Abstract
Introduction: Allogeneic hematopoietic stem cell transplantation (AHSCT) is a curative approach for patients with myelodysplastic syndrome (MDS).
Methods: In this multicenter retrospective study, we analyzed the outcome of adult patients with MDS who underwent AHSCT in Argentina and evaluated the prognostic factors associated with progression-free survival (PFS), overall survival (OS), cumulative incidence (CI) of relapse, and non-relapse mortality (NRM).
Results: We analyzed data from 87 adults (median age: 43 years, range 18–66) who underwent SCT after myeloablative (n = 60) or non-myeloablative conditioning (n = 27), and from related (n = 62) or unrelated (n = 25) donors. For all patients, unadjusted 4-year PFS and OS were 37% and 38%, respectively; no significant differences were found between recipients of related or unrelated donors. One-year CI of relapse and NRM were 21% and 20%, respectively. In the multivariate analysis, intermediate disease risk index (DRI) and acute graft versus host disease AGVHD of all grades (I–IV) were independent variables associated with better PFS and lower relapse CI; only intermediate DRI was associated with better OS.
Conclusions: AHSCT is a feasible procedure in Argentina, with more than 30% of the patients achieving long-term survival. Recipients with unrelated donors had at least similar outcome than those with related donors. DRI may be useful to identify patients at higher risk of relapse after transplantation.
Introduction
Allogeneic hematopoietic stem cell transplantation (AHSCT) is the only curative treatment option for patients with myelodysplastic syndrome (MDS).Citation1,Citation2 Hematopoietic transplantations from related and unrelated donors have acceptable long-term survival, and the use of reduced intensity conditioning has extended the eligibility of patients for AHSCT.Citation3–Citation5
However, relapsed disease remains the main cause of failure. Different factors have been associated with relapsed disease, namely: cytogenetics,Citation6,Citation7 International Prognostic Scoring System (IPSS) criteria,Citation1 WHO classification,Citation8 and disease status at transplantation.Citation5,Citation7,Citation9,Citation10 Therapy-related MDS (t-MDS) may not be an independent factor for poor outcome per se, but rather this is determined by cytogenetic factors.Citation7 Increasingly, cytogenetics has become more specific to predict survival in MDS, from IPSS to the recently published Revised International Prognostic Scoring System (R-IPSS).Citation11,Citation12 For patients undergoing AHSCT, Armand et al. demonstrated the high value of a simple cytogenetic grouping scheme to predict post-transplantation survival.Citation13 According to this, chromosome 7 abnormalities and complex karyotypes (three or more abnormalities) are considered adverse risks; all other karyotypes are considered standard risks.Citation13 By combining this cytogenetic scheme and disease status at transplantation, a new disease risk index (DRI) for patients undergoing AHSCT has been proposed.Citation14 More recently, Della Porta et al. reported that R-IPSS high-risk category and monosomal karyotype were independently associated with relapse and lower overall survival (OS) after transplantation.Citation15
We aimed to study the survival and prognostic factors in Argentinean patients with MDS undergoing AHSCT.
Materials and methods
Patients
Data were collected retrospectively from 87 adult patients who underwent AHSCT between 1995 and 2013 at nine Argentinean centers affiliated to the Argentinean Group of Bone Marrow Transplantation (Grupo Argentino de Trasplante de Médula Ósea, GATMO). We included patients older than or equal to 18 years old with primary MDS, acute myeloid leukemia evolving from preexisting primary MDS (sAML), t-MDS, and mixed myelodysplastic and myeloproliferative syndromes (MDS/MPS). All patients with primary MDS were classified according to the WHO classification into: refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), refractory cytopenia with multilineage dysplasia (RCMD), refractory anemia with excess blasts (RAEB-1 and RAEB-2) and myelodysplastic unclassifiable syndrome (MDS-U). MDS/MPS included chronic myelomonocytic leukemia (CMML) and RA with ringed sideroblasts associated with marked thrombocytosis (RARS-T). Cytogenetic risk stratification was performed according to the categorization proposed by Armand et al. into standard and adverse risks.Citation13 For patients with clonal cytogenetic evolution, the last karyotype before transplant was used.Citation13 Disease status at transplantation was categorized as follows: untreated (supportive care and treatments without chemotherapy or hypomethylating agents), complete remission (CR), and persistent disease (including induction failure, partial remission, or relapse) in patients treated with chemotherapy or hypomethylating agents. We applied the DRI for MDS described by Armand et al. in those patients whose data about cytogenetics and disease status at transplant were available.Citation14 According to this index, patients were categorized as intermediate risk (standard cytogenetics, and untreated or CR at transplant), high risk (standard cytogenetics and persistent disease; or adverse cytogenetics, and untreated or CR at transplant), or very high risk (adverse cytogenetics and persistent disease). Intensity of conditioning regimen was defined according to Bacigalupo et al. as myeloablative (MA) and non-myeloablative (NMA); for analysis purposes, we grouped reduced intensity with NMA.Citation16 Supportive care was defined by the policy of the transplantation center. All patients signed a written consent and the study was approved by the Institutional Ethics Committee for Health Research of the Hospital Privado de Córdoba.
