Abstract
Objectives: The role of CD40–CD154 (CD40L) interaction in T cell-dependent humoral immune response is strongly established. Increased expression of CD154 on stimulated T cells is observed in rheumatic diseases and is associated with disease activity. We investigated the expression of CD154 on T cells from idiopathic thrombocytopenic purpura (ITP) patients and assessed the significance of CD154 expression in disease status.
Methods: We enrolled 59 ITP patients, 23 healthy controls, and 19 patients with non-immune thrombocytopenia. Isolated mononuclear cells were stimulated in RPMI medium containing phorbol myristate acetate (PMA) (5 ng/mL) and ionomycin (500 ng/mL) for 2 h at 37°C. The expression of CD154 on CD4+T cells was evaluated using flow cytometry and serum soluble CD40L levels were measured.
Results: In ITP patients, the percentage of CD4+ CD154+ cells and mean fluorescence intensity (MFI) of CD154 on activated CD4+T cells was not different from that in the healthy controls and non-immune thrombocytopenia patients. Additionally, the percentage and expression level of CD154 was not different between ITP patients with low platelet counts (<50 000/μL) and those with 50 000/μL or more. Soluble CD40L levels were significantly lower in ITP patients with low platelet counts than in healthy controls, but were not correlated with CD154 expression.
Conclusion: Increased CD154 expression on CD4+T cells was not observed in ITP patients and was not related with low platelet counts. Overexpression of CD154 on CD4+T cells is unlikely to be central to the pathogenesis of ITP, and other immune dysfunctions should be targeted for therapy purposes.
Introduction
Idiopathic thrombocytopenic purpura (ITP) is a disorder in which autoantibodies accelerate platelet destruction and interfere with megakaryopoiesis. The production of autoantibodies by B cells is dependent on cellular mechanisms initiated by glycoprotein IIb/IIIa-reactive CD4+T cells.Citation1 Triggering of a T cell-dependent humoral immune response typically involves the interaction of CD154 (CD40 ligand, CD40L) on activated T cells with CD40 present on B cells. CD154 is a molecule that is transiently expressed on activated CD4+T cells and binds to CD40 on B cells, resulting in B cell proliferation and differentiation.Citation2
The CD40–CD154 interaction has been studied in the context of several autoimmune rheumatic diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and psoriatic arthritis.Citation3–Citation5 An increase in expression of CD154 on stimulated T cells is observed in patients with rheumatic disease and is implicated in disease activity. The importance of CD40–CD154 interaction in the pathogenesis of ITP has been recognized and few studiesCitation6–Citation8 suggest that blocking CD40–CD154 interaction can lead to the suppression of autoreactive T cells and therefore could be an effective therapeutic strategy for the treatment of ITP.
However, the assertion that expression of CD154 on activated T cells increases in ITP patients remains contentious. Webber et al.Citation9 reported that the pattern of CD154 expression on stimulated T cells did not differ between ITP patients and healthy controls. Contrary to this, Meabed et al.Citation10 noted that the expression of CD154 on CD4+T lymphocytes was significantly higher in acute and chronic ITP patients than in healthy controls, and it was higher in acute ITP patients than in chronic ITP patients.
Other than T cells, activated platelets also express CD154. A previous study found that CD154 expression on platelets increased in ITP and induced the proliferation of B lymphocytes.Citation6 The involvement of platelet-associated CD154 in the pathogenesis of ITP supports the speculation that CD154 expression on T cells might have a similar role.
Soluble CD40L (sCD40L) in the serum sloughs from activated T lymphocytes and platelets and elevated sCD40L levels have been reported in cases of SLE and RA along with enhanced CD154 expression on T lymphocytes.Citation11,Citation12 On the other hand, studies on sCD40L levels in ITP patients have provided conflicting results. Nagahama et al.Citation13 reported that sCD40L level was significantly higher in ITP patients than in normal controls and non-immune thrombocytopenia patients. Whereas Feng et al.Citation14 demonstrated low levels of sCD40L in ITP patients and suggested the elevation of sCD40L in blood does not give indication of the immune system activation status.
