Abstract
The current status of research and development in Fe-based bulk metallic glasses (BMGs) is reviewed. Bulk metallic glasses are relatively new materials possessing a glassy structure and large section thickness. These materials have an exciting combination of properties such as high mechanical strength, good thermal stability, large supercooled liquid region and potential for easy forming. Ever since the first synthesis of an Fe-based BMG in an Fe–Al–Ga–P–C–B system in 1995, there has been intense activity on the synthesis and characterisation of Fe-based BMGs. These BMGs exhibit some unique characteristics which have not been obtained in conventional Fe-based crystalline alloys. This uniqueness has led to practical uses of these bulk glassy alloys as soft magnetic and structural materials. This review presents the recent results on the glass-forming ability, structure, thermal stability, mechanical properties, corrosion behaviour, soft magnetic properties and applications of Fe-based bulk glassy alloys developed during the last 15 years. This review also highlights the advanced analysis of their properties which has contributed significantly to the progress in understanding and developing of the Fe-based BMGs. The future prospects of Fe-based BMGs have also been presented.
Introduction
Several advanced materials have been developed during the past few decades to meet the ever-growing requirements and demands of the industry.Citation1 Novel and advanced materials have been synthesised which are stronger, harder, stiffer, lighter, highly corrosion resistant, or for use at elevated temperatures. All these have been possible by processing materials through different non-equilibrium processing techniques such as rapid solidification processing, mechanical alloying, plasma processing, spray forming, laser processing and different types of vapour deposition methods.Citation2 Several ‘new’ and advanced materials have also been developed during the last few decades, which include metallic glasses in thin ribbon form,Citation3–Citation5 bulk metallic glasses,Citation6,Citation7 quasicrystals,Citation8,Citation9 high-temperature superconductors, superhard carbo-nitrides, thin-film diamond materials and nanostructured materials.Citation10–Citation13 Metallic glasses are one such class of materials that display interesting combination of properties and have found applications in a variety of industries.Citation3–Citation7
The first synthesis of a metallic glass was achieved in 1960 by rapidly solidifying a metallic liquid of Au–25at-%Si composition at cooling rates of ∼10Citation6 K s−1.Citation14 Since then a very large number of alloy compositions in different alloy systems were synthesised in the glassy state.Citation3–Citation5,7,15–19 However, these metallic glasses were produced in the form of thin ribbons (typically 20–30 μm in thickness), powders or wires, since the critical cooling rate required to form the glassy phases in these alloy compositions was of the order of 105–106 K s−1. And these rates could be achieved only when at least one of the dimensions of the solidified material (section thickness) was a few micrometres in thickness. This limitation on the section thickness has prevented widespread use of these glassy materials in different application fields. For example, glassy alloys in sheet or wire form in the Fe–Si–B and Co–Fe–Si–B systems have been used as soft magnetic materials,Citation20 but there have not been many reports regarding applications of these novel materials in other fields such as structural materials or as functional materials.
Intensive developments during the last 20 years have resulted in the synthesis of metallic glasses with large section thickness or diameter, known as bulk metallic glasses (BMGs).Citation6,Citation7 The observation of the stable phenomenon of supercooled liquid in metallic alloys made it possible to produce glassy alloys in a bulk form with diameters up to ∼72 mm.Citation6,7,21–Citation24 Pioneering work has been done at the Tohoku University in Sendai, Japan and at the California Institute of Technology in Pasadena, California, USA, in this field of study. Research investigations in this field are now being taken up in many other laboratories around the world, notably in China, Korea and India. Bulk metallic glasses have been produced in a large number of alloy systems, for example, those based on Co,Citation25 Cu,Citation26 Fe,Citation27 lanthanum (Ln),Citation28 Mg,Citation29 Ni,Citation30 Pd,Citation31 Pt,Citation32 TiCitation33 and Zr,Citation34,Citation35 with critical rod diameters exceeding 10 mm.Citation36 Bulk metallic glasses have also been synthesised in other alloy systems based on Au,Citation37 Ca,Citation38 Ce,Citation39 Hf,Citation40 Nd,Citation41 Pr,Citation42 SmCitation43 and Y.Citation44 Subsequent efforts by different groups also resulted in the development of BMGs with critical diameters of >20 mm in alloy systems based on Cu,Citation45 Ln,Citation46 Mg,Citation47 Ni,Citation48 Pd,Citation49 PtCitation50 and Zr.Citation51,Citation52 The general features of BMGs have been described in some reviews and books.Citation6,7,22–Citation24
Among the BMG alloys, Fe-based bulk glassy alloys are very attractive because of their excellent soft magnetic properties with rather high saturation magnetisation, high electrical resistivity, high mechanical strength and low materials cost, among others.Citation53 Ever since the first Fe-based bulk glassy alloy was synthesised in the Fe–Al–Ga–P–C–B system in 1995 by a copper-mould casting technique,Citation27 a very large number of Fe-based BMGs including Fe-, Fe–Co-, Fe–Ni- and Fe–Co–Ni-based alloy systems have been developed to date.
This article aims to summarise the details of the developments on the structure, synthesis, properties and applications of Fe-, Fe–Co- and Fe–Co–Ni-based BMGs developed during the last 15 years or so. In spite of the industrial importance of Fe-based alloys, and the possibility of achieving novel attributes for these alloys in the glassy state, there has been only one major review on this topic so far,Citation54 which was written at the early stage of development of Fe-based BMGs. The present article plans to fill that gap by presenting a comprehensive review of the literature in this developing field.
Characteristics of Fe-based BMGs
The first synthesis of an Fe-based BMG with a diameter of 1 mm was reported in 1995 in the Fe–Al–Ga–P–C–B alloy system by Inoue and co-workers.Citation27,Citation55 Since then a large number of Fe-based alloy compositions have been synthesised, with the maximum thickness reaching ∼16 mm.Citation56 presents the details of the composition, synthesis method and the thermal properties of the Fe-based BMGs that have been synthesised till recently.Citation27,55,57–Citation122 The alloy systems have been arranged in such a way that the metalloid content increases on going down the table.
Table 1. Thermal properties of Fe-based BMGs*
Metallic glasses produced by the rapid solidification processing (RSP) method were traditionally classified into the metal–metalloid and metal–metal types. In the metal–metalloid type, the metal content is typically ∼80 at-% and the metalloid content ∼20 at-%. The metal can be either just one element or a combination of more than one element and similarly, the metalloid element also can be either just one or a combination of more than one metalloid element. Typical examples of metal–metalloid type Fe-based metallic glasses produced by the RSP method are: Fe80B20, Fe78B12Si10, Fe80P13C7, Fe40Ni40P14B6, Fe71·3Cr10Mo9P8C1·7 and Fe73·4Cu1Nb3·1B9·1Si13·4. On the other hand, in the metal–metal glasses, there is no such strict compositional requirement. The metal elements can be present in any proportion, and some typical compositions studied include Fe90Zr10 and Fe60Zr40.