Statistical analysis
Non-parametric variables were compared with Mann–Whitney U test. OS and progression-free survival (PFS) were estimated by the Kaplan–Meier method, and comparisons were performed using Log-rank test. Cox-regression analysis was used for multiple regression analysis. Relapse and non-relapse mortality (NRM) were estimated using cumulative incidence analysis and considering competing risks. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method, and multiple comparisons were tested using the Fine-Gray method.Citation17,Citation18 Statistical analyses were performed using EZR V 1.23 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing V 3.0.2).Citation19 All P values were two-sided. P values less than 0.05 were considered to be statistically significant.
Results
Patients and transplant characteristics
Demographic data of 87 consecutive patients are summarized in Table . The distribution by center was heterogeneous with a center including 20 patients and other center including one patient. Conditioning regimen was MA in 60 patients (68.9%): BuCy2 (n = 41), FB4 (n = 13), TBICy (n = 4), BuCyVP-16 (n = 1), and Bu-Mel (n = 1). The rest of the patients (n = 27; 31.1%) received a NMA regimen: FB2 (n = 10), Flu-Mel (n = 13), Flu-Mel-AraC (n = 1), and Flu-TBI (n = 3). Median age of the patients in the MA group was lower than in the NMA group (38 vs. 55 years; P = <0.0001). Acute graft versus host disease (GVHD) prophylaxis was based on tacrolimus in 51 (58.6%) patients and on cyclosporine in 36 (41.4%) patients. Antithymocyte globulin was added in unrelated donors. Donors were human leukocyte antigen (HLA)-identical siblings in 62 cases (71.3%) and unrelated volunteer donors in 25 cases (28.7%). Unrelated donors were matched for 16 patients (64%) and mismatched for nine patients (36%). Most of the patients (75/80; 87.5%) were positive for cytomegalovirus (CMV), and the majority of CMV positive donors were related (52/64; 81.2%). The source of stem cells was peripheral blood in 62 (71.3%) patients and bone marrow in 25 (28.7%) patients. Median time from diagnosis to transplant was 8.8 months (range 1.6–90.8) with significantly longer time for unrelated donors (14.7 vs. 7.2 months; P = 0.001).
Table 1 Patient characteristics (n = 87)*
Graft versus host disease
Acute GHVD of all grades (I–IV) occurred in 54 patients (62%) and grades III–IV in 12 cases (13.7%). Among patients surviving more than 100 days, chronic GVHD appeared in 21/75 (28%) patients (12 cases with moderate and severe disease).
