Abstract
Objectives
This study investigates the link between patient characteristics and mortality in patients with hematological malignancies (HM) in three university-affiliated hospitals in Hunan, China.
Methods
We conducted a detailed retrospective chart review of 121 sequential intensive care unit (ICU) admissions with HM over a 5-year period. Outcome measures were short- and long-term mortality rates and were correlated with physiologic and therapeutic factors. We also evaluate the performance of two severity-of-illness scoring systems in this population, particularly the value and trend of the sequential organ failure assessment (SOFA).
Results
The rates for ICU, 1-month and 6-month mortalities were 60.3, 85.9, and 90.9%, respectively. Invasive mechanical ventilation (IMV) was associated with worse outcomes at all time points. Both acute physiology and chronic health evaluation and SOFA scores had positive correlation with ICU mortality. An increase or no change in SOFA over the course of the admission or during the first 48 hours after admission was the most powerful adverse predictor. IMV use and renal dysfunction had a negative effect on the 1-month survival.
Conclusion
Patients with HM have less access to intensive care resources in Hunan, China. The use of IMV, APACHII at admission, and SOFA trend have a strong predictive value in this population. Based on our results, we propose a panel of parameters for use when considering ICU transfer to guide patient management.
Introduction
With advances in both disease-modifying treatment and supportive care, the prognosis for hematological malignancies (HM) has improved.Citation1,Citation2 More clinicians now believe patients with HM should receive intensive care when they have life-threatening problems and they deserve equal access as patients with other diseases.Citation3
The intensive care unit (ICU) survival of patients with HM has also improved,Citation4 thanks to the improvement in interventions for organ failure and timely interdisciplinary collaboration. In Western countries, the ICU mortality rate of HM decreased from 65–80% in 1980sCitation5–Citation7 to 33.7–56% today.Citation8–Citation10 As reported in several recent studies from North America and Europe, many factors affect the prognosis during the ICU stay, including age, admission diagnosis, sepsis, neutropenia, thrombocytopenia, organ failure leading to the requirement for ventilatory support or hemodialysis, and the patient's overall health status as assessed by scoring systems such as acute physiology and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA).Citation9–Citation13 However, the relative predictive significance of these factors remains controversial.Citation4,Citation14
Although the reluctance to admit patients with hematologic malignancies to the ICU is receding in the West,Citation15 admission policies remain strict in Asian countries partly due to the scarcity of information on the prognostic evaluation of ICU patients with HM.Citation16 We were only able to identify a retrospective study by Yeo et al.Citation17 in Korea on this category of patients. They reported a relatively high ICU mortality for patients with HM, which they attributed to the large proportion of difficult-to-treat acute leukemia patients in their patient sample. Whether their observations and conclusions apply to other Asian populations, particularly the Chinese, is unknown.
In the current study, we first evaluated the ICU access of patients with HM and then conducted a detailed retrospective chart review of 121 ICU admissions with the diagnosis of various HM and analyzed the factors that correlate with short and long-term outcomes. Our findings may help formulate an evaluation system to better assess the prognosis of patients with hematological diseases when considering transferring criteria to the ICU in China.
Methods
Patients and setting
The study design was evaluated by the Ethics Committee of Central South University and deemed exempt from a formal review as no personally identifiable information would be collected. The requirement for informed consent from patients was also waived. Data were collected from three university-affiliated hospitals in Hunan Province, China, from November 2008 to September 2013. The three hospitals have general medical ICUs as well as other specialized ICUs such as coronary care units, neurology care units, and neuroscience intensive care units. Serving as regional referral centers, the general medical ICUs of these hospitals have a total of 77 beds and collectively admit over 4000 critically ill patients each year. The units are equipped with airborne, contact and droplet precaution measures, hemodynamic monitoring, invasive and noninvasive mechanical ventilation, and renal replacement therapy, in addition to the standard supportive care. ICU admission was driven by clinical judgment and the same admission and transfer criteria were applied to hematological patients and other critically ill patients. ICU-level care was considered when a patient needed mechanical ventilation, vasopressor support, or frequent nursing care, monitoring and medication adjustment. The decision to admit or transfer to the ICU was made by the referring department in collaboration with the intensive care specialists and required consent from the patient and/or family member. The same ICU admission protocol was used in all three hospitals.