Solely based on previous observations, it is unclear if CD154 expression on T lymphocytes from ITP patients could induce the production of pathogenic antibodies. In this study, we aimed to determine the relative expression of CD154 on activated T cells from ITP patients compared to controls and to study the impact of CD154 expression on disease severity and response to treatment.
Materials and methods
Patient population and samples
Fifty-nine adult patients were enrolled in this study and the diagnosis of ITP was based on the American Society of Hematology guidelines.Citation15 Of the 59 patients, 21 (age range 19–58 years, 3 men and 18 women) had been newly diagnosed with ITP and 38 (age range 20–85 years, 8 men and 30 women) had chronic ITP with a history of previous treatment. Among the 38 chronic ITP patients, 16 had a platelet count ≥50 000/μL but <100 000/μL, and 22 had platelet counts less than 50 000/μL.
Heparinized whole blood was obtained from ITP patients. Serum was separated from collected blood in serum vacuum tube (Greiner Bio-one GmbH, Kremsmünster, Austria) by immediate centrifugation and then was stored at −70°C until analysis. Patients did not receive therapy for at least 2 weeks prior to analysis. In addition, blood specimens from 23 healthy controls and 19 patients with non-immune thrombocytopenia were collected. Non-immune thrombocytopenia patients included eight cases of liver cirrhosis, six of aplastic anemia, three of myelodysplastic syndrome, and two of hypomegakaryocytic thrombocytopenia.
Platelet counts and immature platelet fraction (IPF) percentages were measured using an automated hematology analyzer (XE-2100, Sysmex Corp, Kobe, Japan) and CD4+T cell counts were quantified using the CELL-DYN Immuno T-cell reagent kit (Abbott Diagnostics, Santa Clara, CA, USA).
Flow cytometric detection of CD154 expression
Heparinized whole blood was diluted with phosphate-buffered saline (PBS; 1:1) and then layered on Ficoll-Paque separation media (GE Healthcare Bio-Science AB, Uppsala, Sweden). Peripheral blood mononuclear cells (PBMC) were separated by centrifugation and were placed in RPMI 1640 culture medium supplemented with 10% fetal calf serum and 2 mM glutamine. The cells were stimulated in 48-well plates (1 × 106 cells/well) using 5 ng/mL phorbol myristate acetate (PMA) (Sigma Aldrich, St Louis, MO, USA) and 500 ng/mL ionomycin (Sigma Aldrich) at 37°C, 5% CO2 for 2 h. The 2 h was optimal to induce CD154 expression preserving CD4 expression based on the preliminary experiments. After stimulation, cells were collected and washed with PBS (1500 rpm for 10 min) and subsequently 5 × 105 cells were resuspended in 100 μL of PBS.
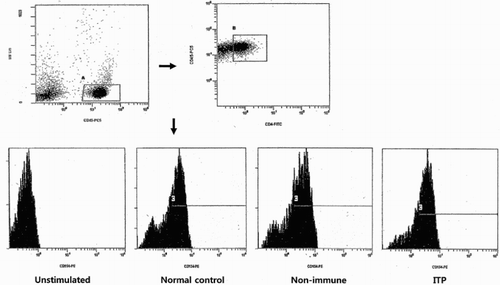
The following monoclonal antibodies (Beckman Coulter, Marseille, France) to human antigens were used: anti-CD4 fluorescein-isothiocyanate (FITC); anti-CD45 phycoerythrin-cyanine 5 (PC5); anti-CD154 conjugated with phycoerythrin (PE). The stimulated cells (100 μL) were stained with 5 μL of anti-CD45-PC5 mAbs, 10 μL of anti-CD4-FITC mAbs, and 10 μL of anti-CD154-PE mAbs. The mixture was incubated for 40 min at room temperature in the dark and then washed three times with PBS prior to analysis. Isotype-matched antibody controls were used to detect nonspecific staining. The samples were analyzed using a Cytomics FC 500 flow cytometer (Beckman). In the flow cytometric analysis, lymphocytes were first gated in a side scatter (SS) and CD45 fluorescence, and then, CD4+T cells was isolated from lymphocytes. The CD154 expression on gated CD4+T cells was analyzed (Fig. ).
