Abstract
Objectives
Thrombocytopenia is common in HIV-infected individuals and often requires a diagnostic bone marrow examination. Interpretation may, however, be limited due to the multifactorial nature of HIV-associated thrombocytopenia and the difficulty in assessing megakaryocyte function morphologically. The immature platelet fraction (IPF) is a parameter which reportedly reflects megakaryocyte activity, with an IPF >7.7% suggesting increased platelet production. The aim of this study was to correlate the IPF with the bone marrow findings as well as other clinical variables of interest in South African patients with HIV-associated thrombocytopenia.
Methods
Seventy-eight HIV-positive patients with thrombocytopenia were enrolled from the Charlotte Maxeke Johannesburg Academic Hospital. The IPF levels were measured using a Sysmex XE-5000 haematology analyzer and were correlated with bone marrow and other findings.
Results
The median IPF was 7.6%, ranging from 1.3 to 44%. It was raised in 78% of patients with immune thrombocytopenia (ITP) (median = 16.3%) and low in 79% of patients with hypocellular marrow (median = 6.5%). Surprisingly, it was highly variable among patients with malignant marrow infiltration and mycobacterial infection of the bone marrow (BMTB) (median = 8.4 and 7.1%, respectively). Mulitivariate linear regression analysis confirmed a significant independent inverse relationship between the IPF and hypocellular marrow (P < 0.0001), a marginally significant positive association with ITP (P = 0.059), and the absence of any relationship with malignant infiltration or BMTB. The IPF had a significant inverse association with the platelet count (P = 0.0006), but was unrelated to the CD4 count and exposure to anti-retroviral therapy. Unexpectedly, it showed a significant positive association with the HIV viral load (P = 0.005). We speculate this to reflect increased megakaryocyte activity in compensation for accelerated platelet clearance due to HIV-driven platelet activation.
Conclusion
This study investigates the role of the IPF in HIV-associated thrombocytopenia, and emphasizes the limitations of morphological analysis in determining megakaryocyte function.
Introduction
Thrombocytopenia is common in the setting of human immunodeficiency virus (HIV) infection, occurring with a prevalence of 3–30%.Citation1,Citation2 It is more prevalent among those with Acquired Immunodeficiency Syndrome (AIDS)Citation1,Citation2 and may be alleviated by antiretroviral therapy (ART).Citation3 The causes for thrombocytopenia are numerous, and often multifactorial. Platelets have an attenuated life-span in HIV-infected individuals,Citation4,Citation5 while their production is compromised due to both HIV-associated stromal cell dysfunctionCitation6 and direct HIV infection of megakaryocytes.Citation7,Citation8 Compounding these pressures are drug-effects (which include megakaryocytic suppression, drug-associated secondary immune thrombocytopenia (ITP), and ineffective haemopoiesisCitation9,Citation10), as well as contributions from opportunistic infections and malignancies. The infective causes are particularly prevalent, and may cause thrombocytopenia by several mechanisms. Mycobacteria and fungal organisms can directly infect the bone marrow causing granulomatous inflammation, while several viral aetiopathogens can either directly infect megakaryocytes,Citation11 cause secondary aplasia,Citation12 or ITP.Citation11,Citation13,Citation14 The mechanistic causes for the latter include cross-reactivity between antibodies to viral proteins with platelet antigens (so-called ‘molecular mimicry’), or adsorption of viral antigens onto the surface of platelets (resulting in bystander antibody-mediated cellular cytotoxicity or immune clearance of platelets).Citation11,Citation14 Bacterial sepsis also causes thrombocytopenia through a number of mechanisms, including a degree of myelosuppression, accelerated platelet consumption due to disseminated intravascular coagulation (DIC), as well as platelet sequestration by activated endothelial cells within the vasculature.Citation15