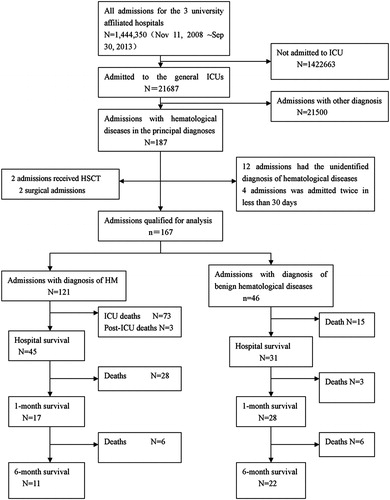
The flow chart in shows the process of case selection for the chart review. Out of the 21 687 ICU admissions between November 2008 and September 2013, 187 listed hematological diseases among the main diagnoses. For patients who had multiple re-admissions to the same ICU, each admission was counted separately, unless it happened within less than 30 days of the prior admission.
Twenty admissions were excluded because the patients received hematopoietic stem cell transplantation (HSCT) (2 admissions), were admitted for surgery (2 admissions), had nonspecific diagnoses of hematological diseases (12 admissions), or were re-admitted within 30 days (4 admissions). The remaining 167 ICU admissions included 46 with benign hematological disorders, and 121 with HM (including seven re-admissions), which were qualified for this study. We excluded the two HSCT patients because their treatment and care deviated significantly from other HM patients, involving high doses of chemotherapy, severe immunosuppression, and isolation, but the sample size was too small to allow for separate analysis.
Data collection
The following background data were collected for each ICU admission: patient's age, sex, principal diagnoses and comorbidities, timing and reason for ICU transfer, lengths of ICU and hospital stays, time lapsed between diagnosis and admission, treatment regimen prior to ICU admission, and the presence or absence of organ failure. As previously described, acute respiratory failure was defined as oxygen saturation less than 90% or PaO2 less than 60 mmHg or severe tachypnea with respiratory rate >30 per minute.Citation18 Shock was defined as systolic blood pressure <90 mmHg or a decrease of over 40 mmHg for systolic blood pressure from the baseline.Citation19 Acute renal failure was defined as decrease in urine output to less than 30 ml/hour or less than 400 ml/day.Citation20 Status of the malignancy was accessed by the most recent bone marrow biopsy data when available. Complete/partial remission and progression/relapse was defined as previously described.Citation17 Briefly, complete remission was defined as less than 5% of blast cells in marrow aspirates with an absolute neutrophil count >1000/microl and platelets >100 000/microl in patients with leukemiaCitation21,Citation22; the negative immunofixation of serum and urine and the disappearance of any soft tissue plasmacytomas, and <5% plasma cells in bone marrow in patients with myelomaCitation23; the disappearance of peripheral and deep lymphadenopathy and other malignant foci in patients with lymphoma.Citation24,Citation25 Progression was defined as persistent disease insensitivity to standard chemotherapy. Relapse was defined as recurrence or progression of disease after a period of remission. Patients diagnosed within the 3 months of the ICU admission were coded as new diagnosis.
Physiologic factors recorded included laboratory tests such as blood counts, electrolytes, coagulation, blood gas, and microbial cultures. Therapy for organ failure included vasopressors, invasive and noninvasive ventilatory support, and dialysis. Neutropenia was defined as a white blood cell count <500/mm3. Vasopressor use was defined as dobutamine, norepinephrine, or epinephrine at any dose or dopamine at a dosage greater than 5 µg/kg/min.Citation17 Systemic fungi-infection treatment was determined clinically according to the revised definition of invasive fungal disease from the European organization for research and treatment of cancer.Citation26
The overall patient condition was assessed using two scoring systems: acute physiologic and chronic health evaluation II (APACHE II) to evaluate the severity of acute illness and SOFA to evaluate organ function. To track the changes in patient condition through the ICU stay, we calculated SOFA scores at Days 1, 3, and ICU discharge. Patients were stratified according to the trend in SOFA scores over time. If the SOFA score decreased by ≥2 points between ICU admission and discharge, the trend was recorded as decrease. Otherwise, the trend was recorded as no-change/increase. To assess the change during the first 48 hours of the ICU stay, we also calculated the change in SOFA score between ICU admission and after the first 48 hours of the ICU stay. Fifty-two patients were excluded from the 48-hour SOFA analysis because they did or were discharged within 48 hours.