Figure 1 CD154 expression on unstimulated CD4+T cells and stimulated CD4+T cells in representative normal control, non-immune thrombocytopenia patient, and idiopathic thrombocytopenic purpura (ITP) patient. Lymphocytes (A) were gated in a side scatter (SS)/CD45 fluorescence dot plot, and then CD4+T cells (B) within indicated lymphocytes were isolated. Histogram displayed fluorescence intensity of CD154 on the X axis and CD4+CD154+T cells (C) were analyzed.
Measurement of serum sCD40L levels
The serum sCD40L levels were measured using an enzyme-linked immunosorbent assay according to the manufacturer's instructions (eBiosciences, San Diego, CA, USA). All samples were diluted in a ratio of 1:5 with the sample diluent provided in the kit and analyzed in duplicates. The average of the optical density values was calculated as the concentration. Values lower than the limit of detection were assigned the same value as the minimum detection limit. The minimum detection limit for sCD40L was 0.06 ng/mL and intra- and inter-assay coefficients of variation were 4.0 and 6.8%, respectively.
Statistical analysis
All statistical analyses were performed using MedCalc Statistical Software 11.3.0.0 (MedCalc Software, Mariakerke, Belgium). Continuous variables are presented as the mean ± SD. We evaluated the intergroup comparisons using either a t-test or a Mann–Whitney U-test. To assess the correlations between two parameters, the linear regressions were calculated. A P-value of less than 0.05 was considered significant.
Results
The characteristics of the patient populations studied are summarized in Table . The mean platelet count and IPF% in ITP patients were 39 ± 27 × 103/μL and 10.9 ± 8.0%, respectively. The IPF% in ITP patients was significantly higher than that in healthy controls and patients with non-immune thrombocytopenia (P < 0.0001, P = 0.0008). In addition, the mean IPF% in ITP patients with low platelet counts (<50 000/μL) was higher than that in ITP patients with platelet counts of 50 000/μL or more (P < 0.0001). We also noted that there was inverse correlation between platelet count and IPF% in ITP patients (r = −0.6251, P < 0.0001). The percentage of CD4+T lymphocytes and CD4+T cell counts in ITP patients were not different from those in healthy controls and non-immune thrombocytopenia patients. Similarly, there was no difference in the percentage of CD4+T lymphocytes and CD4+T lymphocyte counts between the two groups of ITP patients categorized according to platelet count.
Table 1 Characteristics of ITP patients and controls
The percentages of CD154 expression on activated CD4+T cells were 62.6 ± 10.3% in ITP patients, 59.6 ± 14.2% in healthy controls, and 60.5 ± 12.5% in patients with non-immune thrombocytopenia. These results show that CD154 expression was not significantly different between ITP patients and healthy control group or non-immune thrombocytopenia group. The mean fluorescence intensity (MFI) of CD154 on CD4+CD154+T cells and on CD4+T cells in ITP patients was 3.15 ± 0.62 and 2.23 ± 0.60, respectively. The expression level of CD154 in ITP group showed no significant difference compared to control and non-immune thrombocytopenia groups.
In 43 ITP patients who had low platelet counts (<50 000/μL), percentage of CD154 expression on CD4+T cells was 63.4 ± 10.7% and did not differ from that in the controls, non-immune thrombocytopenia patients, and 16 ITP patients with platelet counts of 50 000/μL or more. In ITP patients with low platelet counts, the MFI on CD4+CD154+T cells and CD4+T cells was 3.21 ± 0.62 and 2.24 ± 0.61, respectively. The CD154 expression levels on CD4+CD154+T cells and CD4+T cells in this group were not significantly higher than that in healthy controls, non-immune thrombocytopenia patients, and ITP patients with platelet counts of 50 000/μL or more (Table ).
Table 2 CD154 expression on CD4+T cells and serum soluble CD40 ligand in ITP patients and controls
Among the 21 newly diagnosed ITP patients, 14 received first line treatment, such as corticosteroids or IVIg, and eight responded to the treatment showing a platelet count ≥30 000/μL or a doubling of the baseline platelet counts. The percentage of CD4+CD154+T cells did not differ between the response group and non-response group (60.7 ± 10.1% vs. 65.5 ± 9.3%, P = 0.30), and CD154 MFI on CD4+CD154+T cells in the response group was not significantly different from that in the non-response group (3.02 ± 0.40 vs. 3.35 ± 0.69, P = 0.27).
