Abstract
Objectives
The validity of the three currently used chronic myeloid leukemia (CML) scoring systems (Sokal CML prognostic scoring system, Euro/Hasford CML scoring system, and the EUTOS CML prognostic scoring system) were compared in the CML patients receiving frontline imatinib mesylate.
Patients and methods
One hundred and fourty-three chronic phase CML patients (71 males, 72 females) taking imatinib as frontline treatment were included in the study. The median age was 44 (16–82) years. Median total and on-imatinib follow-up durations were 29 (3.8–130) months and 25 (3–125) months, respectively.
Results
The complete hematological response (CHR) rate at 3 months was 95%. The best cumulative complete cytogenetic response (CCyR) rate at 24 months was 79.6%. Euro/Hasford scoring system was well-correlated with both Sokal and EUTOS scores (r = 0.6, P < 0.001 and r = 0.455, P < 0.001). However, there was only a weak correlation between Sokal and EUTOS scores (r = 0.2, P = 0.03). The 5-year median estimated event-free survival for low and high EUTOS risk patients were 62.6 (25.7–99.5) and 15.3 (7.4–23.2) months, respectively (P < 0.001). This performance was better than Sokal (P = 0.3) and Euro/Hasford (P = 0.04) scoring systems. Overall survival and CCyR rates were also better predicted by the EUTOS score.
Discussion
EUTOS CML prognostic scoring system, which is the only prognostic system developed during the imatinib era, predicts European LeukemiaNet (ELN)-based event-free survival better than Euro/Hasford and Sokal systems in CML patients receiving frontline imatinib mesylate. This observation might have important clinical implications.
Introduction
Very few therapeutic and diagnostic options were present for the patients with chronic myeloid leukemia (CML) prior to the understanding of the Philadelphia chromosome (Ph) and the link between CML and the ABL-BCR genes. Sokal CML prognostic scoringCitation1 and Euro/Hasford CML scoring systemCitation2 have been developed for the estimation of essential prognostic indexes of CML including survival. All of those prognostic scoring systems have been generated in the CML patients treated with interferon alpha and/or hydroxyurea/busulfan.
Once BCR-ABL was implicated in the CML, targeted therapies with tyrosine kinase inhibitors (TKIs) such as imatinib were designed to specifically inhibit the progression of the disease.Citation3 Imatinib mesylate was clinically developed during the 1990s, and was approved in 2001 for the treatment of the patients in all phases of CML.Citation4,Citation5 The success of the long-term results of imatinib made this oral TKI as the standard of care for the initial frontline treatment of CML.Citation6 European LeukemiaNet (ELN) recommendations indicated that initial treatment of chronic phase CML should be imatinib 400 mg daily.
EUTOS CML prognostic scoring system using spleen size and basophils was developed by the ELN in the imatinib era.Citation7 EUTOS is the only CML prognostic system that has been developed in the TKI era.
The aim of this study was to perform a comparative assessment of the Sokal CML prognostic scoring system,Citation1 Euro/Hasford CML scoring system,Citation2 and the EUTOS CML prognostic scoring systemCitation7 in the CML patients receiving front-line imatinib mesylate. Validation of the novel EUTOS system is important for the clinical management of CML patients since there are some contradictions and discrepancies in the literature regarding the power of EUTOS score in CML.Citation8–Citation10
Patients and methods
Study population
CML patients who were commenced imatinib mesylate treatment within their first year of diagnosis in two Turkish hematology centers, Hacettepe University (n = 115) and Ondokuz Mayıs University (n = 28), between 2000 and 2012 were included in the study. Patient characteristics are shown in . No other treatment except hydroxyurea was allowed before imatinib. All patients received imatinib mesylate as supplied by Novartis Oncology (Glivec, Novartis Pharma Stein AG, Stein, Switzerland). The patients' records were reviewed in order to find baseline parameters that are used in the three internationally accepted risk scoring systems for CML (Sokal CML prognostic scoring system,Citation1 Euro/Hasford CML scoring system,Citation2 and the EUTOS CML prognostic scoring system Citation7). These scores were calculated in each CML patient as described in the respective studies.Citation1,Citation2,Citation7 The success of the imatinib treatment was evaluated in each individual case using the European LeukemiaNet (ELN) 2009 criteria.Citation11 The criteria recommended by ELN were used for the definitions of complete hematologic and cytogenetic responses. Event-free survival (EFS) was calculated in all patients from the start of imatinib to the date of failure according to the ELN criteria or stopping treatment due to imatinib intolerance or the date of last follow-up in patients in whom treatment did not fail. OS was calculated from the initial diagnosis to the date of mortality of any reason or the date of last follow-up in surviving patients.
