Abstract
From 2005 to 2011, 25 children of both sexes (13 boys and 12 girls, mean age 7.8 ± 2.5 years, 5–12.4 years) with acute varicella zoster virus (VZV) infection were selected. Five patients showed venous thromboembolism characterized by deep venous thrombosis (DVT). Comparison of activated partial thromboplastin time, antithrombin III, D-dimer, lupus anticoagulant, free S protein (PS), C protein, and antiphospholipid and PS antibodies was performed on children with acute VZV and DVT (group I), acute uncomplicated VZV (group II), and 30 healthy controls of both sexes (15 boys and 15 girls, mean age 7.5 ± 2.6 years, group III). Genetic thrombophilic mutations (Factor V Leiden, MTHFR C677T, and Prothrombin G20210A) were evaluated. Coagulation disorders and PS antibody were found in children with acute VZV (groups I and II). Significant differences were shown among the three groups (P < 0.05). Acute VZV infection could be associated with coagulation disorders and production of inhibitory PS antibodies in many uncomplicated cases.
Introduction
The predominant clinical manifestation of thrombophilia is venous thromboembolism (VTE). Most cases of VTE are due to interactions between genetic predisposition and acquired risk factors. The remaining cases are labeled ‘idiopathic’ or ‘spontaneous’. When recurrent VTE does occur predisposition should be investigated.
Primary thrombophilic states are due to either qualitative or quantitative deficiency of antithrombotic proteins – antithrombin III (ATIII), protein C (PC), protein S (PS) deficiency – or increased level of prothrombotic clotting factors. Many genetic mutations – particularly Factor V Leiden (FVL), MTHFR C677T, and Prothrombin G20210A – have been associated with VTE.
VTE has been considered to have a lower incidence in childhood than in adulthood. Moreover, recent reports and multicenter studies have estimated an incidence of 5:10 000/years,Citation1 but this finding may be dramatically increasing.Citation2
The VTE, comprised of deep venous thrombosis (DVT) and more severe pulmonary embolism (PE) may be the cause of mortality in children.Citation3 Furthermore, individuals who have PE or DVT are often at great risk of chronic thromboembolic pulmonary hypertensionCitation3 and chronic venous insufficiency, respectively.Citation4
Central venous catheterization, malignancies, anemia, infectious diseases such as acute varicella zoster virus (VZV) represent risk factors of VTE in children.
In childhood association of acute VZV infection and thrombophilic alterations, purpura fulminans (PF), PE, and DVT have been frequently reported.Citation1,Citation2
D-dimer, PS and PC reduction, autoantibodies against phospholipids and coagulation proteins, lupus anticoagulant (LA) occurrence have been observed in previously healthy children with acute VZV infection even in uncomplicated cases.Citation5
It has been highlighted that the pattern of immunological response was the same in children with VZV infection with or without thrombotic complications but the intensity of the response was greater in children with VTE.Citation5
The aim of this study was to comparatively evaluate thrombophilic factors among children with acute VZV and symptomatic VTE, acute uncomplicated VZV infection, and healthy children.
Methods
From 2005 to 2011, we have studied 25 children (mean age 7.8 ± 2.5 years, range 5–12.4 years) of both sexes (13 boys and 12 girls) with clinical and immunological evidence of VZV. Five patients showed VTE, particularly saphaenous vein thrombosis.
Antibodies to VZV were evaluated (enzyme-linked immunosorbent assay (ELISA)) as well as the following clotting tests: activated partial thromboplastin time (APTT), ATIII, D-dimer, FVIII, LA, free PS and PC (ELISA), antiphosphilipid antibody (APA) in classes IgM and IgG (ELISA), and IgG and IgM PS antibodies (ELISA).
In children with thrombotic alterations, diagnosis was confirmed by Doppler ultrasonography and magnetic resonance.
Comparison of clotting tests was made between acute VZV patients with and without thrombotic alterations (groups I and II) and in a control population of 30 healthy children (mean age 7.5 ± 2.6 years, range 4.2 ± 12 years, group III) of both sexes (15 boys and 15 girls). Eight children had VZV infection by at least 1 year.
None of control children (group III) were submitted to VZV vaccination.
In children with thrombotic and/or coagulation alterations (groups I and II) and in healthy controls (group III), genetic mutations at nucleotide position C677T of MTHFR, G1691A of FVL, and Prothrombin G20210A (F2) were also evaluated.
Family history of thrombotic events was recorded.
Children with thrombotic complications were treated after coagulation tests by anticoagulants (heparin). Follow-up was made by clotting tests and instrumental analysis over the first year.
Statistical analysis employed the analysis of variance and χ2 test; P values ≤0.05 were considered significant.
All parents gave informed consent. Principles outlined in the Declaration of Helsinki were followed.
Results
None of the family members had thrombotic events.
shows mean values ± DS of APTT, ATIII, PC, PS, and FVIII. Significant differences were found in three groups (P < 0.05).
