Abstract
Introduction
Preeclampsia (PE) is characterized by hypertension and proteinuria after the 20th week gestation. The aim of this study was to investigate whether platelet count (PC) and platelet indices (mean platelet volume (MPV), platelet distribution width (PDW), and plateletcrit (PCT)) could predict severe form of preeclampsia (sPE).
Methods
Three groups were evaluated; G1-pregnant with sPE (N = 29); G2-normotensive pregnant (N = 28) and Group 3: non-pregnant women (N = 30). Platelet parameters were obtained using the same automatic blood cells count. Statistical analysis was performed by analysis of variance, t-test, and receiver operating characteristic (ROC) curve. P ≤ 0.05 was considered significant.
Results
Lower PC and PCT were observed in sPE comparing to normal pregnant (P = 0.031 and 0.035, respectively) and to non-pregnant women (P < 0.001 and 0.004, respectively). PDW was higher in sPE comparing to normotensive pregnant (P = 0.028) and to non-pregnant women (P < 0.001). MPV was higher in sPE comparing to normotensive pregnant and non-pregnant women (P = 0.05 and P < 0.001, respectively). Analysis from the ROC curve and its areas for each variable showed that the parameters have regular diagnostic significance, except for PCT, considered as not good for this purpose.
Conclusion
PC emerges as a good candidate for sPE diagnosis, since it is a simple and habitually done method, with lower cost and greater accessibility in the clinical laboratory. Further studies evaluating sequential PC and platelet indices throughout pregnancy are necessary to clarify the role of platelet parameters in PE development and severity.
Introduction
Preeclampsia (PE) is a multifactorial disease, characterized by the presence of high blood pressure and proteinuria after the 20th week of pregnancy. It is clinically relevant to distinguish between mild and severe preeclampsia (sPE), according to severity of symptoms. This disease can progress to eclampsia (characterized by seizures as a sign of affection of the cerebral vessels), HELLP syndrome (hemolysis, elevated liver enzyme, low platelets) or disseminated intravascular coagulation.Citation1,Citation2 The etiology of the PE is unknown and it is associated with fibrin deposition in the placental and renal microcirculation and intrauterine fetal growth retardation.Citation3,Citation4 PE occurs in 5–8% of pregnancies worldwide and is an important cause of maternal and fetal deaths. Its prevalence varies in different populations and in different ethnic groups.Citation5
Increased plasma levels of platelet activation markers (β-thromboglobulin and platelet factor-4)Citation6,Citation7 and increased expression of activation markers on the surface of plateletsCitation7,Citation8 in preeclamptic women confirm platelet activation in this disease. Besides, an impaired endothelial synthesis of prostacyclinCitation9 and nitric oxide has been related in PE.Citation10,Citation11
Platelet indices are available as part of the data obtained from complete blood count. They include mean platelet volume (MPV), which reflects the marrow bone function, platelet distribution width (PDW), which corresponds to size distribution of platelets; plateletcrit (PCT), which corresponds to the volume that platelets have in 100 mL of total blood; and platelet large cell ratio (PLCR), which reflects the percentage of platelets larger than 12 fL.Citation12,Citation13
Some studies have evaluated the applicability of platelet indices, particularly MPV for the clinical and pathophysiological understanding of vascular diseases, including PE, but their value is not established yet.Citation14–Citation23 It has long been known that platelet volume is a direct indicator of increased platelet synthesis.Citation14,Citation15 In normal pregnancies, a small increase in platelet aggregation is observed, which is compensated by increased synthesis and consequently increased MPV.Citation24 Moreover, it was suggested that in normal pregnancies, changes in platelet volume may be more sensitive than platelet count (PC) as a measure of altered platelet function.Citation25
Although the literature reveals reduction in circulating platelets and platelet activation in PE there is no study assessing and comparing PC and indices from preeclamptic, normotensive pregnant, and non-pregnant women. Therefore, the aim of this study was to investigate in sPE, by automatic cells blood count the PC (PC) and indices (MPV, PDW, and PCT) altogether, in order to improve the knowledge about this multifactorial and complex disease.