Table 1. Characteristics of the CML patients (n = 143)
Calculations of the CML prognostic indexes
Sokal CML prognostic score was calculated using the following formula: Exp 0.0116 × (age in years − 43.4 ) + 0.0345 × (spleen − 7.51) + 0.188 × [ (platelet count/700)2 − 0.563] + 0.0887 × (blast cells − 2.10). Low Sokal risk was <0.8; intermediate Sokal risk was 0.8–1.2 and high Sokal risk was >1.2 (as described in Ref. Citation1).
Euro/Hasford CML score was calculated by means of the following formula: 0.666 when age >50 years + (0.042 × spleen) + 1.0956 when platelet count >1500 × 109/l + (0.0584 × blast cells) + 0.20399 when basophils >3% + (0.0413 × eosinophils) × 100. Low Euro/Hasford risk was <780; intermediate Euro/Hasford risk was 781–1480; and high Euro/Hasford risk was >1480 (as described in Ref. Citation2).
EUTOS CML prognostic score was calculated using the formula, EUTOS score= (7 × basophils) + (4 × spleen size), where the spleen was measured in centimeters below the costal margin and basophils as percentage ratio. A EUTOS score of >87 indicated high risk and <87 low risk. The original equation was; EUTOS score= (0.0700 × basophils) + (0.0402 × spleen size) where the spleen was also measured in centimeters below the costal margin and basophils given as a percentage. For this formula, a EUTOS score >0.8754 indicated high risk and <0.8754 low risk (as described in Ref. Citation7).
Statistical analyses
Both survival analyses and cumulative complete cytogenetic response (CCyR) computations were done by Kaplan–Meier and life table methods. The comparison of the survival rates was calculated by the log rank test. Correlations between different scoring system scores were investigated by the Pearson bivariate correlation analysis. Statistical Packages for the Social Sciences v17.0 (SPSS Inc., Chicago, IL, USA) software was used for all statistical analyses. A P value below <0.05 was considered as statistically significant.
Results
One hundred and forty-three chronic phase CML patients (71 males, 72 females) were included in the study. The median age was 44 (16–82) years. Median total and on-imatinib follow-up durations were 29 (3.8–130) months and 25 (3–125) months, respectively. Median duration between the diagnosis of CML and imatinib start was 0.76 (0–12) months. Starting dose of imatinib was 400 mg in the majority of patients. However, 17 (12%) patients received alternative doses of imatinib mesylate. The complete hematological response (CHR) rate at 3 months was 95%. Eighty-two of 113 (72.6%) evaluable cases attained CCyR on imatinib. Estimated best cumulative CCyR rate at 24 months was 79.6%. Thirty (21%) and 1 (0.7%) cases stopped imatinib treatment due to the failure or intolerance, respectively.
Risk assessments based on the CML prognostic scoring systems
Sokal CML prognostic scoring system
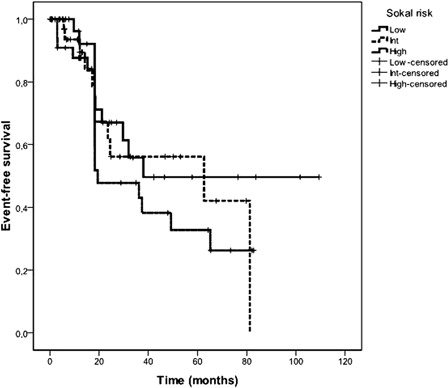
It was possible to calculate Sokal CML score from patient data in 115 patients. Thirty-three (28.6%) patients were found to be low risk, 41 (35.6%) intermediate, and 41 (35.6%) were high Sokal risk ().
Table 2. The proportion of patients, predicted CCyR, and survival rates in each of the risk groups
Euro/Hasford CML prognostic scoring system
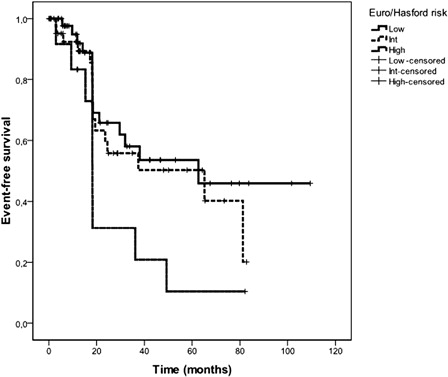
Euro/Hasford score was calculated in 115 patients, too. Fifty-two (45.2%) patients were low risk, 49 (42.6%) intermediate, and 14 (12.2%) were high Euro/Hasford risk ().