Table 1. Clotting tests in children affected by acute VZV and in controls
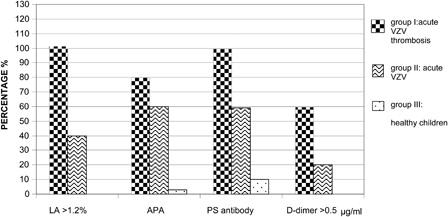
shows the percentage of children with LA (>1.2%), APA, PS antibody, and D-Dimer (>0.5 µg/ml). Significant differences were found among three groups (P < 0.05).
Figure 1. Percentage of children with LA, APA, PS antibody, and D-dimer. LA: group I vs group II P < 0.05. APA: group I vs group II P < 0.05; group I vs group III P < 0.05; group II vs group III P < 0.05. PS antibody: group I vs group II P < 0.05; group I vs group III P < 0.05; group II vs group III P < 0.05. D-dimer: group I vs group II P < 0.05.
None of the patients with acute VZV infection (groups I and II) or healthy children (group III) had genetic thrombophilic risk factor polymorphisms (data not shown).
All patients with DVT and clotting test derangements recovered in the first 6 months. Children with uncomplicated VZV clotting tests normalised in the first month.
Discussion
This case–control study assesses acute VZV infection as a risk factor for VTE.
The relationship between VTE and infectious diseases has been previously reported.
Thromboembolic post-strepthococcical alterations were described in children until 1990 by Francis.Citation6
Successively in children, acute VZV infection has been associated with either VTE due to acquired PS deficiencyCitation6–Citation8 or DVT characterized by spontaneous tibial artery thrombosis, PS deficiency, and LA evidence.Citation9
Josephson et al.Citation5 described ‘The varicella-autoantibody syndrome’ in a wide cohort of children with acute VZV infection complicated by VTE and evidence of autoantibodies including APA and anti PS.
Association of acute VZV infection with DIC, evidence of APA antibodies, LA, and acquired PS deficiency has been reported in a child.Citation10
Other cases of children affected by acute VZV and other infectious diseases with thromboembolic complications have also been reported.Citation11–Citation15
It has been highlighted that 20% of children with DVT develop post-thrombotic syndrome with chronic venous insufficiency.Citation4
In our cohort of patients with acute VZV infection and symptomatic VTE we found significant alterations of clotting tests and particularly reduced values of PC, ATIII, and free PS by inhibitory antibody production (, ).
The APA antibodies and LA (), we found in these patients, represent important thrombophilic risk factors for VTE in children.Citation16,Citation17
The relationship between acute VZV infection and autoimmune disorders in childhood has been frequently observed. Because of the long incubation period of this disease, children affected by VZV infection present at the time of exhantema IgG antibodies that may trigger immunological response as well as IgG-mediated immunothrombocytopenia frequently occurring in children after acute VZV infection.
Furthermore, we observed increased levels of FVIII and D-dimer, the response to acute inflammation. These findings confirm the nature of clinical alterations. LA evidence and PTT elongation are also due to acute infection and/or inflammation.
APA and LA presence are the most common thrombophilic risk factors for VTE in children.Citation16,Citation17 In children affected by systemic lupus erythematosus, the evidence of both LA and activated PC resistance may increase the risk of thrombotic events.Citation18 The D-dimer increase is also associated with thrombotic events.
Increased risk of recurrent thrombotic events has been reported in children with heterozygous and particularly homozygous FVL polymorphism,Citation19 while association of MTHFR C677T polymorphism with higher risk of VTE is controversial.Citation20,Citation21 Recently, we have reported in children with recurrent migraine significant high rate of homozygous FVL and MTHFR C677T polymorphisms.Citation22
Polymorphism of PT G20210A has been also found in children with recurrent VTE. In VZV, acute infection association between PF and heterozygosity for FVL with transient PS deficiency has also been reported.Citation23
Our patients with VTE did not show these genetic mutations or recurrent thrombotic events. Moreover, we have studied only three of the more frequent genetic thromboembolic risk factors, but others – some still unknown – should be investigated. Recently, it has been reported that cystatin-C is associated with risk of VTE in adult subjects with normal kidney function.Citation24
We also observed that patients with uncomplicated acute VZV infection, belonging to II group, showed altered clotting tests (, ). This finding suggests that VZV may be a risk factor of thrombophilia probably interacting with predisposing factors other than those investigated.
We found evidence of low concentrations of APA and PS antibodies in controls (). Autoantibody concentrations were significantly different among the groups (P < 0.005, data not shown). These data are in agreement with another report on a wide cohort of patients and controls.Citation5
Acute VZV infection could be associated with some disorders of coagulation, particularly production of inhibitory PS antibodies, trigging an immunological process without triggering thrombotic events.
Conclusion
Varicella is not always a benign disease, but may lead to serious complications.
In children with acquired anticoagulant deficiency, particularly PS activity may trigger thrombophilic alterations with VTE occurrence. Markers of thrombophilic events could represent an important approach to VTE in childhood by early treatment to avoid post-thrombotic syndrome.
We also think that coagulation tests have a key role as predictors of outcome in children with thrombophilic alterations.