Because of the many possible causes of thrombocytopenia in this setting, bone marrow examination is usually indicated. This may show hyper- or hypocellularity, with or without evidence of bone marrow infiltration. Bare megakaryocyte nuclei are often conspicuous (signifying increased megakaryocyte death), and the haemopoietic precursors morphologically dysplastic.Citation16 As a result, the interpretation of the morphological findings is sometimes difficult, particularly in those patients with apparently adequate megakaryocyte numbers, a degree of haemopoietic disorder and focal marrow infiltration. The immature platelet fraction (IPF) is a parameter which is potentially useful in this regard as a reflection of megakaryocyte function. It quantifies the number of RNA-rich platelets (so-called ‘reticulated platelets’), and is reported as a proportion of the total optical platelet count (IPF%) by Sysmex XE- and XN-series haematology analyzers (Sysmex, Kobe, Japan). Reticulated platelets are newly formed,Citation17,Citation18 and a relationship between their numbers in the blood (as detected by a number of methodologies, including the IPF) and megakaryocytic activity is well established in clinical contexts in which the mechanism causing thrombocytopenia is readily apparent. For example, several authors found the number of reticulated platelets to be significantly higher in patients with ITP, and lower in patients with marrow aplasia.Citation17,Citation19–Citation23
Reticulated platelet numbers have been assessed in two previous studies in the setting of HIV-associated thrombocytopenia, both of which showed the proportion of platelets which were RNA-rich to be elevated in this clinical context. The first examined the platelets of 14 thrombocytopenic HIV-infected individuals using an alternative method to the IPF,Citation24 and the second assessed IPF levels in 50 HIV-infected patients with thrombocytopenia.Citation25 The second group also found the IPF% to be elevated in 34 HIV positive patients with normal platelet counts. This led them to conclude that platelet turnover is increased in HIV-positive individuals, both those with normal platelet counts and those with thrombocytopenia, possibly as a by-product of increased platelet activation and a consequent acceleration of platelet clearance. This hypothesis is supported by the abnormal platelet activation in HIV-positive patients demonstrated by other authors, which is related to the HIV viral load (HIVVL) and the degree of immune activation.Citation26,Citation27 The second group also showed that the IPF% was higher among patients with ITP and active infection, was inversely related to the CD4 count and the platelet count, and was not related to the HIVVL or the use of antiretroviral therapy.Citation25 The findings of the above studies led both groups to conclude that HIV-associated thrombocytopenia is predominantly a product of accelerated platelet destruction, and that platelet production is relatively spared. However, to the best of our knowledge, no study has assessed the relationship between the number of reticulated platelets and the morphological features of the bone marrow in HIV-positive individuals. In this study, we aimed to correlate megakaryocyte function (as reflected by the IPF) with the bone marrow morphological features and other clinical variables of interest in South African HIV-positive patients with thrombocytopenia. In addition, as previous studies have shown that the IPF can be used to predict imminent recovery in the platelet count in some clinical settings (particularly in the context of bone marrow transplantation),Citation28 we aimed to assess the utility of the IPF in predicting the platelet response to therapy in patients with HIV-associated thrombocytopenia.
Materials and methods
Participants were identified through the haematology laboratory at the Charlotte Maxeke Johannesburg Academic Hospital in Johannesburg, South Africa. All adult HIV-positive patients with a platelet count below the lower limit of the laboratory reference range (140 × 109/l) in whom a bone marrow investigation had been performed were considered eligible, and between August 2009 and January 2010, 78 patients were enrolled ().
Table 1. Patient demographic and clinical data (N = 78 unless otherwise specified)
Sample collection and analysis
A venous blood sample was collected from each participant in an EDTA anti-coagulated tube within 24 hours of the bone marrow investigation, and the IPF% measured using a Sysmex XE-5000 haematology analyzer (Sysmex, Kobe, Japan). An IPF% cut-off of 7.7% was selected to discriminate normal from increased platelet production as per Abe et al.Citation19 (normal range = 0.7–5.5% and 0.9–5.3% in South African males and females respectivelyCitation29).