The outcome measures included ICU mortality and long-term mortality rates which were assessed at 1 month (30 days) and 6 months after ICU discharge.
Data analysis
The statistical analysis was done with SPSS, version 17.0. Continuous variables were presented as mean ± standard deviation (SD) or standard error of mean when appropriate. Proportions were presented as percentages. Statistical significance between survivors and non-survivors was tested using student's t-test for continuous variables and Pearson chi-square test or Fisher's exact test for categorical variables. We used logistic regression to examine the relationship between clinical characteristics and ICU survival. Covariance between parameters was checked with Pearson's correlation test. Variables were considered strongly correlated when r ≥ 0.6. In contrast, a correlation r ≤ 0.05 denoted weak correlation. Parameters found to be statistically significant in the univariate analysis were entered into the multivariable model using a step-wise method; P < 0.1 was required to enter the model and P < 0.05 was required for the variable to retain itself within the model. Goodness-of-fit of the model was assessed using the Hosmer–Lemeshow test on all imputed data sets.Citation17 A final bootstrap analysis was performed to confirm the conclusions. Kaplan–Meier survival curves were plotted and compared using a signed log-rank test.
Results
Patient characteristics
summarizes the background data of the 121 ICU admissions of patients with HM included in this study. Majority (71.9%) of the patients were male. The median age was 48 years (range 16–82 years). Acute myeloid leukemia (AML 52.0%) and acute lymphoid leukemia (13.2%) were the most common principal diagnoses. Eighty-six (71.1%) patients were newly diagnosed. Only seven (5.8%) patients were in remission. Acute respiratory failure (57.9%) was the most common reason for ICU admission; others were shock (11.6%), heart failure (5.8%), coma (17.3%), and acute renal failure (1.6%). While in the ICU, 78 patients (64.5%) required invasive mechanical ventilation (IMV), 82 patients (67.8%) needed vasopressor, and 7 (5.8%) required dialysis. Thirty-three (27.3%) patients had positive cultures from a sterile site such as the blood or bronchoalveolar lavage, 79.5% of which grew Gram-negative bacteria. Only one (2.6%) had culture-confirmed fungal infection.Citation26 The ICU mortality was 60.3% and hospital mortality was 62.8%. Most (64.6%, 31/48) of the ICU survivors died within 1 month of discharge. The 6-month overall mortality was 90.9%.
Table 1. Characteristics of 121 ICU admissions with hematologic malignancies (mean ± SD or N and %)
Factors affecting the ICU survival
shows the comparison between ICU survivors and non-survivors and factors associated with ICU survival. There was no significant difference in age, sex, disease status at admission, and reason for ICU admission between the two groups. The proportion of AML was significantly lower among the survivors and non-survivors (37.5 versus 61.6%, P = 0.009). We stratified the patients who received anti-fungi therapy into different groups based on the diagnostic criteria.Citation26 Only those who had confirmed or high suspicion of invasive fungi infection had higher mortality, albeit not statistically significant (OR = 5.676, P = 0.080). With regard to laboratory values, the arterial pH was significantly lower in non-survivors (7.32 ± 0.02 versus 7.44 ± 0.01, P < 0.001). No difference was found in creatinin (Cr), or blood urea nitrogen (BUN).
Table 2. Comparisons of between ICU survivors and non-survivors with HM (n with % or mean ± SEM)
Mortality was significantly higher in patients with neutropenia (OR: 2.364, 95%CI 1.019–5.484, P = 0.042). The need for IMV (OR: 22.500, 95%CI 6.535–77.462, P < 0.001) and the use of vasopressors (OR: 7.891, 95%CI 3.337–18.657, P < 0.001) were also strongly associated with ICU mortality.
