectin, is an activation-dependent MoAb most extensively used in the study of platelet granule membrane proteins.Citation32 It is a constituent of a resting platelet's α-granule membrane.Citation31 It will only be expressed on the surface membrane once the contents of the α-granule are secreted.Citation33–Citation35 For this reason, the MoAb specific for CD62P will not bind to resting platelets, only to degranulated, activated platelets.Citation4 Once activated, platelets also rapidly transfer CD63 from the lysosome-like granules to the platelet surface through the surface canalicular system.Citation2
Van Velzen et al. also found the expression of CD62P and CD63 to be increased when platelets were exposed to thrombin activation in a fixated sample.Citation10
Period between sampling and analysis
CD41 is a calcium-dependent compound of GPIIb/IIIa. The interactions between cells and the cells with the matrix are mediated by GPIIb/IIIa.Citation36,Citation37 CD42b is a receptor for the von Willebrand factor, which plays a critical role in the adhesion of platelets to the wall of injured blood vessels.Citation38
CD41 and CD42b are reliable PSMs. These MoAbs are used to differentiate between platelets and ‘debris’ or fragments.Citation2
As the expression of CD41 and CD42b in the whole-blood samples was similar for immediate analysis of the sample and analysis 24 hours after sampling, we can infer that these platelets identified by CD41 and CD42b expression were not activated after sampling and remained inactive for at least 24 hours. Michelson and associates indicated on several occasions that the binding of CD42b to resting platelets is noticeably increased compared with its binding with activated platelets.Citation39–Citation41 The decreased expression on the surface of activated platelets possibly results from the translocation of this complex to the surface-connected canalicular system membranes.Citation41,Citation42 This shows that CD42b could be a sensitive marker of in vivo activation of platelets.
Although platelet storage has been associated with increased expression of CD62 in the past,Citation3 our results indicate that both an unfixated whole-blood and a finger-prick sample can be stored in the fridge at 6°C if prepared appropriately. The time of analysis, whether it is immediately after the sample was take or 24 hours after sampling, appears to not have an influence on the expression of CD41 and CD42b. However, as the participants were all young and healthy individuals, the effect of age and disease will have to be investigated for this method.
Limitations and possible applications of study
Before conclusions can be drawn from these results, potential limitations need to be addressed. The small sample size, the use of only four MoAbs and the analysis at two time points can possibly constrain the findings of this study. A greater sample size, using different MoAbs and analyzing the samples over a greater time period or at more regular intervals can bring greater insight.
However, although the sample size is limited, the results indicate that the platelets are not additionally activated by this particular methodology. Therefore, these methods may be successfully implemented in clinical studies with antiplatelet agents.
Conclusion
Firstly, the importance of gating strategy for a unfixated sample was established. If all platelets, activated and non-activated, are to be taken into consideration, then the PSM gate should be employed and all samples should be stained with CD41-FITC and not only with the specific phycoerythrin-labeled monoclonal antibodies (including CD41-PE, CD42b-PE, CD62P-PE, and CD63-PE).
Secondly, we can conclude that an unfixated whole-blood and a finger-prick sample are identical with regard to platelet function. Both sampling methods showed little activation when compared with activated whole-blood samples. Therefore, a whole-blood sample and a finger-prick sample can be used interchangeably for flow cytometric analysis of platelets. This is advantageous for research where a sample population needs to be examined over consecutive days or an extending time interval. In these cases, when it is not practical to repeatedly draw blood, a finger-prick will be sufficient.
And lastly, samples can be stored for 24 hours after sampling. Sheath fluid is a sufficient medium for storing unfixated whole-blood and finger-prick samples in the fridge at 6°C for 24 hours, provided that a sufficient amount of citrate is added to the sheath fluid for a finger-prick sample. A volume of 10-μl citrate provides sufficient anticoagulant action for a 20-μl aliquot of finger-prick blood. Both whole-blood samples and finger-prick samples can be kept in the fridge at 6°C for 24 hours before analysis as no platelet activation occurs. This will aid studies where analysis cannot immediately be performed due to traveling distance from flow cytometer or time constraints.
Acknowledgements
The authors acknowledge Peter Smith for his assistance with the statistical analysis.
References
- Marti GE, Stetler-Stevenson M, Bleesing JJH, Fleisher TA. Introduction to flow cytometry. Semin Hematol. 2001;38(2):93–9.
- Ault KA, Mitchell J. Analysis of platelets by flow cytometry. Methods Cell Biol. 1994, 42(Pt B):275–94.
- Lazarus AH, Wright JF, Blanchette V, Freedman J. Analysis of platelets by flow cytometry. Transfus Sci. 1995;16(4):353–61.
- Michelson AD. Flow cytometry: a clinical test of platelet function. Blood. 1996;87(12):4925–36.
- Coller BS, Peerschke EI, Scudder LE, Sullivan CA. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983;61(1):99–110.
- Montgomery RR, Kunicki TJ, Taves C. Diagnosis of Bernard-Soulier syndrome and Glanzmann's thrombasthenia with a monoclonal assay on whole blood. J Clin Invest. 1983;71(2):385–9.
- Gutensohn K, Bartsch N, Kuehnl P. Flow cytometric analysis of platelet membrane antigens during and after continuous-flow plateletpheresis. Transfusion. 1997;37(8):809–15.
- Wall JE, Buijs-Wilts M, Arnold JT, Wang W, White MM, Jennings LK, et al. A flow cytometric assay using mepacrine for study of uptake and release of platelet dense granule contents. Br J Haematol. 1995;89(2):380–5.
- Michelson AD, Barnard MR, Krueger LA, Frelinger AL, Furman MI. Evaluation of platelet function by flow cytometry. Methods. 2000;21(3):259–70.
