Abstract
Objective
To elucidate the protective roles and the underlying mechanism of Tripterygium hypoglaucum Hutch (THH) in mice graft-versus-host disease (GVHD).
Methods
BALB/c (H-2kd) mice were firstly treated with total body irradiation and infused with a mixture of bone marrow and spleen cells from C57BL/6. Then the severity of acute GVHD (aGVHD), chimeras of donor cells, inflammatory cytokines (IFN-γ, IL-4, and IL-10) of plasma, and regulatory T cells were evaluated to elucidate the different drug combinations and concentrations of cyclosporin A (CsA) and THH in preventing aGVHD.
Results
The control group treated with phosphate buffer solution displayed more obvious ruffled hair, hunched posture, diarrhea, reduced weight and more lymphocytes infiltration into the spleen and intestine than these treated with CsA, THH or low-dosed CsA combined with THH, especially those treated with low-dosed CsA combined with THH. No significant differences were observed in the chimeras of donor cells and survival rate among the CsA, THH, or CsA combined with THH-treated groups. Further studies implied that THH might reduce the aGVHD by increasing IL-10, decreasing IL-4, activating Treg cell, and maintaining a relatively high Foxp3 mRNA level.
Conclusion
THH decreased the occurrence of mouse aGVHD and prolonged the survival time by increasing the levels of CD4+/CD25+ T cells, regulating the cytokine secretion and promoting the expression of Foxp3.
Introduction
Hematopoietic stem cell transplantation (HSCT) is probably the most effective cure for hematologic malignancies and some hereditary abnormalities. However, there are several serious complications associated with HSCT, such as severe graft-versus-host disease (GVHD), refractory infection post-transplant, and failure of hematopoietic stem cell engraftment, especially the GVHD that accounts for a substantial portion of early transplant-related morbidity and mortality. GVHD is one of the main factors that affect the long-term survival and quality of life of patients following allogeneic HSCT.Citation1,Citation2 Currently, methotrexate, cyclosporin A (CsA), and glucocorticoidCitation3 were the common drugs used to prevent GVHD. However, these drugs cause adverse reactions, such as damage to liver and kidney functions and an effect on blood glucose levels. Therefore, new drugs to prevent GVHD are urgently needed.
GVHD is characterized by immune damage and is the result of an attack on the target organs of the host and the actions of related cytokines by activated donor T cells.Citation4 Tripterygium hypoglaucum (level) Hutch (THH) is a traditional Chinese herb belonging to the genus Celastraceae. Its main chemical components are alkaloids, terpenes, and pigments.Citation5 THH has been used widely in traditional Chinese medicine for the treatment of various human autoimmune diseases, such as rheumatic arthritis, lupus erythematosus, hyperthyroidism, psoriasis and so on.Citation6–Citation9 It has also been reported that THH has a strong ability to induce chromosomal non-disjunction, chromosomal aberrations, and aneuploidy in mice. Fujita et al.Citation10 found, in addition, that THH can induce C-mitotis, malsegregation, and sister chromatid exchange in mice. It was reported that the use of THH can lead to reversible inhibition of germinal cell development both in human beings and in rats.Citation8–Citation10 Its pharmacological properties include anti-inflammation,Citation6 anti-fertility activities, anti-tumorCitation7,Citation8 and immunosuppression.Citation9 Previous studies have suggested that the contents of tripterifordin and neotripterifordin extracted from THH have a terpene structure (Scheme Ι), which plays important roles in immunosuppression and anti-transplant rejection reaction. Moreover, these compounds were used to treat AIDS.Citation7–Citation10 Currently, THH is also used in treating diseases such as rheumatoid arthritis, chronic nephritis,Citation11,Citation12 and systemic lupus erythematosus. THH has the efficacy of adrenocortical hormones but does not have hormonal side effects, such as rebound and withdrawal symptoms after stopping the drug treatment. In addition, the amount of diterpene lactone compounds in THH is low; therefore, its toxicity is lower than that of Tripterygium wilfordii.
Although previous studies suggested that the THH might be presumed as a drug to reduce the GVHD of the transplantation mice with little adverse side effects, the mechanism by which the THH decreased the aGVHD is still unclear. Thus, the purpose of this study was to use THH to treat a mouse model of acute GVHD (aGVHD) to determine whether it can prevent GVHD and to clarify the underlying mechanisms.