Analysis of the bone marrow aspirate and trephine biopsy morphological features were undertaken by an experienced morphologist. The bone marrow aspirate samples were stained with the May-Grünwald/Giemsa stain, and the trephine biopsies with haematoxylin and eosin. Megakaryocyte numbers and morphology were documented in addition to any other findings judged to be pertinent (such as the presence of a malignant infiltrate or granulomatous inflammation) (). Megakaryocyte dysplasia was quantified as the number of megakaryocytes judged to have abnormal or degenerate morphology out of every 30 megakaryocytes present (as recommended for assessment of megakaryocyte dysplasia in the 4th edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues).Citation30
Patient information was gathered from the hospital file and the laboratory computer system records (). The last available platelet count was documented as a reflection of the platelet response to the therapy initiated by the attending clinician. Patients with a follow-up platelet count performed less than 2 days from the time of the bone marrow aspirate were excluded, as were those patients who received a platelet transfusion. The reported platelet response is the change in platelet count over the course of the patient's hospital stay.
Statistical analysis
Statistical analysis was performed using STATISTICA software, version 11.0 (Stat Soft (Pty) Ltd). Data are presented as medians (interquartile range (IQR)) and proportions as appropriate. Kruskal–Wallis one-way analysis of variance was performed to compare the IPF levels between patients with a variety of bone marrow findings of interest (including the presence of ITP, hypocellular bone marrow, a malignant infiltrate and mycobacterial infection in the marrow) as well as those in whom the marrow showed no specific pathology. This was followed by post-hoc multiple comparisons of the mean rank scores between pairs of groups with a Bonferroni adjustment. All patients who had overlap in their bone marrow findings were excluded from this analysis.
Univariate linear regression was performed to assess the relationship between both the IPF% and the platelet response to therapy with a number of variables of interest. All variables which had a P-value of 0.2 or less in univariate analysis were then included in a multivariate linear model. Because the data were not normally distributed, all continuous variables were log transformed prior to regression analysis. For the patients with an undetectable HIVVL, the low detection limit was used for the purposes of statistical analysis. The reported adjusted β co-efficients are the antilog values of the calculated co-efficients. Residual analysis was performed on all statistically significant results, and any data point with a standard residual of greater than 2.5 was excluded from analysis. P values less than or equal to 0.05 were considered statistically significant.
Ethical clearance
Ethical clearance was obtained from the Human Research Ethics Committee of the University of the Witwatersrand, Johannesburg, and informed consent was obtained from all participants.
Results
General information
Seventy eight patients were enrolled, all but one of whom were of African descent (). A wide variety of HIV-associated pathologies were represented, with an especially high prevalence of mycobacterial infection (). Other infections present included culture positive bacterial sepsis in nine patients (predominantly with gram negative bacilli), and disseminated Cryptococcosis in one patient. Two patients had chronic hepatitis B infection, while serology for HCV was negative in all of the 44 patients tested. A further 13 patients had clinical evidence of infection, the cause for which was not identified.
The treatment initiated by the attending clinician included corticosteroid therapy in 32 (41%) of the patients, anti-Tuberculosis treatment in 38 (49%) and antibiotic therapy in 56 (72%) (including cotrimoxazole in 27 (35%) patients). Platelet response data was available in 58 patients, of whom 43 (74%) showed an improvement in their platelet count (). Twenty of the patients (26%) were receiving ART, with a median duration of 9 weeks (IQR = 2–52 weeks). Unsurprisingly, the HIVVL was significantly lower in patients on ART (P < 0.0001), and was undetectable in four patients, all of whom had been on ART for a sustained period.
The bone marrow aspirate and/or trephine biopsy were adequate for morphological assessment in 72 (92%) of the patients, and among these, megakaryocyte numbers were considered adequate in 63 (88%). The remaining nine patients had either hypocellular marrow with an absolute reduction in all cell lines, or extensive marrow infiltration. Among patients with mycobacterial infection or a malignant infiltrate involving the bone marrow, 93 and 50%, respectively, were judged to have sufficient megakaryocyte numbers.