In principle, BMGs can also be produced in both the metal–metalloid and metal–metal varieties. But it is interesting to note that all the Fe-based BMGs synthesised so far are of the metal–metalloid type. Here again, the metallic component constitutes ∼80 at-% and the metalloid component ∼20 at-%. Furthermore, like in the case of rapidly solidified alloys, the metal component can be either only Fe or a mixture of different elements. Majority of the alloying elements are typically from the Fe-group elements, i.e. Fe, Co and/or Ni. Occasionally, other metallic elements such as Cr, Mn, Al, Ga, Mo, Zr, Nb and Ta are also added, with their concentrations ranging from as small as a few per cent to as large as nearly 15–20% total. Rare-earth elements such as Y, Er, Gd and Tm are also occasionally added with beneficial effects of increasing the glass formability. The metalloid elements added are typically B, C, P and Si, with their total content amounting to nearly 20 at-%. Bulk metallic glasses have also been produced in Nd–Fe–AlCitation123 and Pr–Fe–AlCitation124 even when the Fe content was >50 at-%. But alloy compositions with useful properties were found to be very lean in Fe content. Accordingly, these will not be discussed further in this review.
summarises the alloy compositions of typical Fe-based BMGs with critical diameters of >1 mm together with the calendar years of their first synthesis. From this table, it becomes clear that the different alloy compositions and the critical diameters listed can be basically classified into two groups based on the solute additions. The first group contains Fe and metalloid elements along with Al and Ga as the main metallic solute elements. These alloys, sometimes, also contain the early transition metals (ETMs).Citation55,Citation104,Citation125,Citation126 On the other hand, the second group is composed of Fe and metalloid elements, and sometimes they also contain ETM elements or lanthanum (Ln).Citation91,98,99,103,105,127–Citation130 The second group can also be described as the Fe–ETM/Ln–metalloid type by putting the ETM and Ln together as ETM/Ln due to their similarity in the location in the periodic table. The second group can be more specifically divided into two series by paying attention to the amount of Fe present in the alloy: alloys with Fe ⩾50 at-% (nos. F5–F9) and the others with Fe <50 at-% (nos. F10–F13). The addition of rare-earth elements (e.g. Y and Tm) seems to increase the glass-forming ability (GFA) of these alloys as evidenced by the synthesis of large diameter BMGs reaching up to 16 mm.Citation56,Citation191 It may be emphasised that all the Fe-based BMGs contain metalloid elements such as P, C, B and Si and belong to the metal–metalloid type system. The significant difference in alloy compositions between BMG alloys and the previously reported thin metallic glass ribbons is attributed to the addition of special alloying elements such as Ln, Ga, Zr, Nb and Mo having a significant atomic size difference and large negative heat of mixing between Fe and the metalloid (P, C, B and/or Si) elements.
Table 2. Typical Fe-based BMG alloy systems reported together with the calendar years when the first synthesis was reported
Glass-forming ability
To produce metallic glasses in a reasonable and reliable way, and also to produce them in large quantities, it is essential that we understand the basic reasons regarding ease of glass formation. The ability of a crystalline metallic alloy to transform into the glassy state is defined as the GFA. There has been reasonable success in predicting the compositions and alloy systems in which thin ribbon glasses could be synthesised by RSP methods during the 1970s and 1980s. But with the discovery of BMGs, the activity in this area has been resumed in recent times. Both the alloy systems and their compositions that are likely to be transformed into the glassy condition have been predicted and experimentally verified in some cases. However, as we will see, it has been noted that these predictions seem to have been made based on a limited amount of data from a few alloy compositions and therefore, these do not seem to be universally applicable. The topic of GFA has been covered in detail earlierCitation6,Citation7 and therefore we will not go into many details. But for the sake of completeness and later use, we will briefly summarise the different criteria, especially with reference to BMGs.
The GFA of alloys is determined both by structural and kinetic parameters.Citation18 Here, structural criteria deal with the geometrical arrangement of atoms, bonding and atomic size effects to predict glass formation. On the other hand, the kinetic criterion considers the rate of cooling relative to the kinetics of crystallisation. While both the structural (thermodynamic) and kinetic factors assume importance, it appears that the basic GFA is mostly determined by thermodynamic parameters. This is where structural parameters such as the atomic size and chemical interactions between atoms are important. (It is not, however, implied here that BMGs, and much less the thin-ribbon metallic glasses, are thermodynamically stable). Once these criteria are satisfied, then the actual formation of the BMGs is determined by the kinetic parameters.
In the early years of research on metallic glasses produced by RSP methods, a large number of empirical criteria were proposed to explain the GFA of alloys. Majority of these also appear to be applicable to glass formation in BMG alloy compositions. A very generic and simple criterion proposed was that a glassy phase is obtained only when the liquid is undercooled (e.g. by quickly solidifying above a critical solidification rate, which is dependent on the alloy system and its composition), to a temperature below the glass transition temperature Tg. However, since this is an experimentally determined value (and is time-consuming and difficult to measure) and its estimation is also an involved process, other simple and empirical predictive criteria have been proposed to explain glass formation in alloy systems and these are briefly described below.
| i. | TurnbullCitation131 had suggested, purely on the basis of kinetics of crystal nucleation and the viscosity of melts, that the reduced glass transition temperature Trg, defined as the ratio of Tg to the liquidus temperature of the alloy Tl, should be a good indicator of the GFA of the alloy. The higher the Trg value, the higher the viscosity of the melt and therefore the alloy melt could be easily solidified into the glassy state | ||||
| ii. | it was also suggested that deep eutectic alloy compositions (a deep eutectic is one, wherein the alloy has a eutectic temperature that is much lower than the melting points of the individual metals) are good glass formers. This is because the value of Tg changes slowly and the value of Tl decreases very rapidly with solute content as one approaches the eutectic composition. Accordingly, the Trg value is very high at the ‘deep’ eutectic composition | ||||
| iii. | Egami and WasedaCitation132 had suggested that one of the possible ways by which a crystalline metallic material can become glassy is by the introduction of lattice strain. The lattice strain introduced disturbs the crystal lattice and once a critical strain is exceeded, the crystal becomes destabilised and glassy. In fact, EgamiCitation133 took pains to state that ‘In general, alloying makes glass formation easier, not because alloying stabilises a glass, but because it destabilises a crystal’. Using the atomic scale elasticity theory, these authorsCitation132 calculated the atomic level stresses in the solid solution (the solute atoms are assumed to occupy substitutional lattice sites in the solid solution) and the glassy phase. They observed that in a glass, neither the local stress fluctuations nor the total strain energy vary much with solute concentration, when normalised with respect to the elastic moduli. But in a solid solution, the strain energy was observed to increase continuously and linearly with solute content. Thus, beyond a critical solute concentration, the glassy alloy becomes energetically more favourable than the corresponding crystalline lattice. That is, a minimum solute concentration was necessary in a binary alloy system to obtain the stable glassy phase. And, this minimum solute concentration | ||||
The model based on the atomic size mismatch was subsequently developed by Miracle and co-workers.Citation136–Citation139 The major difference in the models of Egami and Miracle was that while the Egami model assumed that all the solute atoms, irrespective of their size, occupy the substitutional positions in the host lattice, the Miracle model considered that the smaller atoms occupy the interstitial positions while atoms with sizes larger or comparable with those of the solvent atoms occupy the substitutional positions. Furthermore, Miracle proposed that all the solute atoms could be classified into essentially three groups of atoms, which occupy the sites designated as α, β and γ by Miracle.Citation136 Such a designation and grouping of atoms was also found to be helpful in identifying the chemical compositions of alloy systems that led to easy glass formation.