EUTOS CML prognostic scoring system
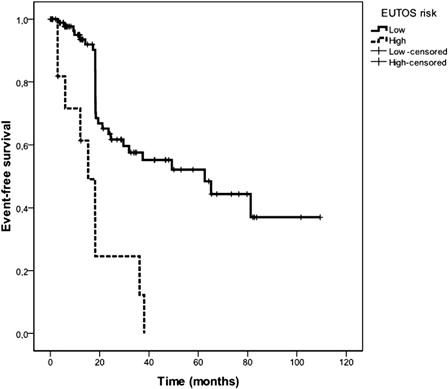
EUTOS scoring was possible in 117 patients. One hundred and three (88%) patients were found to be low risk and 14 (12%) were high EUTOS risk ().
Complete cytogenetic response
Rates of CCyR were 26/30 (86.6%), 23/27 (85%), and 23/37 (62%) in evaluable low, intermediate, and high Sokal risk patients, respectively (P = 0.08). These rates were 32/41 (78%), 31/40 (77.5%), and 7/13 (53.8%) for respective Euro/Hasford risk groups (P = 0.18). Sixty-seven of 83 (80.7%) low and 4/12 (33.3%) high EUTOS risk patients attained CCyR on follow-up (P < 0.001) ().
Event free survival
The 5-year Kaplan–Meier estimated EFS of all CML patients was 48%. The median estimated EFS duration for all patients was 49.2 (20.4–78) months. Five-year probabilities of EFS were 49.6, 56, and 32.8% in low, intermediate, and high Sokal risk patients, respectively () (P = 0.3). The median estimated EFS durations for low, intermediate, and high Sokal risk patients were 38, 62.6 (95% confidence interval= 0–141.8), and 19.4 (0–40) months, respectively. Five-year probabilities of EFS were significantly associated with Euro/Hasford score (low: 53.6%, intermediate: 50.2%, high: 10.4%) () (P = 0.04). The median estimated EFS durations for low, intermediate, and high Euro/Hasford risk patients were 62.6, 65.1 (3.9–126.4), and 18.2 (15.3–21) months, respectively. Five-year probabilities of EFS were highly significantly associated with EUTOS score (low: 96.7%, high: 0%) (). The median estimated EFS durations for low and high EUTOS risk patients were 62.6 (25.7–99.5) and 15.3 (7.4–23.2) months, respectively (P < 0.001) ().
Overall survival
The 5-year Kaplan Meier estimated OS of all patients was 95.7%. Five-year probabilities of OS were 100, 100, and 86.5% in low, intermediate, and high Sokal risk patients, respectively (P = 0.02). The rates were 100, 97.4, and 73.9% in low, intermediate, and high Euro/Hasford risk patients, respectively (P = 0.01). OS was highly significantly associated with EUTOS risk (low: 96.7%, high: 81.5%) (P = 0.001) ().
Correlations
Euro/Hasford scoring system was well-correlated with both Sokal and EUTOS scores (r = 0.6, P < 0.001 and r = 0.455, P < 0.001). However, there was only a weak correlation between Sokal and EUTOS scores (r = 0.2, P = 0.03).
Discussion
In this study, the validity of the three currently used CML scoring systems (Sokal CML prognostic scoring system,Citation1 Euro/Hasford CML scoring system,Citation2 and the EUTOS CML prognostic scoring system Citation7) were compared in the CML patients receiving front-line imatinib mesylate. The definition of ‘event’ during the course of CML under imatinib treatment in this study was based on the definitions of ELN guidelines.Citation11 EFS was significantly associated with the EUTOS score (). Based on our results, EUTOS CML prognostic scoring system predicts ELN-based EFS better than Euro/Hasford and Sokal systems in CML patients receiving front-line imatinib mesylate. OS and CCyR were also significantly and better associated with the EUTOS score.