Moreover, VTE could be the result of interaction between other still unknown genetic factors and environmental risk factors.
The magnitude of risk factors in infectious diseases could also influence VTE events.
Additional multicenter studies on larger cohorts could shed light on the problem. Furthermore, we think that it could be particularly interesting to investigate clotting tests, APA, and PS antibodies in children submitted to VZV vaccination.
References
- Stein PD, Kayali F, Olson Re. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr. 2004;145(4):563–5.
- Raffini L, Huang Y-S, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's Hospital in the United States from 2001 to 2007. Pediatrics 2009;124(4):1001–8.
- Biss TT, Brandao LR, Kahr WH, Chan AK, Williams S. Clinical features and outcome of pulmonary embolism in children. Br J Haematol. 2008;142(5):808–18.
- Goldenberg Na, Donadini MP, Kahn SR, Crowther M, Nowak-Gottl U, Manco-Johnson MJ. Post thrombotic syndrome in children: a systematic review of frequency of occurrence, validity of outcome measures, and prognostic factors. Haematologica 2010;95(11):1952–9.
- Josephson C, Nuss R, Jacobson L, Hacker MR, Murphy J, Weinberg A, Manco-johnson MJ. The varicella-autoantibody syndrome. Pediatr Res. 2001;50(3):345–52.
- Francis RB. Acquired purpura fulminans. Semin Thromb Hemost. 1990;16:310–25.
- Levin M, Eley B, Louis J, Cohen H, Young L, Heyderman R. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127(3):355–63.
- Manco-Johnson MJ, Nuss R, Key N, Moertel C, Jacobson L, Meech S, et al. Lupus anticoagulant and protein S deficiency in children with postvaricella purpura fulminans or thrombosis. J Pediatr. 1996;128:319–23.
- Peyton BD, Cutler BS, Stewart FM. Spontaneous tibial artery thrombosis associated with varicella pneumonia and free protein S deficiency. J Vasc Surg. 1998;27(3):563–7.
- Kurugol Z, Vardar F, Ozkinay F, Kavakli K, Cetinkaya B, Ozkinay C. Lupus anticoagulant and protein S deficiency in a child who developed disseminated intravascular coagulation in association with varicella. Turk J Pediatr. 2001;43(2):139–42.
- Van Der Van A, Van Diest R, Hamulyak K, Maes M, Bruggeman C, Appels A. Herpes virus, cytokines, and altered hemostasis in vital exhaustion. Psychosom Med. 2003;65(2):194–200.
- Nagel MA, Cohrs RJ, Mahalingam R, Wellish MC, Forghani B, Schiller A, et al. The varicela zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology 2008;70(11):853–60.
- Baur A, Pouyau R, Meunier S, Nougier C, Teyssedre S, Javouhey E, et al. [Varicella –associated purpura fulminans and deep vein thrombosis: paediatric case report]. Arch Pediatr. 2011;18(7):783–6.
- Teeninga N, Willemze AJ, Emonts M, Appel IM. [Acute illness following chicken pox: spleen infarction as a complication of varicella zoster infection]. Ned Tijdschr Geneeskd. 2011;155(28):pA2987.
- Lopes da Silva R. Viral-associated thrombotic microangiopathies. Hematol Oncol Stem Cell Ther. 2011;4(2):51–9.
- Manco-Johnson MJ, Nuss R. Lupus anticoagulant in children with thrombosis. Am J Hematol. 1995;48:240–3.
- Male C, Lechner K, Eichinger S, Kyrle PA, Kapiotis S, Wank H, et al. Clinical significance of lupus anticoagulants in children. J Pediatr. 1999;134(2):199–205.
- Male C, Mitchell L, Julian J, Vegh P, Joshua P, Adams M, et al. Acquired activated protein C resistance is associated with lupus anticoagulants and thrombotic events in pediatrics patients with systemic lupus erythematosus. Blood 2001;97(4):844–9.
- Nowak-Göttl U, Junker R, Kreuz W, von Eckardstein A, Kosch A, Nohe N, et al. Childhood Thrombophilia Study Group. Risk of recurrent venous thrombosis in children with combined prothrombotic risk factors. Blood 2001;97(4):858–62.
- Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985;37(1):1–31.
- Meinardi, Middeldorp S, de Kam PJ, Koopman MM, van Pampus EC, Hamulyák K, et al. The incidence of recurrent venous thromboembolism in carriers of factor V Leiden is related to concomitant thrombophilia disorders. Br J Haematol. 2002;116(3):625–31.
- Ferrara M, Capozzi L, Bertocco F, Ferrara D, Russo R. Thrombophilyc gene mutations in children with migraine. Hematology 2012;17(2):115–7.
- Woods C, Johnson C. Varicella purpura fulminans associated with heterozygosity for factor V leiden and transient protein S deficiency. Pediatrics 1998;102:1208–10.
- Brodin EE, Braekkan SK, Vik A, Brox J, Hansen J-B. Cystatin C is associated with risk of venous thromboembolism in subjects with normal kidney fuction – the tromsø study. Hematologica 2012;7:1008–13.