IPF% results in pathologies of interest
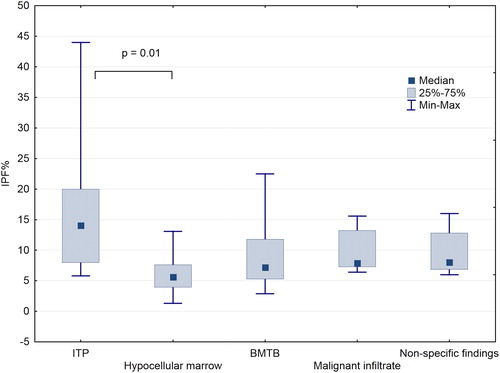
The median IPF% was 7.6% (range = 1.3–44%), and was elevated in 37 (47%) of the patients. It was high in 78% of the patients with ITP (median = 16.3%; IQR = 8.8–17.3%), and low in 57 and 79% of patients with mycobacterial infection involving the bone marrow (BMTB) (median = 7.1%; IQR = 5.3–11.8%) and hypocellular bone marrow (median = 6.5%; IQR = 4.4–7%), respectively (). Surprisingly, the IPF% was raised in six of the eight patients with a malignant marrow infiltrate (median = 8.4%; IQR = 7.6–14.4%), including three of the four patients with apparently extensive marrow infiltration ().
Figure 1. Comparison of the IPF% and various pathologies identified in the bone marrow. A Bonferroni-adjusted Kruskal Wallis analysis showed a significant difference only between patients with ITP and hypocellular marrow (P = 0.01) (ITP, immune thrombocytopenia; BMTB, bone marrow infection by mycobacterial species).
The relationship between the IPF% and variables of interest on linear regression analysis
Univariate linear regression revealed significant inverse relationships between the IPF% and both the platelet count (P < 0.0001) (A) and the presence of hypocellular bone marrow (P = 0.0012), a marginally significant relationship between the IPF% and the HIVVL (P =0.056) (B), and a significant positive association between the IPF% and the presence of ITP (P = 0.0011) ().
Figure 2. Relationship between the IPF% and platelet count (A) and HIV viral load (B) (HIVVL, HIV viral load).
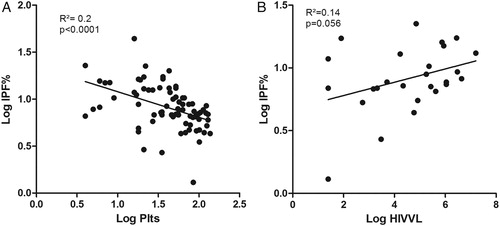
Table 2. Results of univariate regression analysis for those variables with an apparent association with the IPF%
As HIVVL levels were only available in 27 patients, multivariate linear regression was performed with and without the HIVVL included in the model. The model without the HIVVL included showed significant independent associations between the IPF% and the platelet count (P < 0.0001), the presence of ITP (P = 0.021) and the presence of a hypocellular bone marrow (P = 0.001) (A). When the HIVVL was included in the model, it too was independently associated with the IPF% (P = 0.005), while statistical significance of the relationship between the IPF% and the presence of ITP was lost (P = 0.059) (B). No relationship was found between the IPF% and age (P = 0.87), sex (P = 0.22), CD4 count (P = 0.5), the presence of mycobacterial or non-mycobacterial infection at any site (P = 0.95 and 0.53, respectively), or the number of morphologically abnormal/degenerate megakaryocytes (P = 0.55). The IPF% was also unrelated to any treatment initiated prior to the bone marrow aspirate, including ART (P = 0.76), anti-tuberculosis treatment (P = 0.89), corticosteroids (P = 0.50), or antibiotics of any kind (including cotrimoxazole) (P =0.52).
Table 3. Results of multivariate regression analysis showing the relative impact on the IPF% of the variables which appeared to have an association with the IPF% on univariate analysis
Platelet response results
Because the IPF% is predictive of imminent recovery in the platelet count in some clinical contexts, we assessed for a relationship between the platelet response to therapy and the IPF%, as well as several other variables of interest. In univariate analysis, we found the platelet response to be unrelated to the IPF% (P = 0.56), as well as the CD4 count (P = 0.95), the HIVVL (P = 0.21) or the pathology present. However, it had a marginally significant relationship to exposure to ART (P = 0.06), but no other treatment initiated. On further analysis, the platelet response was unrelated to ART exposure within patients with ITP (P = 0.44), mycobacterial infection at any site (P = 0.7) and hypocellular bone marrow (P = 0.39), but had a marginally significant association in patients with non-mycobacterial infection (P = 0.08).