Both the initial APACHE II and SOFA scores on day of ICU admission and change during the ICU stay correlated with survival outcomes (see ). Particularly, ongoing deterioration related to organ failure as indicated by the unchanged/increased SOFA scores during the entire ICU stay (OR: 16.667, 95%CI: 4.587–60.559, P < 0.001) was one of the most powerful predictors for ICU mortality. This relationship with an up-trending SOFA was apparent as early as during the first 48 hours (OR: 6.993, 95%CI: 2.408–19.967, P < 0.001).
Conditions shown to be significant in the univariate analysis were incorporated into the multivariate model (P ≤ 0.1). As we assessed SOFA scores at several different time points as well as their trend during the ICU stay and they all had significant correlation with mortality, only the overall trend in SOFA score, which had the highest odds ratio, was included. After correcting for age and sex, the model had a modest goodness-of-fit (Cox and Snell RCitation2 = 0.556, Nagelkerke RCitation2 = 0.753, Chi-square = 2.627, P = 0.504). When these variables were introduced into a logistic regression model, unchanged/increased SOFA score, APACHE II score on Day 1 and the need for IMV were independently associated with ICU mortality ().
Table 3. Multivariate analysis of factors that were significantly associated with ICU mortality on univariate analysis, using trend in SOFA score
Factors affecting 1-month mortality
illustrates the characteristics of survivors and non-survivors at 1 month after ICU discharge. Non-survivors had significantly higher Cr (151.84 ± 36.87 µmol/l versus 71.47 ± 9.06 µmol/l, P = 0.042) and BUN (12.170 ± 1.52 mmol/l versus 6.60 ± 0.94 mmol/l, P = 0.003). More non-survivors need IMV due to respiratory failure (38.7 versus 5.9%, P = 0.092). However, we did not find that vasopressor use was associated with the 1-month mortality as it was for ICU mortality (OR: 2.250, 95%CI: 0.639–7.923 P = 0.202). We also employed survival analysis to evaluate the effect of IMV and chemotherapy on long-term survival of patients after ICU discharge ( and ). Similar to what was observed with ICU and 1-month mortality, patients who needed IMV during their ICU stay had worse long-term prognosis (6-month mortality rate 71.4 versus 92.3%, P = 0.027) (). Having undergone chemotherapy after discharge was correlated with significantly better prognosis (6-month mortality rate of 38.5% with chemotherapy versus 91.4% without, P < 0.001) ().
Figure 2. Respiratory support and long-term survival of patient with hematologic malignancies admitted to ICU. X-axis denotes time after ICU discharge, in days. Y-axis denotes cumlative survival. (A: dotted line) Patients who have received invasive mechanical ventilation (IMV) during ICU stay. (B: bolded line) Patients who have not received IMV.
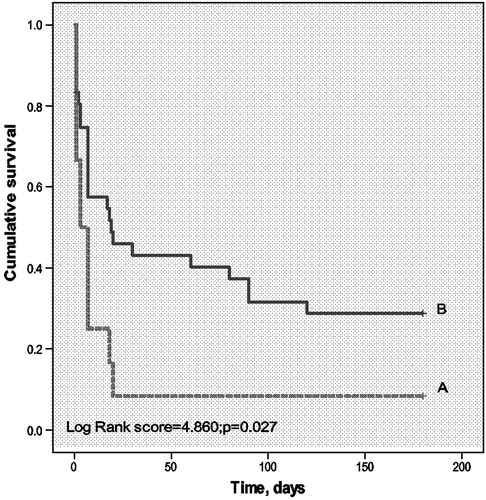
Figure 3. Chemotherapy and long-term survival of patient with hematologic malignancies admitted to ICU. X-axis denotes time after ICU discharge, in days. Y-axis denotes cumulative survival. (D: dotted line) Patients who have not received chemotherapy after ICU discharge. (C: bolded line) Patients who have had chemotherapy within 1 month after ICU discharge.