Materials and methods
Animals and drugs
The recipient mice were 6–8-week-old BALB/c female mice (H-2kd), and the donor mice were 6–8-week-old C57BL/6 male mice. All mice were purchased from the Shanghai Laboratory Animal Center of Chinese Academy of Sciences. These mice were fed for 1 week to let them adapt to the environment. During this period, the mice were fed with acidified water and sterile food. All experiments and protocols were performed according to the Institutional Animal Care and Use Committee guidelines.
The CsA used was brought from Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd and dissolved in a sterile saline solution to 1 mg/ml concentration. The THH was brought from Guangdong Boro Xinfeng Pharmaceutical Co., Ltd and dissolved in a sterile saline solution to 10, 20, and 40 mg/ml concentrations.
Cell preparation and transplantation
Eight- to 10-week-old C57BL/6 (H-2 kb) male mice were sacrificed by cervical dislocation, and the bone marrow mononuclear cells (MNCs) were isolated from the femur and tibia bones to prepare the mouse bone marrow cells and spleen cells and adjusted to 1 × 108 cell/ml. Before transplantation, total body irradiation (TBI) was performed on the recipient mice. After 4 hours, the mixture cells containing 2 × 107 bone marrow cells and 2 × 107 spleen lymphocytes were infused through the tail veins injection.
Treatment
In order to select the doses of THH and CsA, the recipient mice were randomly divided into six groups with 10 animals in each group. TBI was performed on the recipient mice before transplantation. After 4 hours, the 2 × 107 bone marrow cells and 2 × 107 spleen lymphocytes was infused through the tail veins. The drugs were intragastrically administered to recipient mice at 0.2 ml/day at 1–30 days after transplantation (+30 day), while the control group received 0.2 ml/day saline. The THH high-, medium-, and low-dose groups were given 400, 200, and 100 mg/(kg/day) THH, respectively. The CsA high- and low-dose groups were given 10 and 5 mg/(kg/day) CsA, respectively.
In order to study the preventive effects of low-dose THH combined with low-dose CsA on GVHD, the recipient mice were randomly divided into four groups with 20 mice in each group. The recipient mice were given drugs at a rate of 0.2 ml/day by intragastric administration from −1 to +30 days. Group A was defined as the control group and was given 0.2 ml/day saline. Group B was the CsA prevention group and was given 10 mg/(kg/day) CsA. Group C was the THH preventive group and was given 400 mg/(kg/day) THH. Group D was the combined preventive group and was given 100 mg/(kg/day) THH and 5 mg/(kg/day) CsA.
General observations
After transplantation, the peripheral white blood cell count was determined regularly, and the clinical manifestations (such as hunched posture, ruffled hair, and diarrhea), body weight changes, and survival time were detected.Citation13
Pathology
Formalin-fixed liver, skin, and small bowel were embedded in paraffin, cut into 4-μm-thick sections, and stained with hematoxylin and eosin for histological examination. Slides were examined in a blinded manner. Both the tissues from dead and surviving mice were used. A semi-quantitative scoring system was used to assess the following characteristics: dermatopathologist, intestinal pathology, and liver pathology.Citation13 The aGVHD scores contained all the slides observed.
Flow cytometry
To detect the chimerism of the donor cells after transplantation, mouse bone marrow MNCs were isolated 30 days after transplantation, and 2 × 105 MNCs were mixed with FITC-conjugated anti-H-2Kb and PE-conjugated anti-H-2Kd by vortexing. After incubation in the dark for 20 minutes, hemolysin was added, mixed thoroughly and incubated for another 5–8 minutes. After washing twice, the ratio of H-2Kb+ and H-2Kd+ was analyzed by flow cytometry.
To detect changes in Treg cells, peripheral blood was collected from the tail vein at 5, 10, 15, 20, and 30 days after transplantation. The cells were mixed thoroughly with FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 and incubated at room temperature in the dark for 20 minutes. Hemolysin was then added, mixed thoroughly and incubated for another 5–8 minutes. After washing twice, the percentage of CD4+/CD25+ T cells was analyzed by flow cytometry.
Enzyme-linked immunosorbent assay
Mouse peripheral blood plasma was collected at 5, 10, 15, 20, and 30 days after transplantation, and the IFN-γ, IL-4, and IL-10 concentrations were detected by enzyme-linked immunosorbent assay.