The sCD40L levels in ITP patients were 1.47 ± 1.05 ng/mL and lower than those in healthy controls, but the difference was not statistically significant (P = 0.06). Moreover the decrease in sCD40L levels in the ITP group was similar to the decrease in the non-immune thrombocytopenia group. In addition, there was no difference between ITP sub-groups with respect to the platelet counts (P = 0.24). The sCD40L levels did not show any difference between responders and non-responders (1.13 ± 0.85 ng/mL vs. 0.75 ± 0.47 ng/mL, P = 0.36). However, sCD40L levels in ITP patients with low platelets were significantly lower compared to healthy controls (P = 0.047).
In all ITP patients, platelet counts showed significant inverse correlation with IPF, but no significant correlation was found between platelet counts and sCD40L (r = 0.2203, P = 0.09). The percentages of CD154 expression, CD154 MFI on CD4+CD154+T cells, and CD4+T cell counts were also not correlated with platelet counts. We also observed that sCD40L levels were not correlated with percentages of CD154 expression, CD154 MFI on CD4+CD154+T cells, and CD4+T cell counts (Table ).
Table 3 Correlation of platelet counts and serum soluble CD40 ligand with other parameters in ITP patients
Discussion
The CD154 molecule, also known as the CD40 ligand, is barely detectable on resting T cells and is transiently expressed on the surface of activated T cells. The pivotal role of the interaction between CD154 and its counterreceptor CD40 was first discovered in hyper-immunoglobulin M syndrome.Citation16 Since then, the overexpression of CD154 on the surface of CD4+T cells has been reported in many diseases characterized by immune system activation, including rheumatic diseases and human immunodeficiency virus type 1 (HIV-1) infection.Citation3–Citation5,Citation17 It was also noted that this overexpression reduced after antiretrovirus therapy in HIV-1 infection.Citation17
Kuwana et al.Citation1,Citation8 demonstrated that autoreactive CD4+T cells are involved in the pathogenic process of ITP, and CD154 is likely to be a potential target for therapy in refractory ITP. Nevertheless, it remains to be elucidated whether increased expression of CD154 in T lymphocytes contributes to ITP.Citation9,Citation10
In the preliminary experiment, the expression of CD154 in the unstimulated CD4+T cells was not detected and T cell stimulation was necessary to induce CD154 expression. Because it was reported that CD154 expression in T cells was detected at 6 h and was evident at 24 h in both ITP patients and normal controlsCitation9 upon stimulation with PMA and ionomycin, we selected 6 h stimulation. However, the expression of CD4 in gated lymphocytes was significantly weakened after 6 h stimulation. So we incubated PBMC at different time points (2, 4, and 6 h) in culture medium and ascertained that expression of CD154 in CD4+T cells was induced without weakening of CD4 expression after 2 h stimulation. And the expression level of CD154 at different time points was not significantly changed in both ITP patients and normal controls. Based on these data, we finally chose 2 h as an optimal time point.
In this study, we did not find an increase in the expression of CD154 on activated CD4T cells from ITP patients. The percentage of CD4+CD154+T cells in ITP patients was indistinguishable from that in healthy controls and non-immune thrombocytopenia patients, and the expression level of CD154 on CD4+CD154+T cells also did not show any difference between groups. These findings support that T lymphocytes of ITP do not overexpress CD154 and the role of this molecule in the pathogenic antibody production may be minor.
Indeed, percentage of CD4+CD154+T cells and CD154 expression level on CD4+CD154+T cells did not show any significant difference between ITP patients with low platelet counts of less than 50 000/μL and those with platelet counts of 50 000/μL or more. There was also no difference in these parameters between treatment-responsive group and non-responsive group. These data suggest that expression of CD154 on activated CD4T cells is not associated with disease severity and unfavorable prognosis.