Materials and methods
The Ethics Committee of Federal University of Minas Gerais approved the study and informed consent was obtained from all participants.
The present case–control study included 87 women selected from July to October 2010. Three groups were studied, 29 pregnant with sPE (Group 1), 28 normotensive pregnant (Group 2), and 28 non-pregnant women (Group 3). Severe preeclamptic women were selected from Regional Public Hospital of Betim/Brazil. Normotensive pregnant and non-pregnant women were selected from Healthy Center Guanabara, Betim, Brazil. Pregnant were matched for maternal age (24.6 ± 6.1 years), gestational age (36.2 ± 3.8 weeks) and parity, while women of three groups presented similar age (15–39 years) and socioeconomic status. Inclusion criteria for sPE were defined on the basis of systolic/diastolic blood pressure ≥160/110 mmHg and proteinuria ≥2 g/day. For control group the criteria were systolic/diastolic blood pressure ≤120/80 mmHg without a history of hypertension or proteinuria. Only healthy non-pregnant women were included in group 3. Exclusion criteria for the three groups were chronic hypertension, coagulation disturbs, cancer, diabetes, renal, hepatic, and cardiovascular diseases.
Venous blood samples were drawn from all women, in EDTA samples tubes (5 mL). All samples were processed less than 2 hours after venipuncture, using a blood cell counter ABX Pentra XL 80® (Horiba Medical, Kyoto, Japan) for acquiring PC, MPV, PDW, and PCT.
Statistical analysis was performed using SPSS version 13.0 and MedCalc Statistical Package (version 11.3.1.0). Data presented normal distribution and were analyzed by analysis of variance (ANOVA) for comparing the three groups and by t-test for comparing two groups. In order to investigate correlation among indices and between them and both clinical and demographic variables it was used Pearson's correlation coefficient (data normal distribution) and Spearman's correlation coefficient (data not normal distribution). We also evaluated the diagnostic value of PC, MPV, PDW, and PCT separately. Based on calculations of sensitivity and specificity for different cut-off, it was determined the area under the receiver operating characteristic (ROC) curve (AUC) for each variable above. As the biggest AUC is associated to the best parameter for PE diagnosis, a comparison was made among these areas to determine whether there was a significant difference between them. A P < 0.05 was considered significant.
Results
Significant differences were observed among the three groups by ANOVA for PC (P < 0.001), PCT (P = 0.009), PDW (P < 0.001), and MPV (P < 0.001). Means and standard deviations of the variables evaluated are shown on .
Table 1. Values of platelets count, PCT, PDW, and MPV in severe preeclamptic, normotensive pregnant women, and in non-pregnant healthy women. The confidence interval values are in parentheses
Results show a reduction in PC and PCT comparing women with sPE and normotensive pregnant (P = 0.031 and 0.035, respectively) and between sPE and non-pregnant women (P < 0.001 and P = 0.004, respectively). No difference was found comparing normortensive pregnant and non-pregnant women for these two parameters. For PDW an increase was found comparing sPE and normotensive pregnant (P = 0.028) and non-pregnant women (P < 0.001); comparing normotensive pregnant and non-pregnant women (P = 0.001). For MPV an increase was found comparing sPE and normotensive and non-pregnancy women (P = 0.05, P < 0.001, respectively) and normotensive pregnancy and non-pregnancy (P = 0.003). No difference was found comparing sPE and normotensive pregnancy women for this parameter (P = 0.079).
Considering all groups, PC and MPV showed to be inverse and significantly correlated between them (r = −0.647; P < 0,001), as well as PC and PDW (r = −0.657; P < 0.001), while a positive correlation was observed between MPV and PDW (r = 0.916; P < 0.001).
