Abstract
Objectives
To explore the combination therapy of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO, As2O3) on acute promyelocytic leukemia (APL).
Methods
A meta-analysis of six studies was performed. Among 415 included cases, 165 cases were in the ATRA + ATO group, 129 cases in the ATRA-alone group, and 121 cases in the ATO-alone group. The complete remission (CR) rate and incidences of three groups were compared, respectively, between the therapies of ATRA + ATO with ATRA-alone, ATRA + ATO with ATO-alone, and ATRA with ATO.
Results
The assessment results showed that ATRA + ATO therapy significantly improved the CR rate and decreased the incidences of cutaneous reaction compared with ATRA-alone (P < 0.05). However, incidence of liver injury was higher in the ATRA + ATO and ATO-alone groups than that in ATRA-alone group (P < 0.05). Difference in the complications between ATRA + ATO therapy and ATO-alone was not significant (P > 0.05).
Conclusions
In conclusion, we suggest low-dose ATRA and ATO combination therapy may be more effective for the treatment of APL.
Introduction
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia (AML), which represents 10–15% of all types of AML, but up to 32% of that in some regions of China,Citation1 the onset mainly in the age range of 20–50.Citation2 APL has ever been considered the most fatal type of AML because of its severe bleeding tendency and the relevant high mortality.Citation3 Cytogenetically, APL is characterized by a reciprocal chromosomal translocation, t(15; 17), that fuses the promyelocytic leukemia (PML) gene with the retinoic acid receptor α (RARα) gene and generates a chimeric PML-RARα.Citation4 Chimeric protein stops the maturation of myeloid cells at the promyelocytic stage, causing them to fail to differentiate.Citation5
Recent studies suggest that strategies targeting PML-RARα fusion gene may be effective for treatment of APL. For example, treatment with all-trans retinoic acid (ATRA), a natural ligand for the RARs, leads to more than 90% of patients with newly diagnosed APL to achieve complete remission (CR).Citation6 Arsenic trioxide (ATO), an organic arsenic compound, has also shown favorable effects on the treatment of patients with initially diagnosed or relapsed APL in clinical trials, ∼85% CR.Citation7,Citation8 They exert their therapeutic effects on APL by combining the following two mechanisms together: one is promoting the degradation of PML-RARα and terminating transcription inhibition of target genes through various pathways; the other is changing the conformation of PML-RARα fusion protein and leading to an open structure of chromatin.Citation9,Citation10 These two mechanisms finally induce differentiation and apoptosis of APL cells.Citation11,Citation12
Given the similar therapeutic target and mechanism for APL, the synergism between ATRA and ATO may be present. Recently, multiple clinical studies have reported the effects of combination therapy with ATO and ATRA by comparison with ATO- or ATRA-alone therapy. Nevertheless, results of these studies are remarkably inconsistent, which may be related to small sample size.Citation13,Citation14 Therefore, we carried out a meta-analysis to quantitatively combine previous results and comprehensively evaluate the efficacy and safety of ATO–ATRA combination therapy in the treatment of APL.
Materials and methods
Search strategy
PubMed, MEDLINE, the Cochrane Central Register of Controlled Trials, EMBASE, China National Knowledge Infrastructure, Chinese Biology Medicine disc, Wanfang and Weipu Databases were searched for all studies that investigated the therapy of APL with ATO, ATRA, or ATO + ATRA up to December 2012. The following search terms were used: APL AND ATRA AND ATO AND (randomized controlled trial (RCT) OR controlled clinical trial (CCT)). The search was not restricted to specific languages or years of publication.
Literature inclusion and exclusion criteria
The following criteria were used to include published studies: (1) published once; (2) RCTs or CCTs; (3) having definite published year or research year; (4) definite sample size; (5) clear diagnostic criteria for APL; (6) APL adult patients; (7) the evaluation index contained the CR rate (defined as complete disappearance of all such manifestations of disease clinical evidence of APL be absent, untransfused Hb be >100 g/l, neutrophils be >1.5 × 109/l, platelets be >100 × 109/l, and bone marrow morphology reveal normocellularity, with <5% promyelocytes and absence of Auer rod-containing leukemic cells), morbidity of gastrointestinal complications, cutaneous reaction, encephalalgia, and liver damage; (8) scientific data collection methods; (9) correct data analysis method. Studies were excluded if one of the following existed: (1) not providing cases or control source, non-therapeutic clinical studies, animal experiments, non-original literatures, and without definite grouping; (2) unclear diagnostic criteria; (3) having a history of or accompanied with another malignant tumor; (5) unscientific data collection methods; (6) having no control; (7) incorrect data analysis method or method not provided; (8) review; (9) duplicate articles; (10) retrospective articles.