Inoue criteria
Based on the extensive data generated on the synthesis of BMGs for over a decade, InoueCitation140–Citation142 had formulated three basic empirical rules to predict formation of BMGs. These may be stated as:
| i. | the alloy must contain at least three components. The formation of glass becomes easier with increasing number of components in the alloy system | ||||
| ii. | a significant atomic size difference should exist among the constituent elements in the alloy. It is suggested that the atomic size differences should be above ∼12% among the main constituent elements | ||||
| iii. | there should be negative heat of mixing among the (major) constituent elements in the alloy system. | ||||
These rules have been of immense value in identifying alloy compositions for the synthesis of BMGs, even though some apparent exceptions have been found for these empirical rules. For example, BMGs have been reported to form in binary alloy systems such as Ca–Al,Citation38 Cu–Hf,Citation143 Cu–Zr,Citation144 Ni–NbCitation145 and Pd–Si.Citation146 But the section thickness of these glasses is usually small, i.e. typically a maximum of only about 1–2 mm. It may, however, be noted that with the addition of more alloying elements, the GFA of these alloys can improve significantly, resulting in the formation of glasses with larger section thicknesses.
With increasing number of alloy compositions that have been made glassy, it was noted that some of the alloy systems did not follow the above-mentioned Inoue criteria. Therefore, in addition to the above general criteria that have been widely accepted by researchers and mostly followed in a large number of alloy systems, a number of new criteria have been developed since 2003, and these were based on the thermal properties of the alloys and physical characteristics of the component atoms.Citation147–Citation157 These include the so-called α, β, γ, γm, δ, φ, etc. parameters. summarises the different criteria developed to rationalise and predict the GFA of alloys. In spite of this large number of parameters, the predictabilities of glass formation have not significantly improvedCitation158 and it has been difficult to exactly specify which alloy compositions would produce the BMG alloy phases. Furthermore, the applicability of these new criteria appears to be limited in most cases to a few typical alloy systems. That is, majority of these new criteria are followed in a limited number of systems, and not more universally applicable, with the caveat that the γ parameter proposed by Lu and LiuCitation149,Citation150 seems to be applicable in a majority of the alloy systems. The interested reader is advised to refer to a recent critical analysis of the results by Suryanarayana et al.Citation158 and to the book on BMGsCitation7 for full details.
Table 3. Summary of the quantitative criteria proposed to evaluate the GFA of liquid alloys
The three empirical rules proposed by Inoue belong to the structural criteria, and the kinetic criterion involving nucleation and growth controls will be discussed later. In developing and analysing BMG alloys, experimentalists have been using mostly the structural criteria (including the three above empirical rules) rather than the kinetic ones on account of their simplicity and wide applicability to actual alloy systems. Also note that some advanced investigations have been conducted to evaluate the GFA and stability of glassy phases based on statistical- and numerical approaches based on structural criteria.Citation159–Citation162 In these approaches, the second and third factors of the Inoue empirical rules mentioned above or other relevant ones are qualified with corresponding thermodynamic and/or physical quantities such as mixing enthalpy and electronegativity for the second factor. Specifically, the effects of atomic bonding nature and atomic size mismatch were evaluated using factors of mixing enthalpy and mismatch entropy for ternary amorphous alloys,Citation159 mixing enthalpy and atomic size differences,Citation160 composition dependence of mixing enthalpy as a sub-regular solution model,Citation161 and electronegativity and atomic size for a number of bulk glassy alloy systems.Citation162 The results suggest the usefulness and importance of such approaches for further development of BMG alloys in general, and Fe-based bulk glassy alloys in particular, in the near future.
Glass formation by solid-state processing methods
All the criteria described above have been developed to describe the GFA of metallic glasses (and BMGs) processed through the solidification route. Mechanical alloying (MA) is another important non-equilibrium processing method to produce metastable phases in general and amorphous alloys, in particular. Mechanical alloying is a completely solid-state powder processing method that involves repeated cold welding, fracturing and rewelding of powder particles in a high-energy ball mill. The process involves placing the powders and a grinding medium (usually stainless steel balls) in a mill and agitating the whole mixture at a high speed. The repeated mechanical impacts of the grinding medium on the powder particles flatten and cold weld them. Because of this, the powder particles get strain hardened leading to their fracture, on continued milling, resulting in the creation of fresh surfaces. These fresh and active surfaces will again get cold welded when brought together by mechanical impacts. Owing to these repeated processes occurring, the powder particles form a layered structure and the interlamellar distance decreases with milling time. Furthermore, the introduction of crystal defects (dislocations, grain boundaries, stacking faults, etc.) enhances diffusion, further aided by a slight rise in the powder temperature. All these factors lead to alloying between the powder particles and formation of both equilibrium and metastable phases.Citation163–Citation167
A very large number of metallic glasses have been produced in a variety of alloy systems and in different compositions using MA.Citation163,Citation164 However, there have not been many systematic investigations conducted to study the conditions under which amorphous phases are formed by this method. It would be useful and instructive to see if the criteria applicable to the solidification methods would also be applicable to the solid-state processed amorphous alloys or other criteria need to be formulated to predict glass formation in alloy systems processed by MA. A systematic and comprehensive investigation has recently been reported on the glass formation behaviour and stability of several Fe-based glassy alloys processed by MA.Citation168
Based on a systematic and comprehensive investigation on Fe-based alloys of the generic composition of Fe42X28Zr10B20 (where the subscripts represent the composition of the alloy in atomic percentage and X = Al, Co, Ge, Mn, Ni or Sn), it was noted that amorphisation had occurred only in some alloy systems and not in all. For example, amorphisation occurred only in alloy systems with X = Al, Ge or Ni, as evidenced by the presence of a broad diffuse peak centred at the (110)Fe position in the X-ray diffraction (XRD) patterns (). Amorphisation did not occur in alloy systems with X = Co, Mn or Sn ().