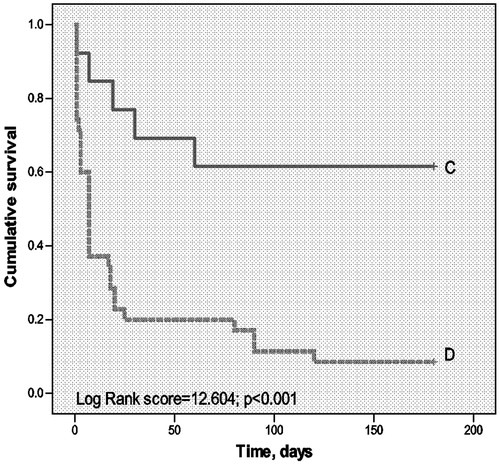
Table 4. Comparisons between 1-month survivors and non-survivors (n and % or mean ± SEM)
Discussion
As the economic development galloped in China, the standard of and access to medical care also saw tremendous improvement in recent years.Citation27 More and more critically ill patients are entering the healthcare system, and the allocation of resources to maximize treatment benefit becomes a necessity. While many studies have examined the prognosis of ICU patients with HM in the West,Citation11,Citation18,Citation28,Citation29 there is still a paucity of data to guide clinicians in China regarding ICU admissions for this specific population of patients.
In this study, we examined the general medical ICU admissions in three university-affiliated hospitals in Hunan Province with a population of 60 million. Collectively, the hospitals have over 9000 beds accommodating 250 000–300 000 inpatient stays each year. During the study period, 21 687 patients (about 1.5% of all admissions) stayed in the 77 beds (0.86% of all inpatient beds) of the three medical ICUs. Compared to Western countries, where medical ICUs occupy 1.8–5% of all inpatient bedsCitation30–Citation32 and receive 1.99–4.1% of inpatient admissions,Citation10,Citation33 the availability of China's medical intensive care resources is relatively poor. Each of these three hospitals also has a specialized hematological ward, totaling 230 beds with over 4000 admissions for patients with HM each year. Meanwhile, only 121 () ICU admissions involving HM occurred during the 5-year study period. In other words, only 0.61% of all inpatients with HM were transferred to ICUs, much lower than what was reported in a previous study from Brazil (5.9%).Citation30,Citation31 This suggests that our patients, especially those with HM, had less access to intensive care. In addition to the lack of overall intensive care resources, we speculate that the presumed poor prognosis for HM, coupled with the high cost of care, a substantial part of which is out-of-pocket, would prevent medical providers and families from transferring patients to the ICU.
Studies from Western countries have showed improved ICU outcomes with mortality rates between 33.7 and 56% in patients with HM in the recent years, including those needing mechanical ventilation and vasopressors.Citation8,Citation10 In our sample, the ICU (60.3%) mortality rate was comparable to some previous studiesCitation8,Citation29 and high than most reports from the West.Citation8,Citation10,Citation12,Citation18 The 1-month mortality (85.9%) was quite high compared to the Western studies.Citation10,Citation18 Both our ICU and follow-up mortality were comparable to the results reported by Yeo et al.Citation17 from Korea (ICU mortality 84.1% and 3-month mortality 93.2%), suggesting that we might have observed a trend common to Asian countries. What factors might contribute to the higher mortality we observed? Part of it may be the patient characteristics. First, acute myeloblastic leukemia accounted for more than half of our ICU admissions (52%). Historically, AML was believed to result in poorer outcomes after admission to the ICU because of the high risk for treatment-related complications.Citation12,Citation13 The Korean study also attributed their findings to the large number of acute leukemia patients.Citation17 We also had fewer patients in remission (5.8%, compare to 23.1% in a prospective study performed by Azoulay et al., for example)Citation18 and mortality was significantly lower in patients in complete remission. Second, more patients in our study experienced complications and organ failures. The proportion of patients needing IMV (64.5%), and vasopressors (67.8%) were significantly higher than prior studies in which only about half of the patients required such measures.Citation10,Citation12,Citation18 Third, our average SOFA (10.75) was higher than most Western studies.Citation34 Geerse et al. reported an in-hospital mortality of 65% with an average SOFA score of 10.1, similar to our results.Citation8 Similarly, APACHE II (20.84 in ours, 19.4 in the Korean retrospective study) scores at admission were also higher in the Asian studies studies.Citation17 Taken together, our patients might have been generally sicker compared to other studies.