PCR
The expression levels of Forkhead/winged helix transcription factor (Foxp3) mRNA in the mouse spleen cells at 5, 10, 15, 20, and 30 days after transplantation were measured. The Foxp3 primers were 5′-CACAACATGGACTACTTCAAGTACCA-3′ and 5′-GGAATAGGCTAC CCGGTAG-3′, respectively. The fluorescence probe was 5′-AATATGCGACCCCCTTTC ACCTATGCC-3′. The PCRs were carried out in ABI Fast 7500 and the reaction conditions were 94°C, 2 minutes, followed by 40 cycles of 94°C for 30 seconds and 55°C for 30 seconds. The standard reaction was performed using the above conditions to obtain the standard curve using the external standard method. The results of all groups were compared.
Statistical analysis
The data are presented as the mean ± standard deviation, and analyzed using SPSS13.0. A comparison among the groups was made by one-way analysis of variance; the survival rate was presented by survival curves by the Kaplan–Meier method and were compared by the log-rank test. P values <0.05 were considered to be statistically significant.
Results
The effects of THH and CsA doses on the survival time of recipient mice after transplantation
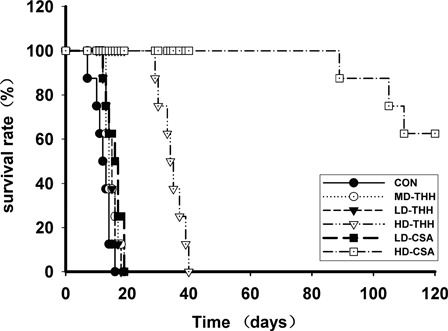
All the recipient mice in the control group died within 20 days after transplantation, with a median survival time of 12.5 days (range: 7–16 days). The median survival times of the THH high-dose group and the HD-CSA group were 34.5 days (range: 29–40 days) and 122.5 days (range: 89–152 days), respectively. This result suggested that the median survival time was significantly prolonged compared with the control group (P < 0.05) (). The median survival times of the THH medium- and low-dose groups and the CsA low-dose group were 15 days (range: 13–19 days), 14 days (range: 12–18 days), and 16.5 days (range: 12–19 days), respectively. These results also indicated that the median survival times of the THH medium-, low-dose groups and the CsA low-dose group were also prolonged compared with that of the control group, although the difference was not statistically significant (P > 0.05) (). Therefore, 400 mg/(kg/day) THH and 10 mg/(kg/day) CSA were used in groups B and C, respectively, and 100 mg/(kg/day) THH and 5 mg/(kg/day) CSA were used in group D.
Figure 1. Survival times of the mice in different drugs and drug concentrations treatment groups after bone marrow transplantation. For the selection of the doses of THH and CsA, the BALB/c recipient mice were randomly divided into six groups with 10 animals in each group. Before transplantation, TBI was performed on the recipient mice. Four hours after irradiation, the cell mixture suspension containing 2 × 107 bone marrow cells and 2 × 107 spleen lymphocytes was infused through the tail veins. The drugs were intragastrically administered to recipient mice in each group at 0.2 ml/day at 1–30 days after transplantation (+30 days); the control group received 0.2 ml/day saline. The THH high-, medium-, and low-dose groups were given 400, 200, and 100 mg/(kg/day) THH, respectively, and the CsA high- and low-dose groups were given 10 and 5 mg/(kg/day) CsA, respectively. The median survival times of the CON, MD-THH, LD-THH, and LD-CSA groups were 12.5, 14, 15, and 16.5 days, respectively. The comparison between any two groups of the CON, MD-THH, LD-THH, and LD-CSA did not show statistical significance. The median survival time of the HD-THH group was 34.5 days, which was significantly different from that of the CON, MD-THH, LD-THH, and LD-CSA groups. The median survival time of the HD-CSA group was 122.5 days, which was significantly different from that of the CON, MD-THH, LD-THH, LD-CSA, and HD-THH groups.
