Abstract
In childhood acute lymphoblastic leukemia (ALL) the reported 5-year event-free survival (EFS) rates are as high as 80%. Since 2004, multiple Egyptian centers shifted protocol of therapy of ALL to the CCG 1991 (the single delayed intensification arm) and CCG 1961 protocol for standard risk and high-risk ALL therapy, respectively, being cost effective. We aimed to evaluate the efficacy and safety of the CCG protocol in treatment of childhood ALL in Ain Shams and Menoufeya University hospitals.
Methods
Fifty-two ALL patients, aged 1–17 years, treated according to the modified CCG protocol in both centers and registered from November 2004 to December 2005 were included. They were classified into three risk groups, standard risk (SR), high-risk standard arm (HR-SA), and high-risk augmented arm (HR-AA).
Results
The mean age at diagnosis was 5.9 + 3.3 years, male/female ratio of 1.6:1, and central nervous system leukemia represented 6%. The 5-year overall survival (OS) and EFS were 84.6% and 67%, respectively. The 5-year OS and EFS were 92.6% and 70% in SR, 68.8% and 55% in HR-SA, 88.9% and 80% in HR-AA patients, respectively. Six patients had grade 3–4 adverse events.
Conclusion
The outcome of HR-SA protocol was inferior to the other two groups, necessitating shift to a more intensified arm with double delayed intensification. The use of minimal residual disease for better risk classification of childhood ALL is recommended in our centers
Keywords:
Introduction
Treatment of childhood acute lymphoblastic leukemia (ALL) is one of the success stories of modern medicine during fifty years. ALL has gone from fatal disease to one with overall cure rate greater than 75%.Citation1 The 5-year event-free (EFS) and overall survival (OS) probabilities in childhood ALL patients treated with total XV were 85.6% and 93.5%, respectively.Citation2 It is very difficult to compare the outcome of different protocols because of difference in patient selection and risk group classification.Citation3
Childhood ALL management in countries with limited health resources face multiple challenges including quality improvement, educational and financial supports, and treatment abandonment.Citation4 Ethnic differences in drug metabolism and toxicity from chemotherapy is also an important point to be considered when adopting protocols of therapy accepted as standard therapy in other countries.Citation5
The current pilot study was intended to evaluate the efficacy and safety of the unified Egyptian protocol of ALL therapy which is a children cancer group ‘CCG’-based protocol adopted by several Egyptian pediatric oncology centers since 2004 being suggested as cost effective.
Methods
A prospective follow-up study was conducted including all eligible ALL patients diagnosed and treated in two pediatric oncology university centers in Egypt during the period from 30 November 2004 to 1 December 2005 and followed for at least 5 years after diagnosis. Approval of the ethical committee of both universities was obtained.
Eligible patients
All ALL patients aged more than 1 year and less than 17 years, diagnosed and treated in the Pediatric Hematology/Oncology Unit, Children's Hospital, Ain Shams University, Cairo; and the Pediatric Hematology/Oncology Unit, Children's Hospital, Menoufeya University registered from 30 November 2004 to 1 December 2005, and giving patient and/or parental consent. Exclusion criteria included patients with mature B cell-ALL, secondary ALL, relapsed ALL, leukemic phase of lymphoma, and patients who received chemotherapy prior to recruitment.
Diagnosis
The diagnosis of ALL was made by cytomorphological and immunological examination of blood and bone marrow smears at the local institution. For the diagnosis of ALL 25% blasts or more in the bone marrow was mandatory. Immunological markers were judged positive if expressed in 20% or more of the malignant cells. ALL leukemia was classified as precursor B-ALL if the malignant cells were positive for TdT, CD19, and HLA-DR (pro-B ALL), or for TdT, CD10, CD19, and HLA-DR (common ALL), or for TdT, CD10, CD19, HLA-DR, and CyIg (pre-B-ALL). T-lineage ALL was defined by positivity for TdT, CD2, cytoplasmic CD3 (CyCD3), and/or CD7.