Data extraction
Two evaluators independently searched and screened literatures. The following data were extracted: (1) basic characteristics of the trails; (2) criteria for included cases, intervention approaches, characteristics of patients, and results measurement index; (3) six indicators for risk of bias assessment, including generation of random allocation, allocation concealment, application of blinding, data integrity, with or without reported results selectively, and other sources of bias.
Statistical analysis
The results of eligible trials were pooled using RevMan5.0 software provided by the Cochrane Collaboration. For continuous data, the mean difference was calculated. Dichotomous data were pooled to obtain a relative risk (RR). Both of their confidence intervals (CIs) were set at 95%. The χCitation2-based Q statistic and ICitation2 were used to assess the statistical heterogeneity among studies. If the result of the Q test was PQ < 0.05 or ICitation2 ≥ 50%, indicating the presence of heterogeneity, a random-effects model was used to estimate the RR; otherwise, when the result of the Q test was PQ ≥ 0.05 and ICitation2 < 50%, indicating the absence of heterogeneity, the fixed-effects model was used.
Afterwards, sensitivity analysis was scheduled to conduct based on the biased estimates of treatment effect and original disease status of the subjects; then the funnel plot analysis was performed to analyze possible publication bias. A P value not more than 0.05 was considered to be statistically significant.
Results
Results of references search
A total of 188 references were initially obtained through searching the databases. After reading title or abstract, 165 articles were excluded. Review of the full text of the 23 articles further excluded 17 references. As a result, a total of six RCTsCitation14–Citation19 relevant studies were included, containing 415 cases. Details of the documents are shown in .
Table 1. Details of included studies
General features of each included group
The average age of the subjects was more than 18 years. The number of the objects in the ATO + ATRA group, the ATRA-alone group, and the ATO-alone group were 165, 129, and 121, respectively. ATO was injected intravenously and ATRA was taken orally. General features including gender, number, and dosages of the drugs in each group are shown in .
Table 2. Characteristics of included studies comparing ATRA + ATO with ATRA-alone and ATO-alone, ATRA with ATO in the treatment of APL
Comparison between ATO + ATRA and ATRA-alone
Four studies analyzed the CR rate.Citation14,Citation16,Citation18,Citation19 The results indicated that ATRA + ATO treatment could induce a significantly increased CR rate compared with ATRA-alone (RR, 1.12; 95% CI, 1.02–1.24; P = 0.02) (A).
Figure 1. Forest plots of the CR rate (A), gastrointestinal complications (B) cutaneous reaction (C), encephalalgia (D), and liver injury (E) in ATRA + ATO therapy and ATRA therapy for APL. RR, relative risk; CI, confidence interval.
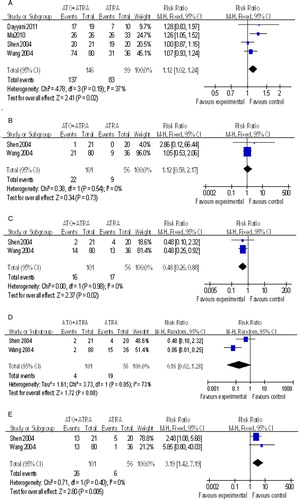
Two papersCitation14,Citation19 reported useful data about the morbidity of gastrointestinal complications. No significant difference in the morbidity of gastrointestinal complications was observed between the ATRA + ATO group and the ATRA group (RR, 1.12; 95% CI, 0.58–2.17; P = 0.73) (B).
Comparison of cutaneous reaction incidences between the two groups were reported in two studies,Citation14,Citation19 showing that incidence of cutaneous reaction was significantly reduced in the ATRA + ATO group (RR, 0.48; 95% CI, 0.26–0.88; P = 0.02) (C).
As shown in D, there was no significant difference in the encephalalgia morbidity between the two groups, which was demonstrated in the two papersCitation14,Citation19 (RR, 0.16; 95% CI, 0.02–1.28; P = 0.08).
Two papersCitation14,Citation19 stated usable data associated with liver injury. Meta-analysis results showed a significantly increased liver injury in the ATRO + ATO group (RR, 3.19; 95% CI, 1.42–7.19; P = 0.005) (E).