Figure 1. a XRD patterns of blended elemental powder mix of Fe42Al28Zr10B20 as a function of milling time. Note that the amorphous phase has started to form on milling for ∼10 h and that the amorphous phase was stable up to ∼40 h.Citation168 b XRD patterns of Fe42Co28Zr10B20 powder mix as a function of milling time. Note that an amorphous phase had not formed in this case; instead only a solid solution phase was obtained on milling for 30 h (Ref. 168)
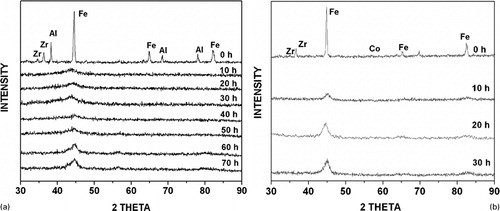
The time required for amorphisation, which was considered a measure of GFA was also different for the different powder blends. presents the results obtained, including the equilibrium number of intermetallic phases present between X and the constituent elements (Zr, Fe or B) in the powder bend.Citation168,Citation169 A close examination of clearly reveals that the ease of amorphisation (i.e. GFA) increased with the number of intermetallics present in the constituent Zr–X binary phase diagrams. This is apparent from the powder blends containing Al or Ni, which amorphised in 10 or 20 h respectively. While the quaternary Fe–Zr–Al–B contains eight intermetallic phases in the binary system between Zr and Al, the Fe–Zr–Ni–B system contains seven intermetallic phases in the binary system between Zr and Ni. Similarly, the Ge-containing system, which also amorphised in 10 h, contains five intermetallic phases between Zr and Ge. The Zr–Co, Zr–Mn and Zr–Sn systems which did not show amorphisation on milling contained five, one and three intermetallic phases respectively. However, when the total number of intermetallic phases was considered, it can be clearly seen that the systems which amorphised on milling contained ⩾10 intermetallic phases in all the constituent binary phase diagrams. That is, if the alloy system contained <10 intermetallic phases, then amorphisation was not observed. Note, however, that when the total number of intermetallics was only 10 (with the Ge-containing alloy), the time required for amorphisation was only 10 h. But this is a special case because Ge is a semi-metal with strong directional bonds. Thus, it becomes easier to amorphise alloys containing Ge (or other semi metals). From a critical analysis of the constituent binary phase diagrams, it also becomes clear that when the phase diagrams contain extensive solid solutions, it will be very difficult to amorphise them.Citation169
Table 4. Summary of the results of amorphisation in the Fe42X28Zr10B20 (where X = Al, Co, Ge, Mn, Ni or Sn) systems*
Amorphisation by MA was reported to occur when the free energy of the crystalline phase GC is higher than that of the hypothetical amorphous phase GA, i.e. GC>GA.Citation170 A crystalline phase normally has a lower free energy than the amorphous phase. But its free energy can be increased by introducing a variety of crystal defects. If an intermetallic has formed, then additional energy can be introduced by disordering the crystal lattice. By this approach, it is then possible to obtain an amorphous phase when(2) where GC represents the free energy of the crystalline phase, GD represents the increase in free energy due to introduction of defects and GA represents the free energy of the amorphous phase.
The magnitude of energy increase is different for different types of defects. As an example, increasing the dislocation density to 10Citation16 m−2 increases the free energy by ∼1 kJ mol−1, while decreasing the grain size down to 1 nm increases the free energy by ∼10 kJ mol−1.Citation171 The only way a solid solution could contribute to an increase in the energy of the system is by grain refinement. But this increase in energy is not sufficiently high to amorphise the system. On the other hand, the presence of intermetallics in an alloy system can contribute to an increase in the energy to favour amorphisation. This is due to two important effects. First, disordering of intermetallics contributes an energy of ∼15 kJ mol−1 of atoms to the system. For example, in strongly ordered intermetallics such as NiAl and γ-TiAl that continue to be in the ordered state till melting, the disordering energy has been estimated to be ∼17·5 kJ mol−1.Citation172 Second, a slight change in the stoichiometry of the intermetallic phase increases the free energy of the system drastically. Additionally, grain size reduction contributes an energy of ∼5 kJ mol−1. Furthermore, disordering of intermetallics has also been shown to be possible by heavy deformation.Citation173 Since MA reduces the grain size to nanometre levels and also disorders the usually ordered intermetallics, the energy of the milled powders is significantly raised. In fact, in most cases, it is raised to a level above that of the hypothetical amorphous phase, leading to the preferential formation of the amorphous phase over the crystalline phase.
Phase diagram features have been used to predict glass formation by RSP and other methods as well. As mentioned above, alloys in the vicinity of deep eutectics exhibit high Trg values, and therefore they exhibit high GFA.Citation131 Furthermore, elemental solids exhibiting a large number of polymorphic phases have been shown to exhibit higher GFA than elements that do not have a large number of polymorphs.Citation174 Difficulty in amorphisation has been observed with RSP in alloys having melting maxima, phase diagrams featuring too many peritectic reactions, high-temperature eutectics and also alloys having positive heats of mixing. However, amorphisation has been observed in most of these cases by MA.Citation163,Citation164 Thus, even though phase diagrams are useful guidelines in choosing alloy compositions for easy glass formation by both the methods, the features to look for appear to be quite different for the RSP and MA methods. It is just fortuitous that some alloy compositions can be amorphised by both the methods.
Structure of the glassy phase
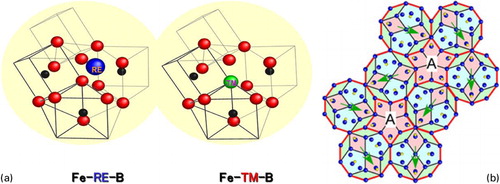
It was mentioned earlier that the Fe-based BMG alloys were composed of multi-component alloy systems which satisfy the three-component rules. The requirement for the three-component rule has been interpreted to originate from the formation, in the supercooled liquid and glassy structure, of unique distorted trigonal prisms and anti-Archimedean prisms consisting mainly of Fe and B or C. These prisms are connected to each other in edge- and face-shared configuration modes through glue atoms of M (M = Ln, Zr, Nb, Mo and Ga) elements.Citation175,Citation176 As an example, shows a schematic illustration of the local atomic configurations in Fe–RE–B (RE = rare-earth metals) and Fe–TM–B (TM = transition metals such as Zr, Nb or Mo) glassy alloys derived from the experimental data using the anomalous XRD, pulsed neutron diffraction, high-resolution TEM and reverse Monte–Carlo computer simulation techniques. Although the maximum size of the structure model was limited to 2–4 nm, due to the lack of periodic atomic configurations, this size corresponds to the upper limit which can be determined by the high density of synchrotron X-ray and the pulsed neutron beam sources. The resulting network-like long-range atomic configurations with attractive bonding nature can effectively suppress the long-range atomic rearrangements of the constituent elements that are necessary for the progress of crystallisation. This can consequently lead to the formation of bulk glassy alloys through stabilisation of the supercooled liquid.