Central nervous system (CNS) involvement was defined as the presence of five or more cells/mm3 in the cerebrospinal fluid with leukemic blasts without major blood contamination (20 or more erythrocytes/mm3), or leukemic mass in the brain diagnosed by cranial computed tomography or brain magnetic resonance imaging and confirmed by biopsy.
Data at diagnosis were collected including the address, sex, date of birth, age at disease onset, peripheral white blood cell (WBC) count, immunophenotyping, CNS status, states of lymph nodes, liver, spleen, testes, bone marrow aspiration, % blast cells at diagnosis, bone marrow (BM) status at day 14 of induction and according to these initial data the eligibility criteria were put and the type of CCG protocol therapy was assigned (M1 marrow with blast count less than 5%, M2 with blast count 5–25%, and M3 marrow with blast count more than 25%).
Risk stratification
Risk stratification was based on clinical data, morphological and immunological studies, day 14 bone marrow response as well as conventional cytogenetics. Standard-risk ALL group involved patients with age 1–9.99 years, WBC count <50 × 109/l, precursor B immunophenotype. High-risk standard arm group involved patients with T-ALL and/or age ≥10 years and/or WBC ≥50 × 109/l. High-risk augmented arm group included patients with CNS disease and/or BM blast day14 > 5% (slow early responders (SER)).
Protocol of therapy
Standard-risk ALL patients were treated according to the CCG 1991 protocolCitation6 using a single delayed intensification (DI) arm. The CCG 1961 protocolCitation7 was adopted for treatment of high-risk ALL patients. The NCI high risk and rapid early responders (RER) patients received the standard regimen of CCG-1961 (standard arm of high-risk protocol, HR-SA); and patients who were SER and patients with initial CNS disease received the augmented regimen of CCG-1961 (augmented arm of high-risk protocol, HR-AA, ).
All three groups used dexamethasone as the sole steroid, single intrathecal therapy (IT) therapy, l-asparaginase (Escherichia coli) doses of 6000 IU/m2 with no high-dose methotrexate (MTX) and monthly pulses of vincristine (VCR) and dexamethasone during maintenance phases.
Standard risk therapy consisted of 3 drugs induction without anthracylines, consolidation involved 6-mercaptopurine, VCR with IT monotherapy and one DI.
High-risk standard arm therapy involved 4 drugs induction with anthracylines added, and one DI. Both groups should have day 14 BM blasts <5% (RER).
The high-risk augmented arm therapy involved 4 drugs induction, with anthracylines added, cranial irradiation during consolidation phase, and escalating IV MTX followed by E. coli asparaginase at 15 000 IU/m2 with Capizzi regimen during interim maintenance phase and double delayed intensification (DDI).
Response to chemotherapy was assessed by BM performed on day 28 of induction, complete response (CR) was considered when M1 marrow was achieved in a normocellular marrow, Partial response when M2 marrow was achieved, and refractory disease when M3 marrow was the result of day 28 BM result.
Statistical analysis
The data were coded, entered, and processed on an IBM-PC compatible computer using SPSS (version 15, Chicago, IL, USA). The level P < 0.05 was considered the cut-off value for significance. Comparisons between categorical variables were made using the χ2 test or Fisher's exact test where appropriate. Student's t-test was used to assess the statistical significance of the difference between two population means in a study involving independent samples. EFS was defined as time-to-relapse or death from any cause (relapse, refractory disease, and infection). OS was the time from start of treatment to death from any cause. EFS and OS were estimated using the Kaplan–Meier survival analysis.
Results
Fifty-two eligible patients were registered in the two centers during the period of the study. According to risk criteria, 27 (51.9%) patients were classified as standard risk (SR), 16 (30.8%) as high-risk standard arm (HR-SA), and 9 (17.3%) as high-risk augmented arm (HR-AA). Among the nine patients receiving the augmented regimen, six (11.5%) patients had day 14 M2/M3 marrow and three had initial CNS3 status; one patient had induction failure and was off protocol, his repeat cytogenetics was positive for t9,22, he received salvage with intensified therapy with imatinib and parents refused stem cell transplantation.