A considerable number of our patients died within 1 month following ICU discharge, which was not seen in other studies. We found three highly associated factors for 1-month mortality among the ICU survivors – the use of mechanical ventilation during the ICU stay, the lack of continued chemotherapy after ICU discharge, and poor renal function represented by elevated Cr and BUN ( and and ). The adverse effect of IMV and renal insufficiency may suggest that some patients were discharged prematurely without receiving adequate treatment to reverse their physiological derangements. Mortality was significantly lower in patients who had further chemotherapy, which may be a reflection of the patient's health status, rather than a causative effect. Patients who did not receive chemotherapy might have been too unwell to do so. However we cannot exclude the possibility that chemotherapy had a protective effect for those patients well enough to stand it. In addition, due to the cultural preference to die at home,Citation35 some patients who would otherwise deteriorate in the ICU were likely discharged home near the end of their life as clinicians and family members became aware of their poor prognosis. Further prospective studies should be done to test these hypotheses.
We confirmed the results from Western countries that organ failure, as assessed by the need for invasive mechanical ventilation, vasopressor therapy, and APACHE II and SOFA scoring systems, correlate with increased ICU mortality for patients with HM (Tables and ).Citation10–Citation13,Citation18 There are also some discrepancies between our results and previous reports. For example, we did not see significant correlation between the time lapsed from admission to ICU transfer and outcome, whereas a recent European prospective multicenter study reported early ICU transfer had better survival.Citation18 One reasonable explanation is that disease development and status of the malignancy may affect the prognosis more than the acute nature of the admission. Alternatively, the early transfer to ICU may reflect more relaxed criteria for acceleration of care so that the patients who were able to benefit from the intensive measures in their study were healthier than ours.
Among the life-sustaining interventions shown previously to be predictive of ICU mortalities were mechanical ventilation, vasopressor therapy, and renal replacement therapy.Citation8,Citation10,Citation17,Citation18,Citation36 Previous studies often did not distinguish between invasive and noninvasive ventilation methods.Citation8,Citation10,Citation18 Here we observed that IMV, namely intubation and tracheostomy, specifically correlated with even higher mortality than noninvasive ventilation, such as bilevel and continuous positive airway pressures (). Moreover, regardless of the timing (P = 0.487) or length (P = 0.914) (Appendix ), IMV was an independent risk factor for ICU mortality in patients with HM. Thus noninvasive mechanical ventilation should be the preferred respiratory support for ICU HM patients, confirming the opinions of Rosario Molina.Citation29 Invasive fungi infection has been reported as another adverse prognosticator in HM patients.Citation37,Citation38 Our study only revealed a non-statistically significant trend, possibly due to the difficulties in detecting invasive fungi infection and the resulting small sample size of confirmed diagnoses.
Both APACHE II and SOFA have been previously applied to hematological patients and separately proved sufficient in predicting outcomes.Citation8–Citation10,Citation17 We reiterated the validity of both scoring systems. SOFA, calculated from a comprehensive index of six organ systems (respiratory, cardiovascular, hepatic, coagulation, neurologic, and renal), was reported to have more predictive power in ICU patients with HM.Citation39 However, controversy exists regarding the timing and interval of SOFA calculation.Citation8,Citation40 Previously, the score at admission,Citation40,Citation41 the highest score during the first week of ICU stay,Citation8 and the change between scores taken at different timesCitation8,Citation41 have all been used without clear suggestion of superiority. In our study, the trend of SOFA from ICU admission to discharge and the change in SOFA scores after 48 hours proved more powerful than any single point-in-time measurement such as the score at admission or the highest score. Based on these results, ICU admission and therapy should not be denied for patients who are very ill and have a high initial SOFA score. We propose that the best time for identifying patients who could maximally benefit from intensive care efforts may be 48 hours after ICU admission, when we can assess the change in SOFA scores since admission and further triage ICU resources.