Figure 2. a Schematic illustration of local atomic structure models for Fe-based BMG alloys in Fe–RE–B (RE = rare earth metals) and Fe–TM–B (TM = Zr, Nb or Mo) systems determined by advanced structural analytical methods. b A preliminary structural model giving the pseudo-tenfold diffraction pattern in which the three types of tiles as structural motifs of Fe23B6 are linked to each otherCitation182
It has been believed for quite some time now that the structure of the liquid, from which the BMG phases form, determines the structure of the glass. Since the liquid contains icosahedral-type clusters, it is also believed that the structure of the glass also contains ‘icosahedral’-type units.Citation177 In fact, several authors have reported that the first phase to precipitate out of the Zr-based BMG alloys during crystallisation is a quasicrystalline phase.Citation178–Citation180 Recent high-resolution TEM investigations have clearly indicated the presence of medium-range order (MRO) in Fe-based glassy alloys.Citation181,Citation182 These authors have reported pseudo-tenfold diffraction patterns during the course of crystallisation of Fe-based BMG alloys. For example, during the course of crystallisation of an Fe48Cr15Mo14C15B6Tm2 BMG, the authors found a nanoscale metastable state with a χ-FeCrMo-like structure, which changed into the [113] zone-axis pattern of the χ-FeCrMo structure on annealing at higher temperatures.Citation181 Similarly, nanoscale quasicrystal-like structural states exhibiting pseudo-tenfold nanobeam electron-diffraction patterns were observed in the course of crystallisation process of an (Fe0·5Co0·5)72B20Si4Nb4 BMG alloy.Citation182 In this case, the metastable structure transformed to the Fe23B6-like intermediate structure and eventually to the Fe23B6 structure. Thus, these metastable states can be considered as the pre-crystallisation stage, similar to the pre-precipitation stage in precipitation-hardenable alloys.Citation183
Existence of the intermediate states between the quasicrystal-like and Fe23B6 structures indicates that the quasicrystal-like structure is an approximant to the Fe23B6 structure. The pseudo-tenfold electron-diffraction patterns are understood to arise from a combination of the three types of tiles found in the Fe23B6 structure. These three types of tiles can produce decagonal units which do not have any icosahedral atomic arrangement. Thus, the presence of icosahedral atomic arrangement is not a prerequisite to the formation of quasicrystal or quasicrystal-like phases.
shows a preliminary structure model giving the pseudo-tenfold diffraction pattern, in which the three types of tiles as structural motifs of Fe23B6 are linked to each other.Citation182 The larger and smaller circles in the model denote the Fe and B atoms respectively. The arrows in the model mean orientations of the structural motifs with five different directions, which contribute to give the isotropic diffraction pattern with 10 strong spots. This report provides the first diffraction evidence for the presence of icosahedral-like structures in an Fe-based bulk glassy alloy.
The diffraction and spectroscopic methods generally provide only the average structural information. Even though a number of different atomic models have been proposed to explain the structure of metallic glasses, direct observation of the local atomic structure in disordered materials has not been achieved till recently. Hirata et al.Citation184 have recently reported direct observation of local atomic configurations in a binary Zr66·7Ni33·3 metallic glass obtained by melt spinning. By employing a state-of-the-art nanobeam electron diffraction technique combined with ab initio molecular dynamics simulation, the authors reported observation of subnanoscale ordered regions. Distinctly symmetric nanobeam electron diffraction patterns were found to originate from individual and interconnected atomic polyhedra. These observations offer compelling evidence of the local atomic order in the disordered metallic glass, which is consistent with the recent cluster models and previous predictions that metallic glasses possess short-range order and MRO as opposed to the long-range periodicity of a crystalline solid.Citation136,Citation137,Citation185
Furthermore, it is important to point out that the spontaneous formation of such unique long-range network-like atomic configurations in special multi-component alloy systems significantly affects the nature of the primary precipitate phase forming from the supercooled liquid. shows the XRD patterns of the [(Fe0·8Co0·1Ni0·1)0·75B0·2Si0·05]96Nb4 BMG alloy subjected to annealing at different temperatures ranging from 853 to 1133 K.Citation70,Citation112 The primary precipitate phase can be identified as (Fe,Co)23B6 with a complex fcc structure and a lattice parameter of ∼1·12 nm. The (Fe,Co)23B6 phase is in a metastable state and changes to a mixture of equilibrium crystalline phases with further increase in the annealing temperature. Thus, the primary precipitate phase in all Fe-based bulk glassy alloys reported to date consists of a complex fcc M23(B,C)6 compound with a large unit cell volume containing a large number of atoms. The local atomic configuration of the M23(B,C)6 phase includes an anti-Archimedean atomic configuration which is similar to that of the Fe–Nb–B glassy alloys.Citation175,Citation176 It is because of the similarity between the local atomic configurations of the supercooled liquid and the primary precipitate phase that this is the first phase to form during crystallisation of the BMG alloys. It is thus interpreted that the spontaneous formation of the long-range network-like atomic configurations in the supercooled liquid in multi-component alloy systems with at least three components is the origin for the high stability of the supercooled liquid against crystallisation in Fe-based BMG alloys.