Table 1. Detailed protocol of treatment of different risk stratification of childhood ALL
The male-to-female ratio of recruited patients was 1.6:1, 10.3% of ALL patients were older than 10 years of age at diagnosis, 32.7% of all patients presented with lymphadenopathy, 71.2% with hepatomegaly, and 67.3% with splenomegaly, whereas CNS disease was present in 5.8% of cases. They had CNS mass in two patients and CSF infiltration in one patient. The mean hemoglobin level of the studied ALL patients was 6.1 g/dl, mean WBC count was 19.9 × 103/dl, and 30.8% of patients presented with a WBC count greater than 50 × 103/l ().
Table 2. Presenting clinical and laboratory data of included ALL patients
Among the studied patients, 73.1% had precursor-B ALL and 26.9% T-ALL, 59.6% of patients had CD10 positivity, and 7.7% had aberrant CD13 and/or CD 33 expression. Patients with T-ALL had a mean age of 7.8 ± 4.6 years, they were significantly older compared to precursor-B ALL (mean age 5.1 ± 2.2 years) (P = 0.041). Patients older than 10 years at diagnosis constituted 66.7% of T-ALL compared to 33.3% in precursor-B patients. Males constituted 31.3% of T-ALL compared to 68.8% in precursor-B patients, with no statistically significant difference. CNS leukemia at diagnosis was present in one patient with T-ALL (7.4%) and two pre-B ALL patients (5.3%). The mean hemoglobin concentration was significantly higher in T-ALL (8.3 ± 2 g/dl) compared to precursor-B ALL 6 ± 1.9 g/dl (P = 0.001), and mean WBC count was significantly higher in T-ALL patients (143.3 ± 135.2 × 103/dl) compared to precursor-B ALL (20.4 ± 27.6 × 103/dl) (P = 0.005). Protocol-assigned risk groups (SR, HR SA, and HR AA) were 71.05, 15.8, and 13.15%, respectively, in precursor-B ALL patients, compared to 0, 71.4, and 28.6% in T-ALL patients (P < 0.001; ).
Table 3. Clinical and laboratory data of ALL patients at presentation according to their immunophenotype
Survival studies
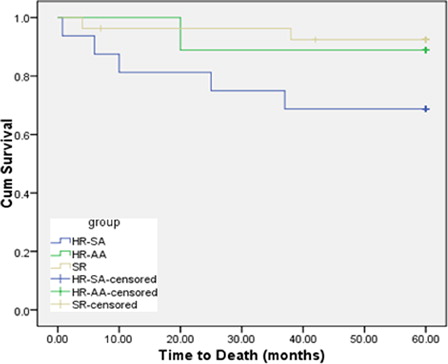
The relapse and death percentage during 5 years follow-up in different risk groups, were respectively, 3 (11.1%) and 1 (3.7%) in SR, 2 (12.5%) and 4 (25%) in HR-SA, 1 (11.1) and 1 (11.1%) in HR-AA, with significantly higher mortality in HR-SA patients (P < 0.05). Kaplan–Meier survival analysis revealed that the probability of OS for the total group of ALL patients at 60 months was 84.6% (SEM 2.267) () while the probability of EFS was 73.4% (SEM 2.292). The 5-year OS was 92.6% (SEM 2.169) for SR patients, 68.8% (SEM 5.456) for HR-SA, and 88.9% (SEM 4.19) for HR-AA patients (), while the 5 years EFS was 70% (SEM 3.371) for SR patients, 55% (SEM 6.195) for HR-SA, 80% (SEM 4.975) for HR-AA patients, The 5-year EFS and OS rates were 63 and 85% for precursor-B ALL, while they were 77 and 77% for T-cell ALL patients, respectively.
Figure 1. OS function from diagnosis (till death) among ALL patients adopting modified CCG-based protocol.