Discussion
In this study, we found a wide variation in the IPF% among patients with HIV-associated thrombocytopenia. We have confirmed the strong negative correlation between the IPF% and the platelet count reported by Garibaldi et al, as well as the tendency for the IPF% to be raised in patients with ITP. The latter was statistically significant in all analyses with the exception of the multivariate model including the HIVVL, possibly because of limited sample numbers. Alternatively, this finding could reflect heterogeneity in the underlying pathogenesis of ITP. Although antibody-mediated immune clearance of platelets is the dominant cause of thrombocytopenia in ITP, some patients also show an inappropriately blunted thrombopoietin response.Citation31 Hence, the IPF% is not invariably raised in patients with ITP, as was the case in two of the nine patients with ITP in our cohort.
Unsurprisingly, we also found the IPF% to have a significant independent inverse relationship with the presence of hypocellular bone marrow, but unexpectedly found a raised IPF% in three of four patients with apparently extensive malignant marrow infiltration. Previous studies have shown that the optical platelet count measured on Sysmex XE analyzers may be inaccurate in patients receiving cytotoxic chemotherapy, possibly due to circulating fragments of apoptotic white blood cells being erroneously counted as platelets.Citation32 As white blood cell cytoplasm is rich in RNA, a spurious elevation of the IPF would be anticipated in this instance. Of the three patients with a high IPF% despite extensive marrow infiltration, two had Burkitt leukaemia/lymphoma, with platelet counts of 5 × 109/l and 18 × 109/l, and IPF levels of 7.8% and 8.8% respectively. As Burkitt leukaemia/lymphoma is associated with a high proliferative index and a brisk rate of tumour apoptosis,Citation33 it is possible that the mildly elevated IPF levels in these cases occurred spuriously due to the presence of circulating fragments of apoptotic tumour cells, despite the absence of a history of chemotherapy exposure. The third patient had extensive marrow infiltration by metastatic carcinoma of the breast, for which chemotherapeutic intervention had already been initiated at the time of the bone marrow investigation.
Like Garibaldi et al., we found that the IPF% was not affected by the use of ART,Citation25 but in contrast to the above study, we found no significant relationship between the CD4 count and the IPF%. However, our study was limited by the fact that 90% of the cohort had AIDS, which may have skewed the results. Also in contrast to the above study, we found a significant positive association between the IPF% and the HIVVL among the 27 patients in whom a viral load was performed. This was an unexpected finding, as HIV directly infects megakaryocytes by means of CD4 molecules expressed on their surface, and a reduction in the viral load would therefore be expected to improve megakaryocyte function. However, as the response to the therapy initiated by the treating physician was not related to either the HIVVL or the IPF%, the lower IPF% among patients with a lower HIVVL may reflect a dampening of HIV-driven platelet activation and clearance, as opposed to megakaryocytic suppression. This hypothesis is supported by the relationship between the HIVVL and the degree of platelet activation shown by other investigators.Citation26,Citation27
Previous studies have shown the IPF% to be predictive of imminent recovery in the platelet count, particularly in the context of bone marrow transplantation.Citation28 However, as alluded to above, our study showed no relationship between the IPF% and the patient's response to the therapy instituted, possibly because of the multifactorial and complex nature of thrombocytopenia in this setting. Interestingly, we found a marginally significant association between the platelet response and exposure to ART. This was most evident among those patients with infection with organisms other than mycobacteria, thus suggesting that ART plays a role in facilitating recovery from infection. The beneficial effects of ART in the setting of ITP demonstrated by other authorsCitation34,Citation35 were not evident in our cohort, possibly because only three patents with ITP were receiving ART.