The low-dose THH combined with low-dose CSA effectively alleviated GVHD
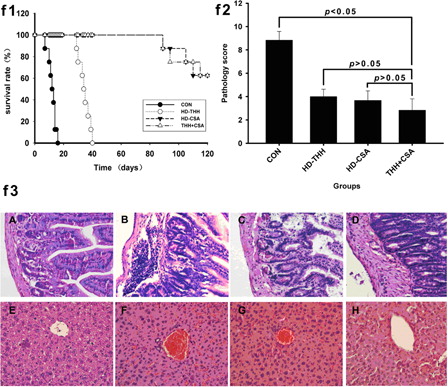
Group A recipient mice started exhibiting typical GVHD manifestations, such as a hunched posture, ruffled hair, diarrhea, and reduced body weight, from +6 days and died; the average survival time was 10.70 ± 1.07 days (). Groups B and C recipient mice started exhibiting GVHD manifestations at +8 − 9 days; the clinical manifestations were mild, and the average survival times were 37.25 ± 3.05 days and 34.15 ± 3.00 days, respectively. The average survival times of these two groups were significantly longer than that of group A mice (P < 0.01) (). No GVHD difference was observed between group B and group C mice (P > 0.05). Group D mice started exhibiting different degrees of hunched posture and ruffled hair, and the average survival time was 40.05 ± 2.54 days. Moreover, the GVHD degree of group D mice was lower than that of other group. The pathological examination implied that the group A mice displayed III–IV grade GVHD. Groups B and C mice had only a small amount of lymphocyte infiltration in the hepatic portal area or intestine, indicating I–II grade GVHD (). Group D mice displayed no obvious abnormalities and only a small amount of lymphocyte infiltration in the intestine, indicating grade I GVHD.
Figure 2. Low-dose THH + CSA effectively prolonged the survival time of mice after transplantation and alleviated the onset of GVHD. To study the preventive function of low-dose THH combined with low-dose CsA on GVHD, the BALB/c recipient mice were randomly divided into four groups with 20 mice in each group. Group A was defined as the control group and was given 0.2 ml/day saline; group B was the CsA prevention group and was given 10 mg/(kg/day) CsA; group C was the THH prevention group and was given 400 mg/(kg/day) THH; group D was the combined prevention group and was given 100 mg/(kg/day) THH and 5 mg/(kg/day) CsA. f1 is the survival time of mice in the different drugs and drug concentrations treatment groups; the median survival times of the CON, HD-THH, HD-CSA, and CSA + THH groups were 12.5, 34.5, 122.5, and 122.5 days, respectively. The median survival time of the CSA-THH group was significantly different from that of the CON and HD-SCA groups but was not significantly different from that of the HD-CSA group. Formalin-fixed liver, skin, and small bowel were embedded in paraffin, cut into 4-μm-thick sections, and stained with hematoxylin and eosin for histological examination. Slides were coded without reference to prior treatment and examined in a blinded manner. A semi-quantitative scoring system was used to assess the degree of aGVHD. A–H represent different groups, A and E: CON group; B and F: HD-THH group; C and G: HD-CSA group; D and H: CSA + THH group. f2 is the pathological score of the mouse liver and intestine at +15 days. f3 is the pathology of the liver and intestine of the different treatment groups on day 15. Groups A, B, C and D mice revealed grade III-IV, I-II, I-II, and I GVHD according to the histopathological criteria.
The low-dose THH combined with low-dose CsA enhanced the implantation of the donor bone marrow and hematopoietic reconstruction
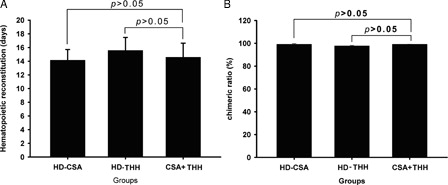
The white blood cell count of the normal BALB/c mice was 9.90 ± 2.09 × 109 cell/l. The total number of white blood cells significantly decreased after transplantation and reached the lowest point at +3 days, gradually increased after +5 days, and finally recovered to >6.00 × 109 cell/l after +15 − 20 days (). The percentage of the H-2b molecule on the bone marrow cell surface at +10 days was >95%, and the percentages of the H-2b molecule in groups B, C, and D at +30 days were 99.18 ± 0.58%, 97.68 ± 0.59%, and 99.15 ± 0.11% (), respectively, indicating that the hematopoietic stem cells had been implanted.
Figure 3. The hematopoietic reconstruction time (A) and chimerism rate (B) were determined in different drugs and drug concentrations treatment groups. (A) The hematopoietic reconstruction time in different drugs and drug concentrations treatment groups. The median time of hematopoietic reconstruction of the HD-CSA, HD-THH, and CSA + THH is given in days. There was no significant hematopoietic reconstruction time difference observed between any two groups. (B) The chimerism rate of the donor at +30 days after transplantation. To detect the chimerism of the donor cells after transplantation, mouse bone marrow MNCs were isolated at 30 days after transplantation, and 2 × 105 MNCs were mixed thoroughly with FITC-conjugated anti-H-2Kb and PE-conjugated anti-H-2Kd by vortexing. After incubation at room temperature in the dark for 20 minutes, hemolysin was added, and the samples were mixed thoroughly and incubated for 5–8 minutes in the dark. After washing twice with 1 × phosphate buffer solution, the ratio of H-2Kb+ and H-2Kd+ was analyzed by flow cytometry. The HD-CSA, HD-THH, and CSA + THH values are given as the control group %. There was also no significant difference in the chimerism rate observed between any two groups.