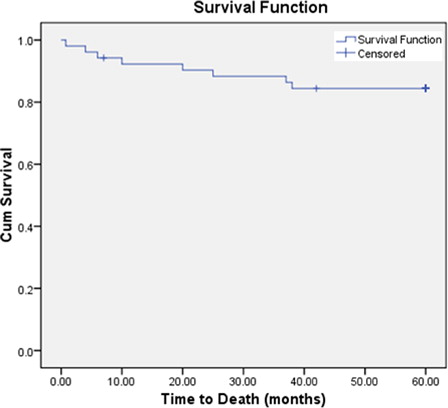
Figure 2. OS function from diagnosis (till death) among ALL patients adopting different arms of CCG.
Seven serious adverse events in six patients (12%) occurred during protocol implementation; four of them happened during induction phase and three during maintenance, and was complicated by mortality in four patients. Causes of death were septic shock (2 patients), massive cerebral thrombosis (one patient) and one patient died with grade-4 hepatotoxicty with hepatic encephalopathy (). Bone marrow relapse occurred in three patients, two of them were in HR-SA and the third was in the SR group. Isolated extramedullary relapse occurred in two patients:testicular in one SR patient and paraspinal soft tissue mass in a patient with HR-AA, while combined bone marrow and testicular relapse occurred in an SR patient.
Table 4. Serious adverse events/relapse data of the studied group
Discussion
Provision of health services for treatment of childhood cancer in Egypt is offered mainly in general and university hospital services and are only partially covered with insurance which increase the challenge of providing supportive care medications and blood products. In order to treat patients free of charge, most cancer-treating centers depend mainly on donations provided individually or from different non-profit organizations. Although most of the patients come from unpriviledged socioeconomic families, compliance did not pose a problem in the studied group, mostly because cancer now forms a public fear due to quite increased incidence over the last years. Inspite of the difficulties, cancer-treating centers are following international protocols; however, modifications may be done to decrease toxicity and/or improve cost-effectiveness of therapy.
The present study was intended to evaluate the outcome of risk-based treatment adopting CCG protocol of childhood ALL in two University centers in Egypt, faced with the challenge of the limited health resources. The 5-year EFS and OS of the studied pediatric ALL patients adopting CCG-based protocols were 73.4 and 84.6%, respectively. In the CCG- series, the reported EFS estimates at 5 years was 75 ± 1% in 1800 seriesCitation8 and 81.9 ± 1.0% in the 1900 series,Citation9 while the estimated 5-year EFS and OS in Jordan adopting modified St Jude-based protocol for ALL therapy was 80 and 89%.Citation10
Standard risk group formed 51.9% of the patients in our study cohort and their 5-years EFS and OS were of 70 and 92.6%, respectively. Standard-risk patients had a higher frequency (63%) in published CCG studies and their 5-year EFS increased from 72 ± 2% in the period 1983–1988, to 81 ± 1% from 1989 to 1995 in CCG 1922 study.Citation8 The successive CCG protocols have omitted the intensive induction-consolidation element of therapy for standard risk and avoided 100 mg/m2 of daunomycin, 2 g/m2 of cyclophosphamide, and 10 hospital days per patient.Citation11 We followed the arm using a single DI. The addition of a single DI phase of therapy was well tolerated and augmented 7-year CCR by 6% with a 7-year OS of 90% in CCG 1881 study.Citation11 Dexamethosone is the sole steroid used. In a previous report of CCG, children with NCI standard-risk ALL assigned to receive dexamethasone had a 6-year isolated CNS relapse rate of 3.7% compared with 7.1% for prednisone. The 6-year EFS was 85% for dexamethasone and 77% for prednisone.Citation12 Patients should have M1 marrow on day 14 to continue on the same regimen being the only prognostic factor of significance in low-risk population.Citation11 Treatment also involved monthly VCR and dexamethasone during maintenance phase, as the CCG-161 trial for lower-risk patients found that addition of monthly VCR and prednisone pulses during maintenance increased the EFS of lower-risk patients from 64 to 77%,Citation13 yet recent systematic review questioned the benefit if intensified therapy was used.Citation14
HR-SA formed 31%, and HR-AA 17% of the studied cohort. The 5 years EFS and OS were 55 and 68.8% for HR-SA group, and 80 and 89% for HR-AA group, respectively. In CCG trials in the period 1989–95, the high-risk group formed 36.9% of ALL patients above 1 year of age with 5-year EFS of 69 ± 1%.Citation8 In CCG 1961 high-risk patients and rapid responder patients randomly assigned to augmented therapy had 5-year EFS of 81.8% compared with 66.8% for patients receiving standard therapy. One versus two interim maintenance and DI courses had no significant impact on EFS.Citation15