As expected, the IPF% was low in the majority of patients with mycobacterial infection in the marrow. However, the presence of an elevated IPF% in 43% of these patients highlights the role of peripherally mediated thrombocytopenia (such as hypersplenism, immune-mediated platelet destruction, or DIC) as the dominant contributor to the thrombocytopenia in some cases with frank marrow pathology. The morphological impression of megakaryocyte adequacy in more than 80% of cases was also disparate with the presence of a low IPF in more than half of the cohort. In addition, the presence of a significant degree of megakaryocyte dysplasia showed no relationship with the IPF (as a reflection of megakaryocyte function). These findings emphasize the limitations of morphological analysis to correctly assess the underlying mechanism of thrombocytopenia, and demonstrate a possible role for the IPF in enhancing the morphologist's ability to interpret the morphological features.
Conclusion
Although the IPF% levels generally correlated well with the morphologically and/or clinically favoured cause of thrombocytopenia in patients with ITP and hypocellular marrow, a number of patients with pathological bone marrow were found unexpectedly to have an elevated IPF%. This study has thus highlighted the multifactorial nature of the causes for thrombocytopenia in patients with AIDS, and emphasizes the limitations of morphological analysis in determining megakaryocyte function. It is also clear that an elevated IPF% does not exclude the presence of marrow pathology in this clinical context, and therefore cannot be advocated as a substitute for a bone marrow investigation in patients with AIDS. Lastly, we have highlighted the possibility of inaccuracy in the IPF% in the event of extensive marrow infiltration by a rapidly proliferating tumour, and shown that the IPF% levels were statistically related to the HIVVL. As the response to the therapy initiated by the treating physician was not related to either the HIVVL or the IPF%, we speculate that the lower IPF% among patients with a lower HIVVL may reflect a dampening of HIV-driven platelet activation and clearance amongst these patients, as opposed to megakaryocytic suppression.
References
- Sloand EM, Klein HG, Banks SM, Vareldzis B, Merritt S, Pierce P. Epidemiology of thrombocytopenia in HIV infection. Eur J Haematol. 1992;48:168–72.
- Sullivan PS, Hanson DL, Chu SY, Jones JL, Ciesielski CA. Surveillance for thrombocytopenia in persons infected with HIV: results from the multistate Adult and Adolescent Spectrum of Disease Project. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:374–9.
- Servais J, Nkoghe D, Schmit JC, Arendt V, Robert I, Staub T, et al. HIV-associated hematologic disorders are correlated with plasma viral load and improve under highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:221–5.
- Van Wyk V, Kotze HF, Heyns AP. Kinetics of indium-111-labelled platelets in HIV-infected patients with and without associated thrombocytopaenia. Eur J Haematol. 1999;62:332–5.
- Cole JL, Marzec UM, Gunthel CJ, Karpatkin S, Worford L, Sundell IB, et al. Ineffective platelet production in thrombocytopenic human immunodeficiency virus-infected patients. Blood 1998;91:3239–46.
- Schwartz GN, Kessler SW, Rothwell SW, Burrell LM, Reid TJ, Meltzer MS, et al. Inhibitory effects of HIV-1-infected stromal cell layers on the production of myeloid progenitor cells in human long-term bone marrow cultures. Exp Hematol. 1994;22:1288–96.
- Sakaguchi M, Sato T, Groopman JE. Human immunodeficiency virus infection of megakaryocytic cells. Blood 1991;77:481–5.
- Zucker-Franklin D, Cao YZ. Megakaryocytes of human immunodeficiency virus-infected individuals express viral RNA. Proc Natl Acad Sci USA 1989;86:5595–9.
- George JN, Raskob GE, Shah SR, Rizvi MA, Hamilton SA, Osborne S, Vondracek T. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med. 1998;129:886–90.
- Carey PJ. Drug-induced myelosuppression: diagnosis and management. Drug Saf. 2003;26:691–706.
- Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood 2009;113:6511–21.
- Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43–70.
- Stasi R, Willis F, Shannon MS, Gordon-Smith EC. Infectious causes of chronic immune thrombocytopenia. Hematol/oncol Clin N Am. 2009;23:1275–97.