Survival
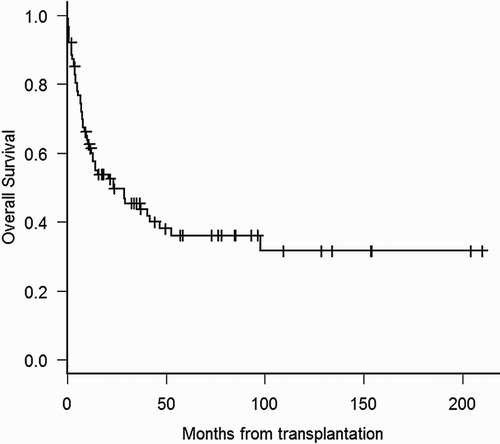
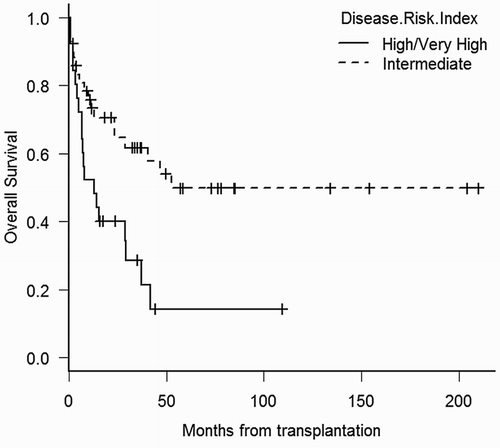
After a median of 4-year follow-up among surviving patients (range 0.3–17 years), 51 patients died at a median of 7.5 months (range 2 days–8 years). For all patients, unadjusted median OS was 23.5 months (95% CI 1.7–45.3 months) and the probabilities of OS at the first and fourth year were 61% (95% CI 50–71%) and 38% (95% CI 27–49%), respectively (Fig. ). Median time of unadjusted PFS was 19.9 months (95% CI 9–31 months), and the probability of PFS at year 1 and 4 were 57% (95% CI 46–67%) and 37% (95% CI 26–48%), respectively. In the univariate analysis, factors associated with a trend towards improved outcome were: age younger than 50 years, less than 5% blasts in bone marrow before transplant, intermediate DRI, and development of acute GVHD (all grades). Among patients surviving more than 100 days, chronic GVHD was associated with better outcome (Table ). In the multivariate analysis for OS (n = 68), patients with intermediate DRI had improved OS with a hazard ratio (HR) of 0.34 (95% CI 0.17–0.71; P = 0.004) (Fig. ). For PFS, multivariate analysis confirmed intermediate DRI (HR 0.32; 95% CI 0.15–0.66; P = 0.002) and acute GVHD all grades (HR 0.33; 95% CI 0.16–0.68; P = 0.003) as variables associated to better PFS.
Table 2 Univariate analysis for overall survival and progression-free survival*
Relapse and non-relapse mortality
Overall, 28 patients relapsed after transplant. Cumulative incidence of relapse was 5%, 21%, and 29% at 100 days, 1 year, and 2 years, respectively. Factors associated with lower relapse incidence were intermediate DRI (Fig. ) and development of both acute and chronic GVHD (Table ). Intermediate DRI and acute GVHD were confirmed to have better outcome in the multivariate analysis (intermediate DRI vs. high/very high DRI, HR 0.20, 95% CI 0.08–0.52, P = 0.0001; and presence vs. absence of GVHD, HR 0.29, 95% CI 0.11–0.74, P = 0.009; chronic GVHD was not included in the model). We also analyzed the influence of the intensity of conditioning in the relapse incidence. There was no significant differences in relapse incidence between MA and NMA according to disease status at transplant (persistent disease 49% vs. 61.1%, P = 0.862; CR: 53% vs. 29%, P = 0.835; and untreated disease: 10% vs. 42%; P = 0.084), DRI (high/very high risk: 45% vs. 74%, P = 0.405; and intermediate risk: 22% vs. 10%; P = 0.611), or percentage of blasts pre-transplantation (<5%: 27% vs. 17%, P = 0.765; and ≥5%: 32% vs. 56%, P = 0.368).
Figure 3 Unadjusted cumulative incidence of relapse according to the disease risk index (P = 0.001).
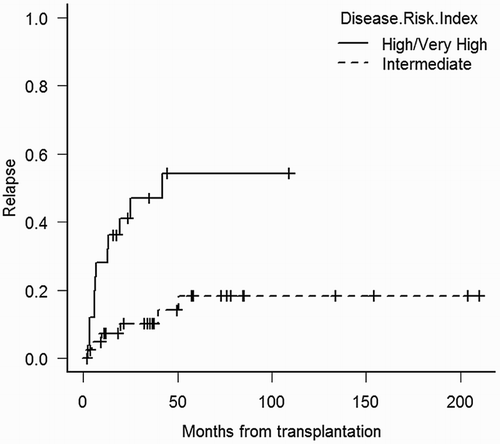
Table 3 Analysis of cumulative incidence of relapse and non-relapse mortality*
Cumulative incidence of NRM was 13%, 20%, 25% at 100 days, 1 year, and 2 years, respectively. No events of NRM were observed after 3 years post-transplantation. Causes of NRM were: infections (n = 12), GVHD (n = 6), hemorrhages (n = 2), brain tumor (n = 1), and other causes (n = 2). No differences were observed in cumulative incidence of NRM between related and unrelated donors, or between MA and NMA conditioning regimen (Table ). No factors were found to be independently associated with a higher NRM.