We shifted six patients adopting standard risk and standard arm of high-risk group to augmented regimen because day 14 BM was M2 or M3. In CCG 1800 series, a rapid early response to induction therapy was a significant favorable prognostic factor among all risk groups. Poor outcome was generally observed for patients of all risk groups who had a slow early response, defined as M3 marrow status (>25% blasts) at either day 7 or 14 of induction therapy.Citation16 Low- or intermediate-risk patients with M3 marrow status at day 14 had a 3.4-fold higher risk of treatment failure compared with patients who achieve M1 (<5% blasts) by day 14, with 5-year EFS dropped from 72 ± 1% in M1, to 60 ± 5% in M2, and 34 ± 7% in M3 marrow.Citation8
The poor outcome of HR-SA may be attributed that, in the present study, the assessment of response was based solely on cytomorphologic examination. It has been reported that the presence or absence and level of residual disease were the most powerful independent prognostic factors compared with immunophenotype, age, risk group, and white-cell count at diagnosis.Citation17,Citation18 Another challenge we are facing that we had the cytogenetic risk stratification based solely on conventional cytogenetics not on reverse transcriptase polymerase chain reaction (RT-PCR and fluorescence in situ hybridization (FISH) to detect either selective favorable and unfavorable molecular translocations and trisomies.
Based on the poor response of the HR-SA group in our series, and the restricted availability of minimal residual disease assessment in all treating centers, the protocol of therapy for HR-SA was changed to include DDI. EFS was reported to be improved on DDI for intermediate risk patients compared to single DI.Citation19
All patients on these three arms received maintenance intrathecal methotrexate and cranial irradiation was reserved for patients with overt CNS disease at diagnosis. Nine patients (18%) received cranial irradiation as part of augmented regimen; three of them received therapeutic (1800 cGY) and six prophylactic (1200 cGY) radiotherapy. An approximate 30% of patients on the CCG-1800 series received cranial irradiation.Citation8
Among our cohort of patients, T-cell ALL patients had a poor outcome and very poor salvage result after relapse. This has pushed our panel of treating oncologists to shift newly diagnosed T-ALL patients to the augmented regimen, supported by published results of CCG-1961 revealing higher survival in the augmented regimen compared to standard one in all high-risk patients.Citation6 CCG studies (1989–1995) had their 5-year EFS of 70 ± 3–80 ± 4% for T-ALL.Citation8 However, the OS and EFS of T-lineage ALL in an Indian study was (61.5 ± 7.6 and 49.9 ± 7.4, respectively).Citation20
Induction-related complications were the highest serious adverse event timing, and sepsis is the most common cause of death especially post-relapse, sepsis remains a serious obstacle to successful cancer treatment in countries with limited resources, partly because of the poor implementation of infection control hospital policies and the patient/family poor understanding of home care especially in families living in slum areas, we are starting an educational program with one of the supporting family groups to teach patients and their families.
We conclude that standard risk ALL patients receiving CCG-based protocol with single DI, and patients classified as high risk and eligible for augmented arm therapy have better survival compared to the high-risk standard arm group receiving a single DI phase. In the HR-SA group, M1 marrow by day 14, indicating simple morphological remission, should be confirmed by minimal residual disease testing for accurate risk stratification and more advanced molecular stratification by RT-PCR and/or FISH techniques. In this HR-SA group, adoption of protocol with DDI was decided aiming to improve their survival. Also, in our study, ALL of T-cell lineage had poor outcome on high-risk standard arm protocol and should be shifted to augmented regimen.
References
- Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349(7):640–9.
- Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;25:360(26):2730–41.