The synergistic effect of the low-dose THH combined with the low-dose CSA on alleviating GVHD by regulating the concentrations of IFN-γ, IL-4, and IL-10
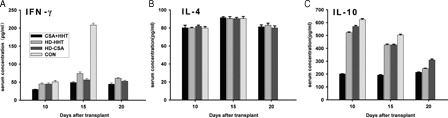
The concentrations of IFN-γ and IL-4 in the mouse plasma peaked at +15 days and started to decrease at +20 days. The concentration of IL-10 peaked at +10 days and gradually decreased thereafter. Compared with group A, the concentrations of IFN-γ in groups B–D significantly decreased (P < 0.05), and the decrease in group C was the most prominent (). However, the changes in the concentration of IL-4 were not significant. The concentrations of IL-10 significantly increased, and the increase in group D was the most prominent. Although the concentration in group D decreased afterwards compared with that in groups B and C, it remained at a higher level at +20 days (P < 0.01).
Figure 4. Changes in the levels of cytokines IFN-γ (A), IL-4 (B) and IL-10 (C) in the different drugs and drug concentrations treatment groups. At 5, 10, 15, 20, and 30 days after transplantation, the IFN-γ, IL-4, and IL-10 concentrations were detected using ELISA. (A) The changes of the IFN-γ concentrations in different treated times and different drug treatment times. The level of IFN-γ in the CON group reached the highest level compared with that in the HD-THH and HD-CSA groups on day 15 and the differences were statistically significant. Although the IFN-γ levels in the CSA + THH group on 10, 15, and 20 days were lower compared with that in the HD-CSA group, the difference was not significant. (B) The changes of the IL-4 concentrations in different treated times and different drug treatment times. There was no significant difference in the levels of IL-4 in each treatment group at days 10, 15, and 20 after transplantation. (C) The changes of the IL-10 concentrations in different treated times and different drug treatment times. The level of IL-10 in the CON group was the highest on day 10 and significantly different when compared with that in the HD-THH and HD-CSA groups. The level of IL-10 in the CSA + THH group on days 10, 15, and 20 was lower and significantly different when compared with that in the HD-CSA group.
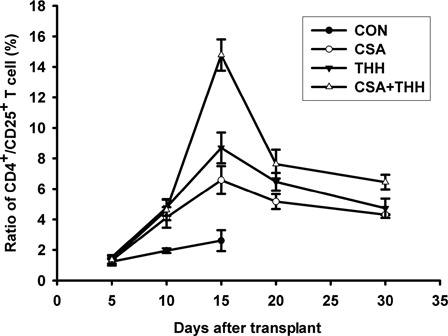
The low-dose THH combined with low-dose CsA reducing GVHD through regulating CD4+/CD25+ T cells (Treg cells)
The number of CD4+/CD25+ T cells in groups B, C, and D began to significantly increase at +10 days compared with that in the control group (P < 0.05). The number of CD4+/CD25+ T cells in groups B, C, and D significantly increased at +15 days compared with that in the control group (P < 0.05) (). The number of CD4+/CD25+ T cells started to decrease at +20 days but those of group D decreased more slowly than those of groups B and C (P < 0.05). The CD4+/CD25+ T-cell ratio in group D remained at a higher level than that in groups B and C at +30 days (P < 0.05). The CD4+/CD25+ T cell ratio after transplantation in groups B, C, and D all increased first and decreased thereafter, whereas the control group had no evident changes.
Figure 5. The changes in the number of CD4+/CD25+ (Treg cells) in the different drugs and drug concentrations treatment groups. To detect changes in the number of Treg cells, peripheral blood was collected at 5, 10, 15, 20, and 30 days after transplantation; mixed thoroughly with FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 by vortexing; and incubated at room temperature in the dark for 20 minutes. Hemolysin was then added, and the samples were mixed thoroughly and incubated in the dark for 5–8 minutes. After washing twice with 1 × phosphate buffer solution, the percentage of CD4+/CD25+ T cells was analyzed by flow cytometry. The number of CD4+/CD25+ T cells in groups B–D was significantly higher than that of the control group on day 10. The number of CD4+/CD25+ T cells in groups B–D was also significantly higher than that of the control group on day 15. Furthermore, the increase in group D was most prominent, followed by groups C and B. The number of CD4+/CD25+ T cells decreased significantly more slowly in group D than in groups B and C. The CD4+/CD25+ T cell ratio in group D was maintained at a higher level than in groups B and C at +30 days (P < 0.05).