Figure 3. X-ray diffraction patterns of [(Fe0·8Co0·1Ni0·1)0·75B0·2Si0·05]96Nb4 bulk glassy alloys subjected to annealing treatments leading to precipitation of the primary crystalline phaseCitation112
![Figure 3. X-ray diffraction patterns of [(Fe0·8Co0·1Ni0·1)0·75B0·2Si0·05]96Nb4 bulk glassy alloys subjected to annealing treatments leading to precipitation of the primary crystalline phaseCitation112](/cms/asset/71ae788f-3b16-477f-b7e0-7ac35c8d0060/yimr_a_11673359_f0003_b.jpg)
In addition to the above-described experimental observations, computational approaches were also undertaken to obtain local atomic configurations in Fe-based BMG alloys. These approaches used the concepts of clusters and their packing leading to MRO in cluster-packed structures. For instance, Kazimirov et al.Citation186 carried out ab initio calculations on Fe–TM–RE–Me BMG alloys (where TM = transition metals such as Mn, Cr and Mo, RE = rare-earth elements such as Er and Y and Me = metalloid atoms such as C and B). Some of the alloys studied included Fe64Mo14C15B7, Fe50Cr15Mo14C15B6 and Fe51Cr14Mo12C15B6Y2. Since atomic diffusion determines the GFA and stability of the glass (slow atomic diffusion may lead to better GFA by reducing the critical cooling rate required to form the glass), the authors calculated the atomic mean-square displacements 〈r(t)2〉. They showed that the 〈r(t)2〉 for C, B and Fe are different and that these atoms diffuse much faster in the Fe79C15B6 alloy than in the Fe49Cr15Mo14C15B6Er1 alloy, explaining why the latter alloy is a better glass former. It may be noted that the Fe49Cr15Mo14C15B6Er1 alloy can be cast into 6 mm diameter rodsCitation97 while Fe79C15B6 can only be made in ribbon form and is not entirely glassy. The role of rare-earth elements in improving the GFA of alloys has also been explained using these results. The slow diffusion of RE atoms has been explained as not just due to the large size of the atom but mostly because RE atoms form structurally complex clusters that slow diffusion down for other chemical species.
Kazimirov et al.Citation186 had also reported the effects of short- and medium-range atomic clustering on the diffusion behaviour and reported that the short-range clusters persisted well into the liquid state. Using these structural models, the authorsCitation186 calculated the bulk moduli as a function of the Er content in these glasses and reported that the bulk modulus decreased with increasing Er content. The bulk modulus values and their variation with composition were consistent with the experimentally determined values.Citation96
Alloy development studies
A large number of Fe-based alloy compositions have been quenched into the glassy state in recent years. All the developments in Fe-based metallic glasses, including the BMGs, can be traced to the early years of research on the binary Fe–B metallic glasses, formed in the form of a thin ribbon by RSP methods. Later developments with respect to alloy development and improvement of mechanical and magnetic properties were also based on the Fe–B system. As mentioned earlier, all the Fe-based BMGs can be classified into three groups, and these can be typically represented by the systems: Fe–M–(P,C,B,Si) (M = Al, Ga, Mo); Fe–B–Si–Nb based; and Fe–Cr–Mo–C–B–Ln. Let us now look at these systems in some detail.
Fe–M–(P,C,B,Si) (M = Al, Ga, Mo) BMGs from Fe–(Al,Ga)–metalloid type
As mentioned earlier, the first synthesis of Fe-based BMGs by the copper-mould casting process was made in the Fe–Al–Ga–P–C–B system in 1995,Citation27 even though the maximum diameter of the glassy alloy was <2 mm. The success of producing Fe-based BMGs was important for significant extension of application fields of BMG alloys as structural and functional materials. This incentive played a trigger effect on the subsequent development of new BMG alloys in the alloy series: Fe–(Cr,Mo)–Al–Ga–P–C–B, Fe–Mo–Ga–P–C–B, Fe–Ga–P–C–B–Si, Fe–Mo–P–C–B–Si, Fe–Cr–Mo–P–C–B–Si, Fe–Co–Ga–P–C–B–Si and Fe–Co–Mo–P–C–B–Si systems.Citation68,Citation104,Citation187 The maximum rod diameter was reported to be 2·5 mm for the Fe–Ga–P–C–B–Si system,Citation126 4 mm for the Fe–Mo–P–C–B–Si systemCitation68 and 6 mm for Fe–Co–Mo–P–C–B–Si system.Citation187
Fe–B–Si–Nb based bulk glassy alloys from the Fe–ETM/Ln–metalloid type
Another type of Fe-based soft magnetic BMG alloy has been developed in the Fe–Co–B–Si–Nb system for the past few years. It was reported in 2002 that the addition of small amounts (2–4 at-%) of Nb to Fe–Co–B–Si amorphous alloys caused a drastic change to the glassy type leading to the appearance of distinct glass transition, followed by a large supercooled liquid region before crystallisation.Citation107 As a result, the glass transition phenomenon was observed over the whole composition range in the [(Fe,Co,Ni)0·75B0·20Si0·05]96Nb4 system.Citation188 The highest Trg of 0·60 and the largest supercooled liquid region ΔTx of ∼65 K were obtained at the Fe–Co-rich composition around [(Fe0·6Co0·4)0·75B0·20Si0·05]96Nb4. The alloy with the highest Trg and the largest ΔTx had the highest GFA which enabled the production of bulk glassy alloy rods with diameters up to 5 mm, as shown in .Citation115 It has also been shown that the use of the B2O3 flux melting treatment causes a further increase in the maximum diameter to ∼9 mm,Citation189,Citation190 indicating that elimination of heterogeneous nucleation sites through refinement of the alloy melt is effective to increase the GFA even for Fe-based BMG systems.