EUTOS CML scoringCitation7 is the only prognostic system developed during the imatinib era. SokalCitation1 was present in the conventional chemotherapy era, whereas Euro/HasfordCitation2 had been developed for the interferon receiving CML patients. There are conflicting results regarding the use of EUTOS system for the prognostic evaluation of CML.Citation8–Citation10 Jabbour and coworkers proposed that there was no difference in the response rates between CML patients with low and high EUTOS score, overall, and within specific therapies. For instance; 8% of the patients with chronic phase CML treated at MD Anderson Cancer Center were in the high EUTOS score and the EUTOS score was not predictive for the outcome in this population.Citation9 Moreover, Marin et al.Citation10 assessed the prognostic value of the EUTOS scoring as applied to an independent cohort of patients with CML.Citation10 Eight-year probability of OS and PFS and 8-year cumulative incidences of CCyR and MMR according to the EUTOS score risk groups were not predictive in this study. Those two studiesCitation9,Citation10 indicated the value of Sokal system as a predictive scoring for their CML patients and also questioned the validity of the EUTOS scoring systemCitation7 which has been established to support clinical decision-making.Citation8 Therefore, EUTOS and Sokal scores do not seem to be parallel for the prediction of outcome in CML patients. It is acknowledged that Sokal prognostic classification, while still providing the most important baseline prognostic factor, may be coming of age, and will be modified or replaced, based on the biological characteristics of leukemia.Citation12 However, the novel EUTOS system also is far being from a universally accepted prognostic system in CML. In the present study, EUTOS and Sokal scores were only weakly correlated. On the other hand, EUTOS system showed significant correlations with the Euro/Hasford score in our CML cohort.
Currently available Sokal,Citation1 Euro/Hasford,Citation2 or EUTOSCitation7 risk stratification scores can be used for the prognostification of the CML patients in the chronic phase. Many investigators use the Sokal score as it has been more consistently predictive of outcome. In the TKI era, it has been shown to correlate with response to imatinib, with EFS, and even with the probability of maintaining a durable molecular response after treatment discontinuation.Citation13 However, in the clinical practice, there is little evidence that Sokal-specific interventions have any additional value over management based on close follow-up of patients and adhering to the relevant treatment goals.Citation13 The EUTOS score depends on only two simple variables; the spleen size and basophils.Citation7 Unfortunately, two independent seriesCitation9,Citation10 have not confirmed its value, albeit with some methodological differences in the analysis.Citation13 Conventional prognostic indicators remain the strongest determinants of allogeneic hematopoietic stem cell transplantation outcomes in advanced phase CML in the imatinib era.Citation14 It is unclear whether currently available Sokal,Citation1 Euro/Hasford,Citation2 or EUTOSCitation7 risk stratification scores may be incorporated to the decision-making algoritms of CML patients prepared for the transplantation versus novel powerful TKI agents and investigational therapy.
In our present study, the EUTOS score was validated in the real-life CML patients outside of clinical studies. Hoffmann et al.Citation8 suggested that the prognostic score should be cross-validated with patients not treated within randomized trials. EUTOSCitation7 risk stratification scores have biological backgrounds. Spleen can provide an alternative niche to Ph-positive stem and progenitor cells. Basophils are morphologically abnormal in CML and represent an important marker for the acceleration of the disease.Citation7 We think that our findings might have important clinical implications. But, they should be confirmed in larger cohorts.
References
- Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63:789–99.
- Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90:850–58.
- Goldman JM. Treatment strategies for CML. Best Pract Res Clin Haematol. 2009;22:303–13.
- Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–52.
- O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004.
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17.
- Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118:686–92.
- Hoffmann V, Baccarani M, Hasford J, Guilhot J, Saussele S, Rosti G, et al. The EUTOS CML score aims to support clinical decision-making. Blood. 2012;119:2966–67.
- Jabbour E, Cortes J, Nazha A, O'Brien S, Quintas-Cardama A, Pierce S, et al. EUTOS score is not predictive for survival and outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors: a single institution experience. Blood. 2012;119:4524–26.
- Marin D, Ibrahim AR, Goldman JM. European treatment and outcome study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol. 2011;29:3944–45.
- Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51.
- Baccarani M, Rosti G, Castagnetti F, Haznedaroglu I, Porkka K, Abruzzese E, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: A European LeukemiaNet Study. Blood. 2009;113:4497–504.
- Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390–7.
- Khoury HJ, Kukreja M, Goldman JM, Wang T, Halter J, Arora M, et al. Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis. Bone Marrow Transplant. 2011;47:810–16.