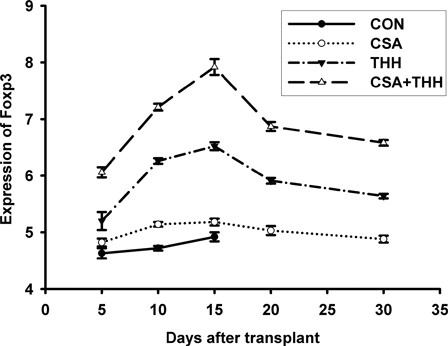
The THH affected Treg cells by regulating the expression of Foxp3 expression
The Foxp3 mRNA levels in the spleen cells of group B, C and D recipient mice increased at +5 days (). No significant difference was observed between the control group and group B (P > 0.05), although the difference in groups C and D was statistically significant (P < 0.01) (). At +10 and +15 days, the expression levels in groups B, C, and D all increased (P < 0.01), especially in group D (P < 0.01). Moreover, the differences among these three groups were significant (P < 0.01). The expression levels peaked at +15 days and gradually decreased thereafter, but the decrease in group D was slower and maintained at a higher level at +30 days and differences were significant (P < 0.01).
Figure 6. The expression changes of Foxp3 in the different drugs and drug concentrations treatment groups. The expression levels of Forkhead/winged helix transcription factor (Foxp3) mRNA in the mouse spleen cells at 5, 10, 15, 20, and 30 days after transplantation were measured on the 7300 real-time fluorescence quantitative PCR. The expression levels of Foxp3 mRNA in the spleen cells of groups B–D recipient mice all increased at +5 days. However, the increase in groups C and D was significantly different from the control groups. The expression levels further increased at +10 days and +15 days. At these time points, the expression levels in groups B, C, and D all increased (P < 0.01), and the increase in group D was the most prominent (P < 0.01). The expression levels in all groups peaked at +15 days and gradually decreased thereafter, and the decrease in group D was slower and was maintained at a higher level at +30 days (P < 0.01).
Discussion
GVHD is a result of an attack on the target organs of the host and the actions of related cytokines by activated donor T cells characterized by immune damage.Citation4 The THH, which is used in Chinese medicine, is an immunosuppressive agent. Its main chemical components are alkaloids, terpenes, and pigments.Citation5 THH has been used widely in traditional Chinese medicine for the treatment of various human autoimmune diseases, such as rheumatic arthritis, lupus erythematosus, hyperthyroidism, psoriasis and so on.Citation6–Citation9 Its pharmacological properties include anti-inflammation,Citation6 anti-fertility activities, anti-tumor,Citation7,Citation8 and immunosuppression.Citation9 Its pharmacological properties include anti-inflammation,Citation6 anti-tumor,Citation7,Citation8 immunosuppression,Citation9 and anti-fertility activities. Now, THH is used in treating diseases such as rheumatoid arthritis, chronic nephritis, and systemic lupus erythematosus, while it had no hormonal side effects such as rebound and withdrawal symptoms after stopping drug treatment.Citation11,Citation12 However, it was still unclear whether the THH prevents GVHD and related mechanisms. Our studies suggested that the THH significantly prolonged the survival time of the recipient mice and alleviated the clinical and pathological manifestations of GVHD. Moreover, the dose-screening experiments indicated that the combination of low doses of THH and CsA might have a synergistic effect while reducing the side effects of CsA at the same time.