Throughout the years of transplantation (1995–2000; 2001–2005; 2006–2010; 2011–2013), no difference was found in terms of relapse incidence or NRM incidence. This may be explained due to the variations in the practice of transplants: percentage of unrelated donors (0%, 12%, 34%, and 48%, respectively; P = 0.0006) and median age of patients at transplant (32, 35, 49, and 44 years old, respectively; P = 0.0001) increased along the years, whereas MA regimens decreased (92%, 82%, 48%, and 78%, respectively; P = 0.1884).
Discussion
Herein, we present the results of 87 adult patients with MDS who underwent AHSCT in Argentina. It was a relatively young cohort with a median age of 43 years (range 18–66), half of the patients had RAEB/sAML subtype, the main (60%) conditioning regimen was MA regimen and there was a considerable proportion of unrelated donors (29%). Despite the heterogeneity of the patient population and preparatory regimens, a 4-year OS of 38% in our series is comparable with previous reports by other authors.Citation20–Citation22 Also, our results confirm that survival of patients with related and unrelated donors yield similar values, even though the time to transplant was longer in cases of unrelated donors.Citation4
We found that the combination of cytogenetics and disease status at transplant, both incorporated in the previously described DRI for MDS,Citation14 was an independent factor to predict PFS, OS, and relapse. Both, cytogeneticsCitation3,Citation6,Citation7 and disease status at transplantCitation1,Citation9,Citation10,Citation21 have been consistently associated with poorer outcome in a separate manner. Poor cytogenetics, defined as chromosome 7 abnormalities and/or complex karyotype, have been associated with higher relapse rate and lower PFS.Citation1,Citation6,Citation7 OS, PFS, and relapse incidence in patients with poor karyotype showed a negative trend; even though, it was not significant. A lower percentage of patients with poor cytogenetics (16%) compared with previously mentioned reports (∼30%) may account for this difference. Definition of disease status at transplant as prognostic factor has been variable. Sierra et al. and Warlick et al. found that more than 5% of blasts in bone marrow before transplant was associated with higher relapse incidence and lower PFS, and Hiramoto et al. reported a cut off of ≥20% blasts associated with worse OS.Citation1,Citation9,Citation21 In our series, ≥5% blasts in bone marrow before transplant was associated with lower OS and PFS but this finding was not confirmed in the multivariate analysis. Busca et al. suggested that advanced disease, defined as relapse/refractory disease (vs. CR/untreated), was an independent variable associated with inferior PFS.Citation10 We applied this definition and included it into DRI, as previously reported.Citation14 The advantage of DRI stratification is probably associated to the identification of patients with good and intermediate cytogenetics but bad response to pre-transplantation treatment. The result of the worst karyotype during the evolution and before transplantation was also considered for the analysis.Citation13
Active treatment with intensive chemotherapy or hypomethylating agents previous to transplant in patients with MDS is controversial, and some authors have suggested that a selection bias may be playing a role in the results of clinical studies.Citation23 In line with this, a clear advantage of MA conditioning versus reduced intensity conditioning has not been demonstrated.Citation24 In our series, MA conditioning only showed a non-significant benefit in patients untreated at transplant and no differences were found regarding the percentage of blasts in bone marrow before transplant. Furthermore, chemotherapy response and hypomethylating response may have different consequences, as it has been demonstrated that failure to respond to hypomethylating agents does not adversely affect the outcome of transplantation.Citation25
Acute GVHD of all grades and chronic GVHD were prognostic factors in our study. Presence of acute GVHD (all grades) was an independent variable associated with better PFS and lower relapse incidence. Since chronic GVHD was not analyzed as a time-dependent covariate, it was not included in the multivariate analysis, but it was associated with better OS, PFS, and relapse incidence in the univariate analysis. Both, acute and chronic GVHD have been associated with inferior relapse incidence and better PFS and OS by other authors, probably as a surrogate marker of the graft versus leukemia effect.Citation19,Citation26–Citation28