Based on calculations of sensitivity and specificity for the different variable cut-off, AUC was determined. The diagnostic value for the variables PC, MPV, PDW, and PCT individually was also evaluated ().
Table 2. Sensibility, specificity, cut-off, AUC of platelet parameters and their classification
The best parameter is that presenting the biggest AUC.Citation26 A comparison of these areas was made to determine whether there was a significant difference among them. P < 0.05 was considered significant. A comparison among these areas showed no significant difference ( and ) and the AUC for PC, MPV, and PDW were considered as regular, while that one for PCT was bad.
Figure 1. Comparison of ROC curve for platelet parameters. PCT, plateletcrit; PDW, platelet distribution width; MPV, mean platelet volume.
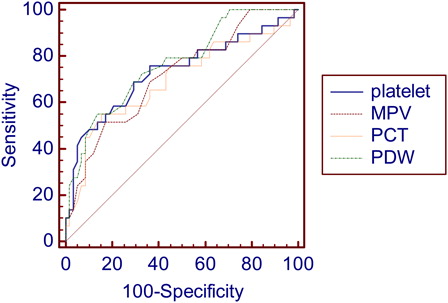
Table 3. Comparison of ROC curve for platelet parameters expressed by difference between areas under curve, CI and significance level
Discussion
The etiology of PE is unknown and no laboratory marker that presents a favorable cost-effectiveness has been proposed in recent decades. Its diagnosis is made primarily based on blood pressure measurement and determination of the proteinuria and clinical data.Citation27
Our results suggest an accelerating replacement of the platelets by the bone marrow in severe preeclamptic women resulting in platelet anisocytosis, based on PDW and MPV values. Larger platelets were also observed during pregnancy, which was revealed by an increased MPV in groups 1 and 2.
In accordance to our findings, several investigators demonstrated lower circulating platelets count and higher MPV values in preeclamptic women.Citation18,Citation21,Citation28–Citation33
Janes and GoodallCitation34 observed thrombocytopenia and macrothrombocytosis in preeclamptic women. GilesCitation15 examined the association between PC and MPV in 5000 pregnant women in early pregnancy and observed that normal PC and increased MPV were associated to PE occurrence. Dundar et al.Citation23 also observed an increase MPV in PE, although few cases have been accompanied by thrombocytopenia. Bath and ButterworthCitation19 confirmed that women with high MPV in the second trimester of pregnancy had an increased risk of developing PE. Similar results were obtained by Walker et al.Citation16 and Järemo et al.,Citation21 which suggest that MPV is a candidate for prediction of sPE. Additionally, MPV increase occurs before the clinical onset of disease symptoms, since it reflects increased platelet turnover.Citation16,Citation21
Although MPV is increased in severe preeclamptic women comparing to normotensive pregnant women, PCT was significantly decreased, which reflects PC reduction rather than MPV increase.
There is some evidence that increase of MPV may precede PE symptoms by approximately 4.6 weeks and the odds ratio of increased platelet size for predicting PE was 2.83.Citation23 Moreover, a longitudinal study reveals that MPV gradually increases in PE comparing to normotensive pregnancy, suggesting that a periodic MPV monitoring may aid the clinicians in predicting PE occurrence.Citation23 However, some researchers found no difference in both PC and MPV values between PE and normotensive pregnant women.Citation35,Citation36 This discrepancy between these studies can be probably explained by the method of MPV measurement. It is known that MPV increases when exposed to EDTA in a time-dependent manner.Citation19 In addition, different hematological counter can yield different MPV results up to 40%.Citation33 Besides, differences in gestational age and PE diagnosis criteria could justify the MPV conflicting results. In our study, the time between sample collection and MPV measurements was less than 2 hours, which may discard in vitro interference in results.