Our study has some limitations. As we took a retrospective approach, we did not have a record of the exact decision-making process for each ICU transfer. We do not know how the clinicians weighed the patients' different physiologic and social characteristics, which may have induced an inherent bias that excluded certain patients. We also do not know the extent of family involvement in the decision-making process. These questions could only be answered by a prospective research. Our research mainly focused on non-transplant HM patients. During the study period, only two patients with HSCT were admitted to the ICUs, whom we purposely excluded from our analysis. Therefore it in unclear whether our observations about mortality predictors would apply to HSCT patients. To assess the ICU outcome of HSCT patients, collaborative studies involving multiple centers are likely needed to provide a large-enough sample size. In addition, China is a big country with a wide range of socioeconomic variations. We only collected data from one of the 33 provinces. However, the three university-affiliated hospitals are among the largest and most technologically advanced tertiary care centers in China with a large catch-man and referral area spanning several provinces in the Central and South regions. We believe the hospitals represent the intermediate level of medical care accessible to the average Chinese citizen and the results are generalizable to other Chinese populations. More importantly, to our knowledge, this is the first study to investigate the prognostic factors for HM patients admitted to the ICU in Chinese population. We hope that the comprehensive series of these clinical determinants studied in our project can guide Chinese physicians to make clinically appropriate and cost-effective decisions about ICU admissions.
In conclusion, we propose that the decision for the ICU admission of hematological patients should be based on the extent of organ dysfunction instead of the underlying disease, both APACHE II score at admission and trends in SOFA score should be used to monitor patient condition to encourage early intervention.
Disclaimer statements
Contributors JL and QC contributed equally to this paper.
Funding NIH Grant ♯1 R25 TW007700-01A1.
Conflicts of interest None.
Ethics approval None.
References
- Brenner H, Gondos A, Arndt V. Recent major progress in long-term cancer patient survival disclosed by modeled period analysis. J Clin Oncol. 2007;25:3274–80.
- Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet 2002;360:1131–5.
- Hill QA. Intensify, resuscitate or palliate: decision making in the critically ill patient with haematological malignancy. Blood Rev. 2010;24:17–25.
- Azoulay E, Soares M, Darmon M, Benoit D, Pastores S, Afessa B. Intensive care of the cancer patient: recent achievements and remaining challenges. Ann Intensive Care. 2011 2011-01-20; 1:5.
- Lloyd-Thomas AR, Wright I, Lister TA, Hinds CJ. Prognosis of patients receiving intensive care for lifethreatening medical complications of haematological malignancy. BMJ (Clinical research ed.). 1988;296:1025.
- Schuster DP, Marion JM. Precedents for meaningful recovery during treatment in a medical intensive care unit: outcome in patients with hematologic malignancy. Am J Med. 1983;75:402–8.
- Yau E, Rohatiner AZ, Lister TA, Hinds CJ. Long term prognosis and quality of life following intensive care for life-threatening complications of haematological malignancy. Br J Cancer. 1991;64:938.
- Geerse DA, Span LFR, Pinto-Sietsma S, van Mook WNKA. Prognosis of patients with haematological malignancies admitted to the intensive care unit: sequential organ failure assessment (SOFA) trend is a powerful predictor of mortality. Eur J Intern Med. 2011;22:57–61.
- Hampshire PA, Welch CA, McCrossan LA, Francis K, Harrison DA. Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: a secondary analysis of the ICNARC case mix programme database. Crit Care. 2009;13:R137.
- Bird GT, Farquhar-Smith P, Wigmore T, Potter M, Gruber PC. Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: a 5 yr study. Br J Anaesthesiol. 2012;108:452–9.
- Evison J, Rickenbacher P, Ritz R, Gratwohl A, Haberthur C, Elsasser S, et al. Intensive care unit admission in patients with haematological disease: incidence, outcome and prognostic factors. Swiss Med Wkly. 2001;42:4–73.
- Rabbat A, Chaoui D, Montani D, Legrand O, Lefebvre A, Rio B, et al. Prognosis of patients with acute myeloid leukaemia admitted to intensive care. Br J Haematol. 2005;129:350–7.
- Thakkar SG, Fu AZ, Sweetenham JW, Mciver ZA, Mohan SR, Ramsingh G, et al. Survival and predictors of outcome in patients with acute leukemia admitted to the intensive care unit. Cancer. 2008;112:2233–40.
- Farquhar-Smith WP, Wigmore T. Outcomes for cancer patients in critical care. Curr Anaesthesia Crit Care. 2008;19:91–5.
- Smedira NG, Evans BH, Grais LS, Cohen NH, Lo B, Cooke M, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990;322:309–15.
- Sirio CA, Tajimi K, Tase C, Knaus WA, Wagner DP, Hirasawa H, et al. An initial comparison of intensive care in Japan and the United States. Crit Care Med. 1992;20:1207–15.