Figure 4. Compositional dependence of the maximum diameter obtained in [(Fe1−x−yCoxNiy)0·75B0·20Si0·05]96Nb4 BMG alloys produced by the copper-mould casting methodCitation115
![Figure 4. Compositional dependence of the maximum diameter obtained in [(Fe1−x−yCoxNiy)0·75B0·20Si0·05]96Nb4 BMG alloys produced by the copper-mould casting methodCitation115](/cms/asset/55b63b3a-3ad2-4eb8-a0e9-5ae436f0d611/yimr_a_11673359_f0004_b.jpg)
Fe–Cr–Mo–C–B–Ln BMGs from non-ferromagnetic Fe–ETM/Ln–metalloid type
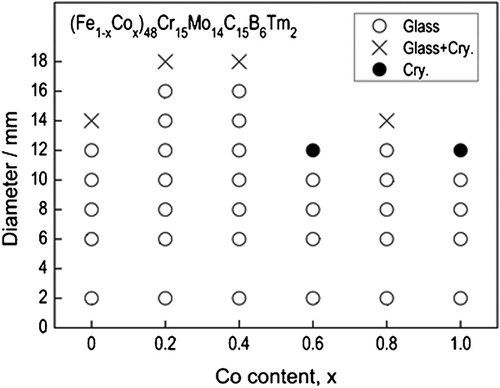
By altering the Fe content in Fe-based BMG alloys, the magnetic nature of the glassy phase changes from ferromagnetic to non-ferromagnetic type at room temperature. It is also noted that in these cases, the maximum diameter of the BMG alloy rod increases to high values reaching as much as 16 mm.Citation99,Citation103,Citation119,Citation191 For instance, as a forerunner for the non-magnetic type Fe-based BMG alloys, it was reported that Fe48Cr15Mo14C15B6Er2 is formable as a BMG alloy with a critical diameter of 12 mm,Citation99 exceeding 1 cm. Subsequently, the largest diameter BMG rod of 16 mm was produced in the Fe41Co7Cr15Mo14C15B6Y2 alloy.Citation103 The fact that partial replacement of Fe by Co increases significantly the critical diameter has also been confirmed in the Fe48Cr15Mo14C15B6Tm2 glassy alloy.Citation98 shows the XRD patterns of the cast (Fe0·8Co0·2)48Cr15Mo14C15B6Tm2 alloy rods with different diameters ranging from 6 to 18 mm. No distinct diffraction peak due to a crystalline phase is seen for the rod specimens with diameters <16 mm, indicating that the critical diameter of the glassy alloy was as large as 16 mm. shows the change in the critical diameter for the formation of a glassy phase in the cast (Fe1−xCox)48Cr15Mo14C15B6Tm2 alloys as a function of the Co content.Citation191 The critical diameter is 12 mm for the Co-free alloy, increases to 16 mm for the alloys with x = 0·2 and 0·4 and then decreases to 10 mm with further increase in the Co content. It is noticed that the Co–Cr–Mo–C–B–Tm alloy also has a large critical diameter of 10 mm.Citation191 The effectiveness in increasing the GFA of alloys by partial replacement of Fe with Co seems to result from the necessity of the long-range atomic rearrangement of the constituent elements required for the simultaneous precipitation of Fe23(C,B)6 and Co23(C,B)6 phases from the supercooled liquid for the Fe–Co–C–B–Tm alloys. It has also been found that the critical diameter increases further to 18 mm by more fine-tuning of the composition of the alloy to (Fe0·8Co0·2)47Cr15Mo14C15B6Tm3.Citation192 Considering that the critical diameter of Fe50Cr15Mo14C15B6 is ∼2 mm, addition of 2–3 at-%Tm is concluded to be very effective in increasing the GFA. Furthermore, the addition of 2%Tm does not cause distinct change in the melting temperature. Therefore, the significant effectiveness for glass formation is presumably due to the increase in the ease of formation of the network-like long-range atomic configurations due to the coexistence of two kinds of glue atoms (Mo and Tm). This may be in addition to the atomic size effect since Tm has the largest atomic size as compared with all other constituent elements.
Figure 5. X-ray diffraction patterns of cast (Fe0·8Co0·2)48Cr15Mo14C15B6Tm2 BMG alloy rods with diameters of 6–18 mm
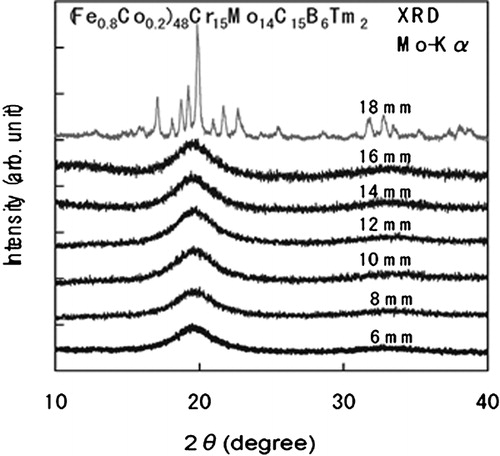
Figure 6. The critical diameter for the formation of a glassy phase as a function of Co content for (Fe1−xCox)48Cr15Mo14C15B6Tm2 BMG alloysCitation191
Role of alloying elements on GFA
The synthesis of the Fe80B20 glass (commercially referred to as METGLAS alloy no. 2605), with a high strength of ∼3630 MPa (370 kgf mm−2), triggered the further development in metal–metalloid type of Fe-based metallic glasses by maintaining a standard composition of ∼80 at-% of the transition metal(s) and ∼20 at-% of the metalloid element(s). All subsequent investigations were concerned with alloy modifications in terms of changing the nature and relative proportions of the transition metal and/or metalloid elements. The goal has always been to increase the GFA (to synthesise larger diameter BMG alloy rods) and improve the mechanical (simultaneous increase in strength and ductility) and magnetic properties (higher saturation magnetisation, high permeability and low magnetostriction). Recent results on increasing the GFA will be briefly described below.
In a series of developments of Fe-based BMG alloys, it is widely accepted that addition of small amounts of some specific alloying elements greatly affects the GFA. For instance, it was reportedCitation193 that 6 at-% addition of Y as well as Sc, Dy, Ho and Er in ternary Fe72M6B22 alloys made it possible to produce BMG alloys with 1 mm in diameter. On the other hand, other elements such as Zr, Hf, Nb, Ta and lanthanide elements ranging from La to Tb did not contribute to the formation of BMG alloys. Here, special attention should be paid to the recent report,Citation92 investigating the role of Nb, Zr and Nb+Zr on GFA of Fe–Nb–Zr–B alloy system. The authors found that the optimal glass formers were located at Fe71Nb6B23 and Fe77Zr4B19 in the ternary systems, and Fe71Nb4·8Zr1·2B23 in the quaternary system. Accordingly, the authors were able to produce 1·5 mm diameter rod in the Fe–Nb–B system, 1 mm diameter rod in the Fe–Zr–B system and 2 mm diameter rod in the Fe–Nb–Zr–B system. This report suggests that Nb and Y are effective alloying elements to improve GFA, supporting the recent trends to add Y and Nb together in Fe-based BMG alloys.Citation194 A similar early study discussing the effect of Y on the enhancement of GFA of Fe-based alloys was reported by Lu et al.Citation57,Citation150 in which they reported that addition of Y to Fe–Cr–Co–Mn–Mo–C–B alloy enhances the GFA. It was mentioned that while the critical diameter for glass formation was only <7 mm for the Y-free alloy, it increased to ⩾12 mm for the alloy containing 1·5 at-%Y.Citation150 Similarly, while the Tm-free Fe50Cr15Mo14C15B6 alloy could be cast into rods of <2 mm in diameter, the alloy containing 2 at-%Tm could be cast into rods of 12 mm diameter.Citation98 Replacement of Fe with Co increased the critical diameter to 16 mm.Citation191 These reports support that the effects of Y and Tm on enhancing the GFA are, in part, due to the large atomic size differences between the principal elements among Fe, Y (or Tm) and B. In a framework for the development of Fe-based BMG alloys containing Y, it was also reportedCitation194 that replacement of Fe with Zr or Co significantly affected the GFA and the soft magnetic properties. The effects of addition of minor amounts of solute elements in Fe-based and other glassy alloys were more widely and thoroughly reviewed.Citation195,Citation196 The principal aspects of the formation and properties of Fe-based BMG alloys have been highlighted in these reviews as follows:
| i. | by the addition of some metals with high melting temperature, such as Zr, Nb, Ta, W and Mo, Fe-based BMGs with 5 mm diameter can be obtained by Cu-mould castingCitation197 | ||||
| ii. | with the proper rare-earth element additions, the Fe- as well as Mg-based BMGs can be successfully fabricated by a conventional Cu-mould casting method even in air atmosphere | ||||
| iii. | a minor Ni addition can significantly enhance the soft magnetic properties of Fe-based glass-forming alloys without deteriorating their high GFA | ||||
| iv. | the addition of metalloid elements usually makes the Fe-based BMGs more brittle | ||||
| v. | enhancement of GFA by the additions of C, B and Y, each at levels of <5 at-%, in Fe-based BMG alloys, has been shown to occur in several instances.Citation195 | ||||
Phase stability and evolution of glassy alloys associated with crystal nucleation and growth of undercooled alloys have been reviewed by Perepezko,Citation198 even though this was with special reference to Al-based glassy alloys. This approach involving nucleation and growth controls belongs to the kinetic criteria for formation of glassy alloys towards the structure criteria mentioned earlier. Besides, in a framework of phase evolution of Fe-based BMG alloys, Nouri et al.Citation199 investigated the effects of changes in test temperature on the microhardness/strength of Fe48Mo14Cr15Y2C15B6 BMG alloys by paying attention to structure evolution under a variety of different test conditions over the temperature range from room temperature to 620°C. Although a very high microhardness value (e.g. >12 GPa) was exhibited at room temperature, significant hardness reductions were exhibited near Tg. In addition, the effect of exposure time (up to 5 h) at elevated temperatures on the evolution of microhardness/strength was also evaluated for selected temperatures. Such results are of great value when using Fe-based BMG alloys as structural materials, in particular, at elevated temperatures ranging from room to glass transition temperatures.