- van Velzen JF, Laros-van Gorkom BAP, Pop GAM, van Heerde WL. Multicolor flow cytometry for evaluation of platelet surface antigens and activation markers. Thromb Res. 2012;130(1):92–8.
- Shattil SJ, Cunningham M, Hoxie JA. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987;70(1):307–15.
- Goodall AH, Appleby J. Flow-cytometric analysis of platelet-membrane glycoprotein expression and platelet activation. Methods Mol Biol. 2004;272:225–53.
- Pham A, Wang J. Bernard-Soulier syndrome: an inherited platelet disorder. Arch Pathol Lab Med. 2007;131(12):1834–6.
- Robert S, Poncelet P, Lacroix R, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? Thromb Haemost. 2009;7(1):190–7.
- Aurich K, Spoerl MC, Fürll B, Sietmann R, Greinacher A, Hosten N, et al. Development of a method for magnetic labeling of platelets. Nanomedicine. 2012;8(5):537–44.
- Chow S, Hedley D, Grom P, Magari R, Jacobberger JW, Shankey TV. Whole blood fixation and permeabilization protocol with red blood cell lysis for flow cytometry of intracellular phosphorylated epitopes in leukocyte subpopulations. Cytom A. 2005;67(1):4–17.
- Prabhala RH, Neri P, Bae JE, Tassone P, Shammas MA, Allam CK, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107(1):301–4.
- Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Hohm SA, Lahey R, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107(5):2162–9.
- Weil R. Sodium citrate in the transfusion of blood. JAMA. 1983;250(14):1901–4.
- Ejim OS, Powling MJ, Dandona P, Kernoff PBA, Goodall AH. A flow cytometric analysis of fibronectin binding to platelets from patients with peripheral vascular disease. Thromb Res. 1990;58(5):519–24.
- Wehmeier A, Tschope D, Esser J, Menzel C, Nieuwenhuis HK, Schneider W. Circulating activated platelets in myeloproliferative disorders. Thromb Res. 1991;61(3):271–8.
- Ault KA, Rinder HM, Mitchell JG, Rinder CS, Lambrew CT, Hillman RS. Correlated measurement of platelet release and aggregation in whole blood. Cytometry. 1989;10(4):448–55.
- Matzdorff AC, Kühnel G, Kemkes-Matthes B, Pralle H. Quantitative assessment of platelets, platelet microparticles, and platelet aggregates with flow cytometry. J Lab Clin Med. 1998;131(6):507–17.
- Hübl W, Assadian A, Lax J, Meixner U, Fang IF, Hagmüller G, et al. Assessing aspirin-induced attenuation of platelet reactivity by flow cytometry. Thromb Res. 2007;121(1):135–43.
- Rand ML, Kuhle S. Platelets and platelet function testing in children. Prog Pediatr Cardiol. 2005;21(1):63–9.
- Rubens FD, Labow RS, Waghray G, Robblee J. The importance of sampling site in the measurements of whole-blood platelet flow cytometry. J Cardiothorac Vasc Anesth. 1998;12(3):309–13.
- Reed GL. Platelet secretory mechanisms. Semin Thromb Hemost. 2004;30(4):441–50.
- Fox JEB, Lipfert L, Clark EA, Reynolds CC, Austin CD, Brugge JS. On the role of the platelet membrane skeleton in mediating signal transduction. J Biol Chem. 1993;268(34):25973–84.
- Becker RC, Tracy RP, Bovill EG, Mann KG, Ault K. The clinical use of flow cytometry for assessing platelet activation in acute coronary syndromes. Coron Artery Dis. 1994;5(4):339–45.
- Yano Y, Kambayashi J, Kawasaki T, Sakon M. Quantitative determination of circulating platelet microparticles by flow cytometry. Int J Cardiol. 1994;47(SUPPL.):S13–20.
- Woods J, Wolff LE, Keller DM. Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not other extracellular proteins. J Biol Chem. 1986;261(32):15242–51.
- Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto C, et al. CD antigens 1993. Blood 1994;83(4):879–80.
- Hsu-Lin SC, Berman CL, Furie BC. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984;259(14):9121–6.
- McEver RP. Properties of GMP-140, an inducible granule membrane protein of platelets and endothelium. Blood Cells 1990;16(1):73–83.
- Stenberg PE, McEver RP, Shuman MA. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101(3):880–6.
- Jennings LK, Phillips DR. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982;257(17):10458–66.
- Peerschke EIB. Platelet membrane glycoproteins: Functional characterization and clinical applications. Am J Clin Pathol. 1992;98(4):455–63.
- Ruggeri ZM. The platelet glycoprotein Ib-IX complex. Prog Hemost Thromb. 1991;10:35–68.
- Michelson AD. Thrombin-induced down-regulation of the platelet membrane glycoprotein Ib-IX complex. Semin Thromb Hemost. 1992;18(1):18–27.
- Michelson AD, Barnard MR. Thrombin-induced changes in platelet membrane glycoproteins Ib, IX, and IIb-IIIa complex. Blood. 1987;70(5):1673–8.
- Michelson AD, Benoit SE, Furman MI, Barnard MR, Nurden P, Nurden AT. The platelet surface expression of glycoprotein V is regulated by two independent mechanisms: proteolysis and a reversible cytoskeletal-mediated redistribution to the surface-connected canalicular system. Blood. 1996;87(4):1396–408.
- Hourdille P, Heilmann E, Combrie R, Winckler J, Clemetson KJ, Nurden AT. Thrombin induces a rapid redistribution of glycoprotein Ib-IX complexes within the membrane systems of activated human platelets. Blood. 1990;76(8):1503–13.