- Schrappe M, Camitta B, Pui CH, Eden T, Gaynon P, Gustafsson G, et al. Long-term results of large prospective trials in childhood acute lymphoblastic leukemia. Leukemia. 2000;14(12):2193–4.
- Kulkarni KP, Arora RS, Marwaha RK. Survival outcome of childhood acute lymphoblastic leukemia in India: a resource-limited perspective of more than 40 years. J Pediatr Hematol Oncol. 2011;33(6):475–9.
- Phan VH, Moore MM, McLachlan AJ, Piquette-Miller M, Xu H, Clarke SJ. Ethnicdifferences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol. 2009;5(3):243–57.
- Matloub Y, Angiolillo A, Bostrom B, Hunger SP, Stork LC, Angiolillo A, Sather H, et al. Double delayed intensification (DDI) is equivalent to single DI (SDI) in children with National Cancer Institute (NCI) standard-risk acute lymphoblastic leukemia (SR-ALL) treated on Children's Cancer Group (CCG) clinical trial 1991 (CCG-1991). Blood. 2006;108(11):A-146.
- Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2008;1:111(5):2548–55.
- Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD, et al. Children's Cancer Group trials in childhood acute lymphoblastic leukemia: 1983–1995. Leukemia. 2000;14(12):2223–33.
- Gaynon PS, Angiolillo AL, Carroll WL, Nachman JB, Trigg ME, Sather HN, et al. Children's Oncology Group.Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983–2002: a Children's Oncology Group Report. Leukemia. 2010;24(2):285–97.
- Halalsheh H, Abuirmeileh N, Rihani R, Bazzeh F, Zaru L, Madanat F. Outcome of childhood acute lymphoblastic leukemia in Jordan. Pediatr Blood Cancer. 2011;57(3):385–91.
- Hutchinson RJ, Gaynon PS, Sather H, Bertolone SJ, Cooper HA, Tannous R, et al., Children's Cancer Group/Children's Oncology Group. Intensification of therapy for children with lower-risk acute lymphoblastic leukemia: long-term follow-up of patients treated on Children's Cancer Group Trial 1881. J Clin Oncol. 2003;1;21(9):1790–7.
- Bostrom BC, Sensel MR, Sather HN, Gaynon PS, La MK, Johnston K, et al. Children's Cancer Group. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2003;15;101(10):3809–17.
- Bleyer WA, Sather HN, Nickerson HJ, Coccia PF, Finklestein JZ, Miller DR, et al. Monthly pulses of vincristine and prednisone prevent bone marrow and testicular relapse in low risk childhood acute lymphoblastic leukemia: a report of the CCG-161 study by the Children's Cancer Study Group. J Clin Oncol. 1991;9:1012–21.
- Eden TO, Pieters R, Richards S. Childhood Acute Lymphoblastic Leukaemia Collaborative Group (CALLCG). Systematic review of the addition of vincristine plus steroid pulses in maintenance treatment for childhood acute lymphoblastic leukaemia – an individual patient data meta-analysis involving 5,659 children. Br J Haematol. 2010;149(5):722–33.
- Nachman JB, La MK, Hunger SP, Heerema NA, Gaynon PS, Hastings C, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children's oncology group. J Clin Oncol. 2009;1;27(31):5189–94.
- Gaynon PS, Desai AA, Bostrom BC, Hutchinson RJ, Lange BJ, Nachman JB, et al. Early response to therapy and outcome in childhood acute lymphoblastic leukemia: a review. Cancer. 1997;1;80(9):1717–26.
- van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352:1731–8.
- Cave H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer – Childhood Leukemia Cooperative Group. New Engl J Med. 1998;339:591–8.
- Lange BJ, Bostrom BC, Cherlow JM, Sensel MG, La MK, Rackoff W, et al. Children's Cancer Group. Double-delayed intensification improves event-free survival for children with intermediate-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood. 2002;1;99(3):825–33.
- Arya LS, Padmanjali KS, Sazawal S, Saxena R, Bhargava M, Kulkarni KP, et al. Childhood T-lineage acute lymphoblastic leukemia: management and outcome at a tertiary care center in North India. Indian Pediatr. 2011;48(10):785–90.