Previous studies suggested that type I T-cell cytokines and the inhibitory type II cytokines had a close relation to the occurrence of aGVHD. The cytokine hypothesis proposed by Antin and FerraraCitation14 suggests that the imbalance between the aGVHD promoting type I T-cell cytokines and the inhibiting type II cytokines is the underlying mechanism for aGVHD. Moreover, they also proposed that the occurrence of aGVHD is a three-step process. Our study indicated that THH treatment decreased the secretion of the TH1-type cytokine IFN-γ which induces the expression of MHCII moleculesCitation15, thus inhibiting the activation of donor T cells. IL-2 belongs to the TH2 class cytokines.Citation16,Citation17 These cytokines can inhibit the production of TH1 cytokines, further reduce IFN-γ secretion, and reduce T-cell activation to alleviate aGVHD.Citation14 This study also showed that the combined low-dose CsA and THH treatment significantly reduced the IFN-γ concentration and increased the peak value of IL-10 which remained at a high level at +20 days. Wan et al.Citation18 stimulated human monocytes using lipopolysaccharide to increase the levels of IL-8 and TNF-α and then treated the cells with a decoction of THH, which led to the secretion of IL-8 and TNF-α significantly decreased. Therefore, they concluded that THH can inhibit the secretion of cytokines, such as TNF-α, by monocytes and macrophages that interfere with the expression of intracellular adhesion molecule 1 (ICAM-1) in the vascular endothelial cells, thus alleviating inflammatory responses and restoring the normal cytokine network. CsA mainly affects step 2 in the reaction cascade by inhibiting the production of IL-2.Citation19 THH contains some contents that can also affect step 1 reaction cascade to some extent by regulating inflammatory cytokines, such as TNF-α and IL-8, and adhesion molecules, such as ICAM-1. The combined treatment of these two drugs can therefore affect both steps 1 and 2 in the reaction cascade at the same time to have a synergistic effect. Previous studies suggested that the contents of tripterifordin and neotripterifordin extracted from THH have a terpenes structure (Scheme Ι), which plays important roles in the immunosuppression and anti-transplant rejection reaction.Citation7–Citation10 This study also suggested that the THH could reduce the degree of GVHD by regulating the production of T cells and the cytokines.
In recent years, studying the roles of regulatory T cells in immune tolerance has led to considerable interest.Citation20 Studies have shown that Tregs established and maintained immune nonresponsiveness to its own components and negatively controlled different immune responses against non-self antigens.Citation21 The CD4+/CD25+ Treg is an important sub-group of Tregs and is involved in the induction and maintenance of immune tolerance.Citation22 Vela-Ojeda et al.Citation23 showed that the CD4+/CD25+ Tregs could prevent mouse GVHD. Previous studies suggested that the triptolidein in the THH might affect the content of the CD4+/CD25+ Tregs, and this study also indicated that the THH has a stronger CD4+/CD25+ Treg induction function than CsA.
In order to determine whether the THH protective effect on mouse GVHD was associated with Tregs at the gene level, the expression of Foxp3 in the spleen cells of the recipient mice was detected. Foxp3 is specifically expressed in Tregs and the expression level of Foxp3 in activated CD4+/CD25− T cells is less than 1% of that of CD4+/CD25+ Tregs.Citation24 Khattri et al.Citation25 also showed that the expression level of Foxp3 is involved in the function of the peripheral CD4+ Tregs. Furthermore, the expression of Foxp3 is presumed negatively correlated with the severity of GVHD.Citation26 Our results showed that the expression of Foxp3 in the low-dose THH and CsA combination treatment group had a rapid increase and peaked at +30 days, remaining at a high level thereafter. The changes of the Foxp3 expression displayed similar trend to the changes of the CD4+/CD25+ T cells. CsA inhibits the calcineurin activated by antigens to inhibit the transcription of genes, such as IL-2. The decrease of IL-2 production and secretion blocked the growth and differentiation of the IL-2-dependent T cells. Foxp3 can directly or indirectly inhibit the expression of effectors genes (such as IL-2),Citation27 act as an adaptor protein to recruit other inhibitor molecules bind to target genes, or up-regulating the expression of cytokines, such as TGF-β and IL-10, or inhibiting the IL-4 and IFN-γ from participating in immune regulation, thus alleviating GVHD.Citation23–Citation26 The combination of THH and CsA not only augmented their inhibitory function on IL-2 expression but also improved immune functions to alleviate GVHD by increasing the expression of TGF-β and IL-10.
In summary, THH prevented GVHD in the mouse allogeneic bone marrow transplantation model. The combination treatment with a low dose of THH and CsA had a synergistic effect to reduce the required dosage and side effects of CsA.
References
- Couriel D, Caldera H, Champlin R, Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101:1936–46.
- Martin PJ, Schoch G, Fisher L, Byers V, Anasetti C, Appelbaum FR, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–72.