According to our data, severe preeclamptic women had significantly higher MPV, but only a minority developed thrombocytopenia. This might be explained by a quick platelet turnover, which is the result of continuous platelet consumption in the peripheral blood followed by continuous production in the bone marrow.Citation37 Järemo et al.Citation21 demonstrated that platelet volume increase was related to the presence of hypertension rather than platelet alterations and attributed this finding to disturbed platelet density distributions. High-density platelets have an elevated volume and it is reasonable to assume that platelet activation and subsequent granules release disturbs platelet density distributions.Citation21
Our results are consistent to the previous ones that establish relation of PC and MPV. Although PC has been found still within normal range, our results demonstrated a decreased PC with elevated MPV. It can be speculated that unknown pathophysiologic events other than platelet consumption might explain such finding. In addition, in one study it was suggested that changes in platelet volumes may be more sensitive than PC as a measure of altered function in normotensive pregnancy,Citation25 and it was also reported that MPV correlated with the severity of PE.Citation30,Citation31,Citation38
Previous retrospective and longitudinal studies have investigated the ability of MPV in the prediction of PE. A longitudinal study suggested that pregnant women with high MPV in the second trimester in a single random blood sample are at risk of PE.Citation18 Another longitudinal study proposed that a cut-off value of ≥10 fL for MPV may be added as a part of the significant parameters that are able to predict unfavorable neonatal outcome in women affected by altered uterine artery Doppler velocimetry.Citation39 A relatively large cross-sectional study provided evidence that reduced PC and elevated MPV have 90% sensitivity and 83.3% specificity in predicting PE.Citation20
On the other hand, Walker et al.Citation16 in a study conducted with 300 pregnant women between 28 and 30 weeks of pregnancy, showed no changes in MPV during pregnancy in women who developed PE mild or moderate, while in the severe PE group, there was a significant increase in the MPV. Similar results were obtained by Ahmed et al.Citation18 These investigators reported that a value of MPV ≥ 11.0 at 28 weeks of gestation is associated with high incidence of PE.
To better analyzing our data, we used the ROC curve, which constitutes an authoritative method for the analysis of accuracy of biomarkers. In addition, the deflection point in the ROC curve provides the best cut-off point for categorization of variables in logistic models. The discriminating power of the test (or the overall accuracy) can be measured by calculating the area under the ROC curve. Medronho et al.Citation40 categorized AUC according to the quality of diagnosis and this score (expressed in words ‘Excellent, Good, Regular and Bad’) is used to assess the quality of the studied parameters. In our study, this analysis showed that the platelet parameters had regular diagnostic significance, except for PCT, considered as ‘Bad’ for this purpose. A comparative analysis among the areas under ROC curves showed no statistically significant difference.
Currently, one the most important goals in obstetrics is the identification of pregnant with an increased PE risk. Besides, the definition of sensitive and specific biomarkers would allow not only the detection of patients at risk of PE, but it would also allow a close surveillance, a precise PE diagnosis and a timely pregnancy intervention. In this context, PC emerges as a good candidate, since it is a simple and habitually done method, with lower cost and greater accessibility in the clinical laboratory. It is known that severe PE is associated with platelet activation and significant hemostatic abnormalities, which result in serious complications for the mother and newborn. Despite the mild value of the platelet parameters to severe PE diagnostic/prognostic, our preliminary study is the first one investigating these parameters together. Certainly, the small number of participants, as well as the enrollment of women at a late gestational age, limits definitive conclusions. Further studies evaluating sequential PC and platelet indices throughout pregnancy are necessary to clarify the role of platelet parameters in PE development and severity.
Acknowledgement
The authors thank FAPEMIG, CNPq, PROPq/UFMG/Brazil, and Gynecology and Nursing staffs of Maternidade do Hospital Público Regional de Betim/Brazil. LMSD and MdGC are grateful to CNPq Research Fellowship (PQ).