Comparison between ATO + ATRA and ATO-alone
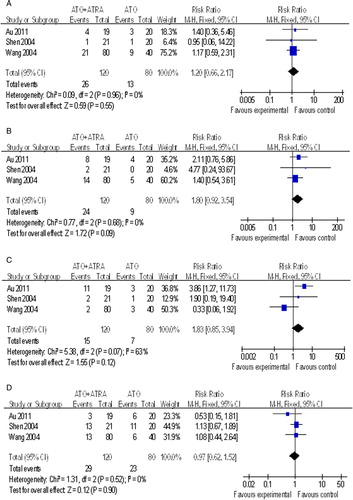
In previous studies, three papersCitation14,Citation15,Citation18 compared the incidences of gastrointestinal complications (RR, 1.20; 95% CI, 0.66–2.17), cutaneous reaction (RR, 1.80; 95% CI, 0.92–3.54), encephalalgia (RR, 1.83; 95% CI, 0.85–3.94), and liver injury (RR, 0.97; 95% CI, 0.62–1.52) between ATO + ATRA and ATO-alone. Differences were not statistically significant (P > 0.05, ).
Comparison between ATO and ATRA
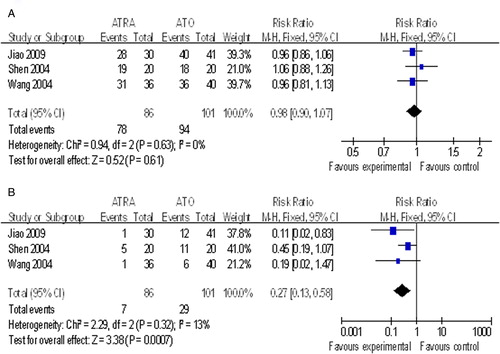
There was no significant difference in the CR rates between ATO and ATRA treatment for APL (RR, 0.98; 95% CI, 0.90–1.07; P = 0.61).Citation14,Citation17,Citation19 However, incidence of liver injury was lower after ATRA-alone therapy compared with ATO-alone therapy (RR, 0.27; 95% CI, 0.13–0.58, P = 0.0007, ).
Biased estimates and sensitivity analysis
The assessment of publication bias could not be conducted due to the number restriction of included trials.
Discussion
In this study, we carried out a meta-analysis of six RCT studies to assess the efficacy and safety of ATO/ATRA combination therapy. The results showed that ATRA + ATO treatment relatively improved the CR rate and decreased the incidences of cutaneous reaction compared with ATRA-alone, indicating ATRA seems to exert a synergistic effect when combined with ATO.Citation20 However, incidence of liver injury was higher in the ATRA + ATO group and the ATO-alone group than that in ATRA-alone. Previous studies have suggested that liver dysfunction is the most important toxic effect of ATO in APL manifested by increases in alanine aminotransferase, aspertate aminotransferase, and γ-glutamyl transferase.Citation21,Citation22 Furthermore, ATO may increase liver mitochondrial reactive oxygen species formation, lipid peroxidation and mitochondrial membrane potential collapse, cytochrome c release and mitochondrial swelling, ultimately resulting in liver cell death.Citation21 Therefore, a combination of low-dose ATO and sequential ATRA may be more safer.Citation23
However, the interpretation of this finding should be cautious, since the included case quality and number are limited. The main impact factors were as followed: (1) potential publication bias: the publication bias could not be analyzed by funnel plot analysis. The possibility of potential publication bias may not be eliminated, since it was much easier to be released for positive results of clinical trials compared with negative ones; (2) the total number of cases was only 415. Therefore, the samples selected may display a poor typicality, which may influence the popularization and application of estimation results for treatment effect. The comprehensive evaluation on safety of treatments may be restricted. Combination therapy with ATRA–ATO for APL required further improvement on experimental design based on risk of bias assessment for this system; (3) subgroup analysis stratified by age and gender was not performed; (4) this study only focused on the adult newly diagnosed with APL, but not children or relapsed APL. The following aspects need further emphasis and more attention in the future study: (1) the follow-up situation are recommended to be the main outcome indicators by extension of the follow-up time to ensure the quality of clinical trials for leukemia; (2) popularization of registration system of clinical trials would promote the publication of the negative results; (3) clinical trial reports should refer to the international CONSORT (Consolidated Standards of Reporting Trials) statement.
In conclusion, the results of this study suggest that both ATRA and ATO are associated with favorable therapeutic effects for APL. ATRA + ATO combination therapy is more effective than ATRA-alone or ATO-alone on the treatment of APL, although at the cost of high incidence of liver injury. However, due to the above limitations, more clinical trial studies with reliable designs are needed to further confirm the clinical efficacy and long-term safety of ATO and ATRA combination therapy for APL.
Acknowledgments
This article was supported by National Natural Science Foundation of China (NSFC) (30973495).
References
- Lu DP. Therapeutics of leukemia. Beijing: Science Press; 1922, p. 152.