- Liebman HA. Viral-associated immune thrombocytopenic purpura. Hematology (Am Soc Hematol Educ Program). 2008;2008:212–8
- Warkentin TE, Aird WC, Rand JH. Platelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology (Am Soc Hematol Educ Program). 2003;2003:497–519
- Bain BJ. The haematological features of HIV infection. Br J Haematol. 1997;99:1–8.
- Ault KA, Knowles C. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp Hematol. 1995;23:996–1001.
- Dale GL, Friese P, Hynes LA, Burstein SA. Demonstration that thiazole-orange-positive platelets in the dog are less than 24 hours old. Blood 1995;85:1822–5.
- Abe Y, Wada H, Tomatsu H, Sakaguchi A, Nishioka J, Yabu Y, et al. A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF). Thromb Res. 2006;118:463–9.
- Briggs C, Kunka S, Hart D, Oguni S, Machin SJ. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol. 2004;126:93–9.
- Kienast J, Schmitz G. Flow cytometric analysis of thiazole orange uptake by platelets: a diagnostic aid in the evaluation of thrombocytopenic disorders. Blood 1990;75:116–21.
- Richards EM, Baglin TP. Quantitation of reticulated platelets: methodology and clinical application. Br J Haematol. 1995;91:445–51.
- Rinder HM, Munz UJ, Ault KA, Bonan JL, Smith BR. Reticulated platelets in the evaluation of thrombopoietic disorders. Arch Pathol Lab Med. 1993;117:606–10.
- Robinson M, Machin S, Mackie I, Harrison P. Comparison of glycocalicin, thrombopoietin and reticulated platelet measurement as markers of platelet turnover in HIV+ samples. Platelets 2001;12:108–13.
- Garibaldi B, Malani R, Yeh HC, Lipson E, Michell D, Bennett M, et al. Estimating platelet production in patients with HIV-related thrombocytopenia using the immature platelet fraction. Am J Hematol. 2009;84:852–4.
- Holme PA, Muller F, Solum NO, Brosstad F, Froland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 1998;12:79–89.
- Mayne E, Funderburg NT, Sieg SF, Asaad R, Kalinowska M, Rodriguez B, et al. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr. 2012;59:340–6.
- Zucker ML, Murphy CA, Rachel JM, Martinez GA, Abhyankar S, McGuirk JP, et al. Immature platelet fraction as a predictor of platelet recovery following hematopoietic progenitor cell transplantation. Lab Hematol. 2006;12:125–30.
- Botma J, Mogongoa LF, Jaftha AD, Janse van Rensburg W. Reference ranges for platelet indices using Sysmex XE -2100 blood analyser. Med Technol SA 2012;26:17–21.
- Brunning R, Orazi A, Germing U, Le Beau MM, Porwit A, Baumann I, et al. Myelodysplastic Syndromes/neoplasms, overview. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Steil H, Thiele J, Vardiman JW editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008. pp. 88–93
- Gernsheimer TB. The pathophysiology of ITP revisited: ineffective thrombopoiesis and the emerging role of thrombopoietin receptor agonists in the management of chronic immune thrombocytopenic purpura. Hematology (Am Soc Hematol Educ Program) 2008. 2008;219–26
- Segal HC, Briggs C, Kunka S, Casbard A, Harrison P, Machin SJ, et al. Accuracy of platelet counting haematology analysers in severe thrombocytopenia and potential impact on platelet transfusion. Br J Haematol. 2005;128:520–5.
- Leoncini L, Raphaël M, Stein H, Harris NL, Jaffe ES, Kluin PM. Burkitt lymphoma. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp. 262–4.
- Gentile I, Bonadies G, Buonomo AR, Minei G, Borrelli F, Foggia M, et al. Resolution of autoimmune thrombocytopenia associated with raltegravir use in an HIV-positive patient. Platelets 2013;24:574–7.
- Carbonara S, Fiorentino G, Serio G, Maggi P, Ingravallo G, Monno L, et al. Response of severe HIV-associated thrombocytopenia to highly active antiretroviral therapy including protease inhibitors. J Infect 2001;42:251–6.