References
- National Heart, Lung, and Blood Institute National High Blood Pressure Education Program. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:1–22
- Sibai BM, Caritis SN, Thom E, Klebanoff M, Mcnellis D, Rocco L, et al. National Institute of Child Health And Human Development Network of Maternal-Fetal Medicine Units Prevention of pre-eclampsia with low-dose aspirin in healthy, nulliparous pregnant women. N Engl J Med. 1993;329:1213–8.
- Gilabert J, Estellés A, España F, Grancha S, Aznar J. Modificaciones de la hemostasia en obstetricia. Rev Iber Tromb Hemost. 1995;8:102–12.
- Estelles A, Gilabert J, Grancha S, Yamamoto K, Thinnes T, Espana F, et al. Abnormal expression of type 1 plasminogen activator inhibitor and tissue factor in severe pre-eclampsia. Thromb Haemost. 1998;79:500–8.
- Trogstad L, Magnus P, Stoltenberg C. Pre-eclampsia: risk factors and causal models. Best Pract Res Clin Obstet Gynaecol. 2011;30:1–14.
- Inglis TCM, Stuart J, George AJ, Davies AJ. Hemostatic and rheological changes in normal pregnancy and pre-eclampsia. Br J Haematol. 1982;50:461–5.
- Konijnenberg A, Stokkers EW, Post JAMVD, Schaap MCL, Boer K, Bleker OP, et al. Extensive platelet activation in preeclampsia compared with normal pregnancy: enhanced expression of cell adhesion molecules. Am J Obstet Gynecol. 1997;176:461–9.
- Janes SL, Kyle PM, Redman C, Goodall AH. Flow cytometric detection of activated platelets in pregnancy women prior the development of preeclampsia. Thromb Haemost. 1995;74:1052–63.
- Fitzgerald DJ, Stephen SE, Mulloy K, Fitzgerald GA. Decreased prostacyclin biosynthesis preceding the clinical manifestation of pregnancy-induced hypertension. Circulation 1987;75(5):956–63.
- Pinto A, Sorrentino R, Sorrentino P, Guerritore T, Miranda L, Biondi A, et al. Endothelial-derived relaxing factor relesead by endothelial cells of umbilical vessels and its impairment in pregnancy-induced hypertension. Am J Obstet Gynecol. 1991;164:507–13.
- Seligman SP, Buyon JP, Clancy RM, Young BK, Abramson SB. The role of nitric oxide in the pathogenesis of preeclampsia. Am J Obstet Gynecol. 1994;171:944–8.
- Lind SE, Marks PW, Ewenstein BM. Haemostasis. In: , Handin RI, Lux SE, Stossel TP (eds.). Blood: principles, practice of hematology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2003. p. 959–1080.
- Uysal P, Tuncel T, Olmez D, Babayigit A, Karaman O, Uzuner N. The role of mean platelet volume predicting acute exacerbations of cystic fibrosis in children. Ann Thorac Med. 2011;6(4):227–30.
- Bessman JD, Williams LJ, Gilmer PR. The inverse relation of platelet size and count in normal subjects, and an artifact of other particles. Am J Clin Pathol. 1981;76(3):289–93.
- Giles C. The platelet count and mean platelet volume. Br J Haematol. 1981;48:31–7.
- Walker JJ, Cameron AD, Bjornsson S, Singer CR, Fraser C. Can platelet volume predict progressive hypertensive disease in pregnancy? Am J Obstet Gynecol. 1989;161(3):676–9.
- Bessman JD, Williams LJ, Gilmer PR. Platelet size in health and hematologic disease. Am J Clin Pathol. 1982;78(2):150–3.
- Ahmed Y, van Iddekinge B, Paul C, Sullivan HF, Elder MG. Retrospective analyses of platelet numbers and volumes in normal pregnancy and in preeclampsia. Br J Obstet Gynaecol. 1993;100(3):216–20.
- Bath PM, Butterworth RJ. Platelet size: measurement, physiology and vascular disease. Blood Coagul Fibrinolysis 1996;7(2):157–61.