- Ribeiro RC, Rego E. Management of APL in developing countries: epidemiology, challenges and opportunities for international collaboration. ASH Educ Program Book 2006;2006:162–8.
- Chang H, Kuo MC, Shih LY, Dunn P, Wang PN, Wu JH, et al. Clinical bleeding events and laboratory coagulation profiles in acute promyelocytic leukemia. Eur J Haematol. 2012;88:321–8.
- Kakizuka A, Miller W Jr, Umesono K, Warrell R Jr, Frankel S, Murty V, et al. Chromosomal translocation t (15; 17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor, PML. Cell 1991;66:663–74.
- Wang Z-Y, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 2008;111:2505–15.
- Tallman MS. Treatment of relapsed or refractory acute promyelocytic leukemia. Best Pract Res Clin Haematol. 2007;20:57–65.
- Douer D, Tallman MS. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J Clin Oncol. 2005;23:2396–410.
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–60.
- Zhang X-W, Yan X-J, Zhou Z-R, Yang F-F, Wu Z-Y, Sun H-B, et al. Arsenic trioxide controls the fate of the PML-RARα oncoprotein by directly binding PML. Science 2010;328:240–3.
- Tomita A, Kiyoi H, Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) in acute promyelocytic leukemia. Int J Hematol. 2013;97:717–25.
- Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RARα protein in acute promyelocytic leukemia cells. J Natl Canc Inst. 1998;90:124–33.
- Zheng P-Z, Wang K-K, Zhang Q-Y, Huang Q-H, Du Y-Z, Zhang Q-H, et al. Systems analysis of transcriptome and proteome in retinoic acid/arsenic trioxide-induced cell differentiation/apoptosis of promyelocytic leukemia. Proc Natl Acad Sci USA 2005;102:7653–8.
- Raffoux E, Rousselot P, Poupon J, Daniel M-T, Cassinat B, Delarue R, et al. Combined treatment with arsenic trioxide and all-trans-retinoic acid in patients with relapsed acute promyelocytic leukemia. J Clin Oncol. 2003;21:2326–34.
- Shen Z-X, Shi Z-Z, Fang J, Gu B-W, Li J-M, Zhu Y-M, et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA 2004;101:5328–35.
- Au W-Y, Kumana CR, Lee HK, Lin S-Y, Liu H, Yeung DY, et al. Oral arsenic trioxide-based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10-year follow-up study. Blood 2011;118:6535–43.
- Dayyani F, Kantarjian H, O'Brien S, Pierce S, Jones D, Faderl S, et al. Outcome of therapy – related acute promyelocytic leukemia with or without arsenic trioxide as a component of frontline therapy. Cancer 2011;117:110–5.
- Jiao L, Wang S, Zhuang J, Zhao Y, Zhou D, Xu Y, et al. Comparison of efficacy and adverse effects between arsenic trioxide and all-trans retinoic acid in patients with acute promyelocytic leukemia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2009;31:555–8.
- Ma X, Ren H, Cen X, Qiu Z, Wang W, Ou J, et al. Efficacy analysis of sequential treatment with chemotherapy, ATRA and As2O3 for acute promyelocytic leukemia. Zhonghua Xue Ye Xue Za Zhi 2010;31:328.
- Wang G, Li W, Cui J, Gao S, Yao C, Jiang Z, et al. An efficient therapeutic approach to patients with acute promyelocytic leukemia using a combination of arsenic trioxide with low-dose all-trans retinoic acid. Hematol Oncol. 2004;22:63–71.
- Wang H, Chen X-Y, Wang B-S, Rong Z-X, Qi H, Chen H-Z. The efficacy and safety of arsenic trioxide with or without all-trans retinoic acid for the treatment of acute promyelocytic leukemia: a meta-analysis. Leuk Res. 2011;35:1170–7.
- Wang H, Xi S, Liu Z, Yang Y, Zheng Q, Wang F, et al. Arsenic methylation metabolism and liver injury of acute promyelocytic leukemia patients undergoing arsenic trioxide treatment. Environ Toxicol. 2013;28:267–75.
- Hao L, Zhao J, Wang X, Wang H, Wang H, Xu G. Hepatotoxicity from arsenic trioxide for pediatric acute promyelocytic leukemia. J Pediatr Hematol Oncol. 2013;35:e67–70.
- Lin J, Zhu H, Li S, Fan H, Lu X. Complete remission of acute promyelocytic leukemia in a very elderly patient after treatment with low dose arsenic trioxide and sequential retinoic acid: a case report. Ann Hematol. 2014;93(2):335–6.