Solanilla et al.Citation6 studied in vitro culture of B lymphocytes and autologous platelets and reported that surface expression of platelet CD154 increased in ITP and platelet-associated CD154 was implicated in the production of autoantibodies. Unlike platelet-associated CD154, we failed to demonstrate the change in expression of CD154 on activated T lymphocytes. It must be acknowledged that CD154 expression on activated platelets was not measured in this study, as we assumed that T lymphocytes do not share the same expression derangement of surface molecule with platelets, and CD154 on T lymphocytes may not be a pathogenic in ITP.
A soluble isoform of CD40L that results from the cleavage of the surface molecule expressed on activated T cells and platelets can be detected in the serum of healthy individuals. The levels of sCD40L have been reported to be elevated in patients with SLE, RA, Sjogren's syndrome, systemic vasculitis, and lymphoproliferative disorders.Citation11,Citation12,Citation18,Citation19 To the best of our knowledge, data regarding serum sCD40L levels in ITP are limited and inconsistent.
In our study, sCD40L levels in ITP patients were lower than those in normal controls, which was consistent with previous reports.Citation14,Citation20 Moreover, sCD40L levels in ITP patients with low platelet counts were significantly lower than those in control patients. Previous studies demonstrated that platelets are the main source of sCD40L and over 95% of sCD40L in plasma originates from platelets.Citation21,Citation22 Although significant correlation between platelet counts or IPF and sCD40L was not observed in this study, we hypothesize that low levels of sCD40L in ITP result from low platelet counts.
In HIV infection, sCD40L levels significantly correlate with CD4+T cell countsCitation23 and reflect the presence of activated CD4+T cells. In contrast, we have shown here that sCD40L levels were not related to CD154 expression on CD4+T cells or CD4+T cells counts. Our findings indicate that the main source of sCD40L in ITP seems to be platelets, not T lymphocytes, and that serum sCD40L levels do not indicate the presence of activated T cells.
In summary, we confirmed that CD4+T lymphocytes from ITP patients do not overexpress CD154 and CD154 expression level may not be related to the disease severity or to treatment efficacy. Therefore, it is unlikely that overexpression of CD154 on CD4+T cells plays a central role in the development of ITP and other immune dysfunctions should be targeted for therapy.
Disclaimer statements
Contributors JK designed the research study and analyzed the data; IS and DJ performed the research; KK and SK reviewed the manuscript.
Funding None.
Conflicts of interest The authors have no conflict of interests to declare.
Ethics approval The authors received IRB approval for this study.
References
- Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102(7):1393–402. doi: 10.1172/JCI4238
- Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114(9):1198–208. doi: 10.1172/JCI23411
- Koshy M, Berger D, Crow MK. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996;98(3):826–37. doi: 10.1172/JCI118855
- Berner B, Wolf G, Hummel KM, Muller GA, Reuss-Borst MA. Increased expression of CD40 ligand (CD154) on CD4+T cells as a marker of disease activity in rheumatoid arthritis. Ann Rheum Dis. 2000;59(3):190–5. doi: 10.1136/ard.59.3.190
- Daoussis D, Antonopoulos I, Andonopoulos AP, Liossis SN. Increased expression of CD154 (CD40L) on stimulated T-cells from patients with psoriatic arthritis. Rheumatology 2007;46(2):227–31. doi: 10.1093/rheumatology/kel229
- Solanilla A, Pasquet JM, Viallard JF, Contin C, Grosset C, Dechanet-Merville J, et al. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood 2005;105(1):215–8. doi: 10.1182/blood-2003-07-2367
- Patel VL, Schwartz J, Bussel JB. The effect of anti-CD40 ligand in immune thrombocytopenic purpura. Br J Haematol. 2008;141(4):545–8. doi: 10.1111/j.1365-2141.2008.07039.x
- Kuwana M, Nomura S, Fujimura K, Nagasawa T, Muto Y, Kurata Y, et al. Effect of a single injection of humanized anti-CD154 monoclonal antibody on the platelet-specific autoimmune response in patients with immune thrombocytopenic purpura. Blood 2004;103(4):1229–36. doi: 10.1182/blood-2003-06-2167
- Webber NP, Mascarenhas JO, Crow MK, Bussel J, Schattner EJ. Functional properties of lymphocytes in idiopathic thrombocytopenic purpura. Hum Immunol. 2001;62(12):1346–55. doi: 10.1016/S0198-8859(01)00348-2
- Meabed MH, Taha GM, Mohamed SO, El-Hadidy KS. Autoimmune thrombocytopenia: flow cytometric determination of platelet-associated CD154/CD40L and CD40 on peripheral blood T and B lymphocytes. Hematology 2007;12(4):301–7. doi: 10.1080/10245330701383957
- Vakkalanka RK, Woo C, Kirou KA, Koshy M, Berger D, Crow MK. Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheum. 1999;42(5):871–81. doi: 10.1002/1529-0131(199905)42:5<871::AID-ANR5>3.0.CO;2-J
- Tamura N, Kobayashi S, Kato K, Bando H, Haruta K, Oyanagi M, et al. Soluble CD154 in rheumatoid arthritis: elevated plasma levels in cases with vasculitis. J Rheumatol. 2001;28(12):2583–90.