- Howarth S, Marshall LR, Barr AL, Evans S, Pontre M, Ryan N. Platelet indices during normal pregnancy and pre-eclampsia. Br J Biomed Sci. 1999;56(1):20–2.
- Järemo P, Lindahl TL, Lennmarken C, Forsgren H. The use of platelet density and volume measurements to estimate the severity of preeclampsia. Eur J Clin Invest. 2000;30(12):1113–8.
- Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:1–30.
- Dundar O, Yoruk P, Tutuncu L, Erikci AA, Muhcu M, Ergur AR, et al. Longitudinal study of platelet size changes in gestation and predictive power of elevated MPV in development of pre-eclampsia. Prenat Diagn. 2008;28(11):1052–6.
- Subbs TM, Lazarchick J, van Dorsten JP, Cox J, Loadholt CB. Evidence of accelerated platelet production and consumption in nonthrombocytopenic preeclampsia. Am J Obstet Gynecol. 1986;155(2):263–5.
- Tygart SG, Mcroyan DK, Spinnato JA, Mcroyan CJ, Kitay DZ. Longitudinal study of platelet indices during normal pregnancy. Am J Obstet Gynecol. 1986;154(4):883–7.
- Martinez EZ, Louzada-Neto F, Pereira BB. A curva ROC para testes diagnósticos. Cadernos Saúde Coletiva. 2003;11(1):7–31.
- Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Ann Rev Pathol. 2010;5:173–92.
- Trudinger BJ. Platelets and intrauterine growth retardation in preeclampsia. Br J Obstet Gynaecol. 1976;83(4):284–6.
- Redman CWG, Bonnar J, Beilin L. Early platelet consumption in preeclampsia. Br Med J. 1978;1:467–9.
- Giles C, Inglis TC. Thrombocytopenia and macrothrombocytosis in gestational hypertension. Br J Obstet Gynaecol. 1981;88(11):1115–9.
- Glanville T, Walker JJ. Pre-eclampsia: etiology and clinical practice. Cambridge University press, United Kingdom; 1989. p. 357–68
- Redman CW. Platelets and the beginnings of preeclampsia. N Engl J Med. 1990;323(7):478–80.
- Hutt R, Ogunniyi SO, Sullivan MH, Elder MG. Increased platelet volume and aggregation precede the onset of preeclampsia. Obstet Gynecol. 1994;83(1):146–9.
- Janes SL, Goodall AH. Flow cytometric detection of circulating activated platelets and platelet hyper-responsiveness in pre-eclampsia and pregnancy. Clin Sci. 1994;86(6):731–9.
- Makuyana D, Mahomed K, Shukusho FD, Majoko F. Liver and kidney function tests in normal and pre-eclamptic gestation – a comparison with non-gestational reference values. Cent Af J Med. 2002;48( 5–6):55–9.
- Ceyhan T, Beyan C, Başer İ, Kaptan K, Güngör S, Ifran A. The effect of pre-eclampsia on complete blood count, platelet count and mean platelet volume. Ann Hematol. 2006;85(5):320–2.
- Piazze J, Gioia S, Maranghi L, Anceschi M. Mean platelet and red blood cell volume measurements to estimate the severity of hypertension in pregnancy. J Perinat Med. 2006;34(3):246–7.
- Missfelder-Lobos H, Teran E, Lees C, Albaiges G, Nicolaides KH. Platelet changes and subsequent development of pre-eclampsia and fetal growth restriction in women with abnormal uterine artery Doppler screening. Ultrasound Obstet Gynecol. 2002;19(5):443–8.
- Gioia S, Piazze J, Anceschi MM, Cerekja A, Alberini A, Giancotti A, et al. Mean platelet volume: association with adverse neonatal outcome. Platelets 2007;18(4):284–8.
- Medronho RA, Boch KV, Luiz RR, Werneck GL. Epidemiologia. 2nd ed. São Paulo: Atheneu; 2009. 685p.