No factors were associated with NRM in our study. Neither the conditioning regimen nor the type of donor affected the incidence of NRM. Guardiola et al., in a multicenter retrospective study of 234 patients who underwent transplantation from HLA-identical siblings, showed a significantly reduced 2-year NRM using peripheral blood vs. bone marrow as stem cell source.Citation29 Our series was heterogeneous in terms of stem cell source, and the outcome was not different between bone marrow and peripheral blood. In agreement with this, a recent analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) has shown no difference in efficacy between bone marrow and peripheral blood stem cells, except for patients with chronic myeloid leukemia.Citation30 Age at transplantation has been suggested as a predictive factor for NRM. We found that patients ≥50 years old did not have significant higher values of NRM and lower OS. Sierra et al., in a retrospective analysis from IBMTR, demonstrated that patients older than 45 years of age had the highest relative risk of NRM.Citation1 More recent data by Shimoni et al. also showed that patients ≥50 years old had inferior OS and that NRM was higher in patients with hematopoietic cell transplant-co-morbidity index greater than 2.Citation31
We had a considerable number of missing data that may diminish the statistical power of our results. Cytogenetics and blasts number before transplantation were missing in 19 (22%) and 24 (28%) patients, respectively. Also, the retrospective nature of the study, along with the long period of analysis, may explain the lower rate of chronic GVHD (28%) compared to previous reports.Citation20 Other factors already known to be associated with outcome in patients with MDS who underwent AHSCT, such as CD34+ and CD3+ doses and age of donors, were not analyzed in this study.Citation5,Citation32 CMV status in our population merits further comment. Most of the patients (75/80; 87.5%) were CMV seropositive. This figure is similar to the previously published data for Argentinean patients undergoing AHSCT.Citation33 Also, almost all cases had CMV seropositivity of the donor and/or the recipient (79/80; 98.7%). CMV seropositivity has been associated with an adverse prognosis after allogeneic transplantation decreasing OS.Citation34 Thus, this may be an important issue in populations with high prevalence of CMV infection.
Conclusion
In this series of Argentinean patients with MDS who underwent AHSCT, a considerable number of patients achieved long-term survival. The DRI for MDS may be a useful tool to identify patients with higher post-transplant relapse risk.
Disclaimer statements
Contributors Conception and design: ALB, JA, and MVP. Data analysis and interpretation: ALB, JA, MV, and MMR. Manuscript writing: ALB, MMR, and MCF. Provision of study materials or patients: JA, MCF, MMR, GR, GK, JHM, JA, GJ, JJG, and JMR. Final approval of manuscript: ALB, MMR, GR, GK, VM, MCF, SS, JHM, JA, GJ, JMR, GK, JJG, and MVP.
Funding None.
Conflicts of interest The authors have stated that they have no conflicts of interest.
Ethics approval The study was approved by Institutional Ethics Committee for Health Research of the Hospital Privado de Córdoba.
References
- Sierra J, Pérez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood 2002;100(6):1997–2004.
- Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Pérez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004;104(2):579–85. doi: 10.1182/blood-2004-01-0338
- Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood 2002;100(4):1201–7. doi: 10.1182/blood-2002-02-0527
- Ho AY, Pagliuca A, Kenyon M, Parker JE, Mijovic A, Devereux S, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood 2004;104(6):1616–23. doi: 10.1182/blood-2003-12-4207
- Kröger N, Zabelina T, de Wreede L, Berger J, Alchalby H, van Biezen A, et al. Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia 2013;27(3):604–9. doi: 10.1038/leu.2012.210
- Nevill TJ, Fung HC, Shepherd JD, Horsman DE, Nantel SH, Klingemann HG, et al. Cytogenetic abnormalities in primary myelodysplastic syndrome are highly predictive of outcome after allogeneic bone marrow transplantation. Blood 1998;92(6):1910–7.
- Chang C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood 2007;110(4):1379–87. doi: 10.1182/blood-2007-02-076307
- Alessandrino EP, Della Porta MG, Bacigalupo A, Van Lint MT, Falda M, Onida F, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Blood 2008;112(3):895–902. doi: 10.1182/blood-2008-03-143735
- Warlick ED, Cioc A, Defor T, Dolan M, Weisdorf D. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant 2009;15(1):30–8. doi: 10.1016/j.bbmt.2008.10.012
- Busca A, Pecoraro C, Giaccone L, Bruno B, Allione B, Corsetti MT, et al. Allogeneic stem cell transplant for adults with myelodysplastic syndromes: relevance of pre-transplant disease status. Leuk Lymphoma 2014;55(4):863–9. doi: 10.3109/10428194.2013.816422
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International Scoring System for evaluating prognosis in myelodysplastic syndromes. Blood 1997;89(6):2079–88.
- Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised International Prognostic Scoring System for myelodysplastic syndromes. Blood 2012;120(12):2454–65. doi: 10.1182/blood-2012-03-420489
- Armand P, Deeg HJ, Kim HT, Lee H, Armistead P, de Lima M, et al. Multicenter validation study of a transplantation-specific cytogenetics grouping scheme for patients with myelodysplastic syndromes. Bone Marrow Transplant. 2010;45(5):877–85. doi: 10.1038/bmt.2009.253
- Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 2012;120(4):905–13. doi: 10.1182/blood-2012-03-418202
- Della Porta MG, Alessandrino EP, Bacigalupo A, van Lint MT, Malcovati L, Pascutto C, et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood 2014;123(15):2333–42. doi: 10.1182/blood-2013-12-542720
- Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004
- Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statist Med. 1999;18(6):695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. doi: 10.1038/bmt.2012.244
- Valcárcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26(4):577–84. doi: 10.1200/JCO.2007.11.1641
- Hiramoto N, Kurosawa S, Tajima K, Okinaka K, Tada K, Kobayashi Y, et al. Positive impact of chronic graft-versus-host disease on the outcome of patients with de novo myelodysplastic syndrome after allogeneic hematopoietic cell transplantation: a single-center analysis of 115 patients. Eur J Haematol. 2014;92(2):137–46. doi: 10.1111/ejh.12214
- Robin M, Porcher R, Adès L, Raffoux E, Michallet M, François S, et al. HLA-matched allogeneic stem cell transplantation improves outcome of higher risk myelodysplastic syndrome A prospective study on behalf of SFGM-TC and GFM. Leukemia. 2015; http://doi.org/10.1038/leu.2015.37. [Epub ahead of print].
- Kindwall-Keller T, Isola LM. The evolution of hematopoietic SCT in myelodysplastic syndrome. Bone Marrow Transplant. 2009;43(8):597–609. doi: 10.1038/bmt.2009.28
- Luger SM, Ringdén O, Zhang MJ, Pérez WS, Bishop MR, Bornhauser M, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203–11. doi: 10.1038/bmt.2011.69
- Oran B, Popat U, Andersson B, Champlin R. Allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. 2013;13(Suppl. 2):S282–8. doi: 10.1016/j.clml.2013.07.012
- Castro-Malaspina H, Harris RE, Gajewski J, Ramsay N, Collins R, Dharan B, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood 2002;99(6):1943–51. doi: 10.1182/blood.V99.6.1943
- Laport GG, Sandmaier BM, Storer BE, Scott BL, Stuart MJ, Lange T, et al. Reduced intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14(2):246–55. doi: 10.1016/j.bbmt.2007.11.012
- Gyurkocza B, Gutman J, Nemecek ER, Bar M, Milano F, Ramakrishnan A, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(4):549–55. doi: 10.1016/j.bbmt.2014.01.009
- Guardiola P, Runde V, Bacigalupo A, Ruutu T, Locatelli F, Boogaerts MA, et al. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor–mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood 2002;99(12):4370–8. doi: 10.1182/blood.V99.12.4370
- Eapen M, Logan BR, Appelbaum FR, Antin JH, Anasetti C, Couriel DR, et al. Long-term survival after transplantation of unrelated donor peripheral blood or bone marrow hematopoietic cells for hematologic malignancy. Biol Blood Marrow Transplant. 2015;21(1):55–9. doi: 10.1016/j.bbmt.2014.09.006
- Shimoni A, Shem-Tov N, Volchek Y, Danylesko I, Yerushalmi R, Nagler A. Allo-SCT for AML and MDS with treosulfan compared with BU-based regimens: reduced toxicity vs reduced intensity. Bone Marrow Transplant. 2012;47(10):1274–82. doi: 10.1038/bmt.2012.4
- Servais S, Porcher R, Xhaard A, Robin M, Masson E, Larghero J, et al. Pre-transplant prognostic factors of long-term survival after allogeneic peripheral blood stem cell transplantation with matched related/unrelated donors. Haematologica 2014;99(3):519–26. doi: 10.3324/haematol.2013.089979
- Basquiera AL, Pizzi S, Correas AG, Longo PG, Goldman WC, Prates MV, et al. Allogeneic hematopoietic stem cell transplantation in pediatric myelodysplastic syndromes: a multicenter experience from Argentina. Pediatr Blood Cancer 2015;62(1):153–7. doi: 10.1002/pbc.25238
- Schmidt-Hieber M, Labopin M, Beelen D, Volin L, Ehninger G, Finke J, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood 2013;122(19):3359–64. doi: 10.1182/blood-2013-05-499830