- Nagahama M, Nomura S, Kanazawa S, Ozaki Y, Kagawa H, Fukuhara S. Significance of chemokines and soluble CD40 ligand in patients with autoimmune thrombocytopenic purpura. Eur J Haematol. 2002;69(5–6):303–8. doi: 10.1034/j.1600-0609.2002.02774.x
- Feng X, Scheinberg P, Samsel L, Rios O, Chen J, McCoy Jr. JP, et al. Decreased plasma cytokines are associated with low platelet counts in aplastic anemia and immune thrombocytopenic purpura. J Thromb Haemost. 2012;10(8):1616–23. doi: 10.1111/j.1538-7836.2012.04757.x
- Neunert C, Lim W, Crowther M, Cohen A, Solberg Jr. L, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011;117(16):4190–207. doi: 10.1182/blood-2010-08-302984
- Notarangelo LD, Peitsch MC, Abrahamsen TG, Bachelot C, Bordigoni P, Cant AJ, et al. CD40lbase: a database of CD40L gene mutations causing X-linked hyper-IgM syndrome. Immunol Today 1996;17(11):511–6. doi: 10.1016/S0167-5699(96)80904-2
- Sousa AE, Chaves AF, Doroana M, Antunes F, Victorino RM. Early reduction of the over-expression of CD40L, OX40 and Fas on T cells in HIV-1 infection during triple anti-retroviral therapy: possible implications for lymphocyte traffic and functional recovery. Clin Exp Immunol. 1999;116(2):307–15. doi: 10.1046/j.1365-2249.1999.00872.x
- Younes A, Snell V, Consoli U, Clodi K, Zhao S, Palmer JL, et al. Elevated levels of biologically active soluble CD40 ligand in the serum of patients with chronic lymphocytic leukaemia. Br J Haematol. 1998;100(1):135–41. doi: 10.1046/j.1365-2141.1998.00522.x
- Goules A, Tzioufas AG, Manousakis MN, Kirou KA, Crow MK, Routsias JG. Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. J Autoimmun. 2006;26(3):165–71. doi: 10.1016/j.jaut.2006.02.002
- Fan Y, Ge Y, Zhu H, Wang Y, Yang B, Zhuang Y, et al. Characterization and application of two novel monoclonal antibodies against CD40L: epitope and functional studies on cell membrane CD40L and studies on the origin of soluble serum CD40L. Tissue Antigens 2004;64(3):257–63. doi: 10.1111/j.1399-0039.2004.00257.x
- Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation 2002;106(8):896–9. doi: 10.1161/01.CIR.0000028962.04520.01
- Viallard JF, Solanilla A, Gauthier B, Contin C, Dechanet J, Grosset C, et al. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood 2002;99(7):2612–4. doi: 10.1182/blood.V99.7.2612
- Sipsas NV, Sfikakis PP, Kontos A, Kordossis T. Levels of soluble CD40 ligand (CD154) in serum are increased in human immunodeficiency virus type 1-infected patients and correlate with CD4(+) T-cell counts. Clin Diagn Lab Immunol. 2002;9(3):558–61.