- Yeo CD, Kim JW, Kim SC, Kim YK, Kim KH, Kim HJ, et al. Prognostic factors in critically ill patients with hematologic malignancies admitted to the intensive care unit. J Crit Care. 2012;27:731–9.
- Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium – a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol. 2013;31:2810–8.
- Estenssoro E, González F, Laffaire E, Canales H, Saenz G, Reina R, et al. Shock on admission day is the best predictor of prolonged mechanical ventilation in the ICU. CHEST J. 2005;127:598–603.
- Broden CC. Acute renal failure and mechanical ventilation: reality or myth? Crit Care Nurse. 2009;29:62–75.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Lymphoblastic Leukemia. Version 3.2013 [accessed 2014 March 23]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/all.pdf.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Acute Myeloid Leukemia. Version 2.2014 [accessed 2014 May 29]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Multiple Myeloma. Version 2.2014 [accessed 2014 March 23]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's Lymphomas. Version 2.2014 [accessed 2014 March 23]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hodgkin Lymphoma. Version 2.2014 [accessed 2014 March 28]. Available from http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf.
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813–21.
- Yip WC, Hsiao WC, Chen W, Hu S, Ma J, Maynard A. Early appraisal of China's huge and complex health-care reforms. Lancet 2012;379:833–42.
- Cherif H, Martling C, Hansen J, Kalin M, Björkholm M. Predictors of short and long-term outcome in patients with hematological disorders admitted to the intensive care unit for a life-threatening complication. Support Care Cancer. 2007;15:1393–8.
- Molina R, Bernal T, Borges M, Zaragoza R, Bonastre J, Granada RM, et al. Ventilatory support in critically ill hematology patients with respiratory failure. Crit Care. 2012;16:R133.
- Merz TM, Schär P, Bühlmann M, Takala J, Rothen HU. Resource use and outcome in critically ill patients with hematological malignancy: a retrospective cohort study. Crit Care. 2008;12:R75.
- Soares M, Salluh JIF, Torres VBL, Leal JVR, Spector N. Short- and long-term outcomes of critically ill patients with cancer and prolonged ICU length of stay. CHEST J. 2008;134:520–6.
- Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35:808–14.
- Capelastegui A, Espana PP, Quintana JM, Areitio I, Gorordo I, Egurrola M, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27:151–7.
- Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent J. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13:R15.
- Broad JB, Gott M, Kim H, Boyd M, Chen H, Connolly MJ. Where do people die? An international comparison of the percentage of deaths occurring in hospital and residential aged care settings in 45 populations, using published and available statistics. Int J Public Health. 2013;58:257–67.
- Azevedo LC, Caruso P, Silva UV, Torelly AP, Silva E, Rezende E, et al. Outcomes for patients with cancer admitted to the ICU requiring ventilatory support: results from a prospective multicenter study. Chest J. 2014;146:257–66.
- Massion PB, Dive AM, Doyen C, Bulpa P, Jamart J, Bosly A, et al. Prognosis of hematologic malignancies does not predict intensive care unit mortality. Crit Care Med. 2002;30:2260–70.
- Vandijck DM, Benoit DD, Depuydt PO, Offner FC, Blot SI, Van Tilborgh AK, et al. Impact of recent intravenous chemotherapy on outcome in severe sepsis and septic shock patients with hematological malignancies. Intensive Care Med. 2008;34:847–55.
- Cornet AD, Issa AI, Loosdrecht AA, Ossenkoppele GJ, Van Schijndel RJ, Johan Groeneveld AB. Sequential organ failure predicts mortality of patients with a haematological malignancy needing intensive care. Eur J Haematol. 2005;74:511–6.
- Silfvast T, Pettilä V, Ihalainen A, Elonen E. Multiple organ failure and outcome of critically ill patients with haematological malignancy. Acta Anaesthesiol Scand. 2003;47:301–6.
- Lamia B, Hellot MF, Girault C, Tamion F, Dachraoui F, Lenain P, et al. Changes in severity and organ failure scores as prognostic factors in onco-hematological malignancy patients admitted to the ICU. Intensive Care Med. 2006;32:1560–8.