- Blazar BR, Murphy WJ. Bone marrow transplantation and approaches to avoid graft-versus-host disease (GVHD). Philos Trans R Soc Lond B Biol Sci. 2005;360:1747–67.
- Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307.
- Huang WH, Zhang R, Guo BL, Si JP. Study on total alkaloids content and its influential factors in medicinal materials of Tripterygium. Zhongguo Zhong Yao Za Zhi. 2008;33:15–8.
- Cheng DW, Zou Y, Qian N, Wang CL, Tian YB, Wang DL, et al. Effects of compound preparation of Cordyceps sinensis and Tripterygium hypoglaucum on survival time of pigskin after allogeneic transplantation. Zhong Xi Yi Jie He Xue Bao. 2006;4:185–8.
- Liu SX, Cao J, Yuan J, Huang P, Shua PQ, Honma M. Effects of total alkaloids of Tripterygium hypoglaucum Hutch on tk gene of mouse lymphoma cells. Zhongguo Zhong Yao Za Zhi. 2003;28:954–7.
- Zhuang WJ, Fong CC, Cao J, Ao L, Leung CH, Cheung HY, et al. Involvement of NF-kappaB and c-myc signaling pathways in the apoptosis of HL-60 cells induced by alkaloids of Tripterygium hypoglaucum (level.) Hutch. Phytomedicine. 2004;11:295–302.
- Pu LX, Zhang TM. Effects of triptolide on T lymphocyte functions in mice. Zhongguo Yao Li Xue Bao. 1990;11:76–9.
- Fujita R, Duan H, Takaishi Y. Terpenoids from Tripterigyum hypoglaucum. Phytochemistry. 2000;53:715–22.
- Zhong J, Xian D, Xu Y, Liu J. Efficacy of Tripterygium hypoglaucum Hutch in adults with chronic urticaria. J Altern Complement Med. 2011;17:459–64.
- Wu X, Xu J, Luo X. Effects of Tripterygium hypoglaucum on serum IL-1, IL-6, and TNF-alpha in chronic nephritis rats. Zhongguo Zhong Yao Za Zhi. 2010;35:3354–6.
- Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173:5467–75.
- Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–8.
- Pollack BP, Sapkota B, Cartee TV. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res. 2011;17:4400–13.
- Raju K, Rabinovich BA, Radvanyi LG, Spaner D, Miller RG. A central role for IL-2 in fate determination of mature T cells–I: role in determining the Th1/Th2 profile in primary T cell cultures. Int Immunol. 2001;13:1453–9.
- Poudrier J, Owens T. Th1 and Th2 help for B cells: differential capacity for induction of autonomous responsiveness to IL-2. Int Immunol. 1995;7:1021–7.
- Wan CF, Li ZH, Xu GJ, Liu SS, Qi XY. Effects of depressive disorder on monocytic expression of CD(40) and plasma IL-8 concentration in senile coronary heart disease patients. Zhonghua Yi Xue Za Zhi. 2011;91(35):2459–63.
- Hess AD, Fischer AC, Beschorner WE. Effector mechanisms in cyclosporine A-induced syngeneic graft-versus-host disease. Role of CD4+ and CD8+ T lymphocyte subsets. J Immunol. 1990;145:526–33.
- Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–7.
- Wolf M, Schimpl A, Hunig T. Control of T cell hyperactivation in IL-2-deficient mice by CD4(+)CD25(-) and CD4(+)CD25(+) T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol. 2001;31:1637–45.
- Tawara I, Shlomchik WD, Jones A, Zou W, Nieves E, Liu C, et al. A crucial role for host APCs in the induction of donor CD4+CD25+ regulatory T cell-mediated suppression of experimental graft-versus-host disease. J Immunol. 2010;185:3866–72.
- Vela-Ojeda J, Montiel-Cervantes L, Granados-Lara P, Reyes-Maldonado E, Garcia- Latorre E, Garcia-Chavez J, et al. Role of CD4+CD25+highFoxp3+CD62L+ regulatory T cells and invariant NKT cells in human allogeneic hematopoietic stem cell transplantation. Stem Cells Dev. 2010;19:333–40.
- Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–23.
- Khattri R, Kasprowicz D, Cox T, Mortrud M, Appleby MW, Brunkow ME, et al. The amount of scurfin protein determines peripheral T cell number and responsiveness. J Immunol. 2001;167:6312–20.
- Miura Y, Thoburn CJ, Bright EC, Phelps ML, Shin T, Matsui EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–93.
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35.