Mechanical properties
Metallic glasses, and more specifically the BMGs, have been known to exhibit very high strength and hardness and wear resistance in comparison with their crystalline counterparts. Therefore, in view of their low cost, Fe-based BMGs can be exploited for structural applications. Associated with the high strength is the drawback that the Fe-based metallic glasses have little or no plasticity. However, materials used for structural applications are required to have some plasticity and therefore recent research efforts have been directed to develop BMG alloys that possess simultaneously both high strength and some amount of plasticity. The plasticity of the BMGs can be evaluated by measuring the amount of plastic deformation that the material undergoes beyond the elastic limit. The mechanical properties of BMGs have been recently reviewed.Citation200–Citation202
As mentioned earlier, majority of the developments in Fe-based BMG alloys have been based on the early years of research on binary Fe–B metallic glasses, formed in the form of thin ribbons by RSP methods. A special attribute of the Fe80B20 glass is its high strength, and it was reportedCitation203 that among the metallic glasses, Fe80B20 glass exhibits the highest strength of ∼3630 MPa (370 kgf mm−2), which is superior to another type of Fe-based metallic glass (Fe80P16C3B1 METGLAS alloy no. 2615) with a yield strength of 2440 MPa (249 kgf mm−2) and to other Ni-based metallic glasses commercialised as METGLAS series. The Fe80B20 glass was known as the strongest metallic glass found in the middle of 1970s, and this situation continued till the beginning of the twenty-first century until the development of a Co43Fe20Ta5·5B31·5 BMG alloy in 2004 with a yield strength of >5000 MPa.Citation204 Thus, the history of the development of Fe-based BMGs has been very closely linked with the high mechanical strength of Fe-based metallic glasses. lists the mechanical properties of some typical Fe-based BMG alloys. It may be noted from this table that the yield strength of the Fe-based BMGs is very high.
Table 5. Mechanical properties of some typical Fe-based BMG alloys*
The research on mechanical properties of Fe-based BMGs has been a major topic of research during the last decade or so. An important aspect of this research has been on improving the plasticity of these alloys, since Fe-based glassy alloys that belong to the metal–metalloid type are intrinsically brittle in nature due to the covalent-like bonding between metal–metalloid atomic pairs. These efforts to improve the plasticity of Fe-based BMG alloys, and also BMG alloys in general, are aided by alloy design principles through modification of alloy compositions to alter their fracture behaviour. Some of these studies are presented below with the aim of identifying their essential features. The studies can be grouped under three categories, namely alloying additions, compositional modifications to modify the shear and elastic moduli and the Poisson’s ratio, and development of composites. However, it should be realised that the first two aspects are intimately related to each other.
It has been reportedCitation206 that the chemistry of the alloy basically determines its ductility and fracture toughness. The Fe–Mn–Mo–Cr–C–B system was shown to exhibit very low toughness, approaching values typical of the inherently brittle oxide glasses (0·06 kJ m−2). But by replacing Mn with Er and increasing the Cr content from 4 to 15 at-%, the fracture toughness was increased significantly (from ∼5 to 26 MPa m1/2). Similarly, in the Fe–Mo–C–B system, the addition of Cr and Er increased the shear and elastic modulus and reduced the compressive plasticity.Citation95 However, replacing Er with P improved the compressive plasticity. Similarly, substitution of carbon for boron in Fe49Cr15Mo14C13B8Er1 and (Fe0·9Co0·1)58·5Cr6Mo14C15B6Er0·5 steels resulted in a decrease in the shear modulus, while the bulk modulus remained essentially constant. These modified alloys exhibited high fracture strengths and some compressive plasticity.Citation97
The relationship between ductility and alloy design was provided by Gu et al.,Citation109 who reported that the ductility of Fe-based BMG alloys can be improved by partial replacement of the elements that enhance the nature of ionic and covalent bonds. The analysis based on first-principles electronic structure calculations was also performed for ductile Fe–Cr–Mo–P–C–B amorphous steels. Furthermore, it was pointed out that the enhanced ductility of amorphous steels was attributed to the decrease in the shear modulus in the ductile region, where the Poisson’s ratio is 0·33–0·34. The relationship between the mechanical properties and relevant physical quantities, such as shear modulus and Poisson’s ratio, has also been clarified empirically by Gu et al.,Citation96 suggesting that the onset of plasticity in the Fe65Mo14C15B6 BMG alloy doped with lanthanides was associated with an increase in the Poisson’s ratio. In fact, Chen et al.Citation207 had earlier pointed out way back in 1975 that ‘it is the high Poisson’s ratio (ν) which is responsible for the ductile behaviour of many metallic glasses. The decreasing ν with falling temperature, together with a relatively lower ν (<0·40) results in a rapid increase in the fracture strength and the brittle behaviour of Fe-based glasses’. Recent workCitation208 has further indicated that the levels of compressive plasticity may be affected by various test details (e.g. alignment, stress concentrations, etc.) and that fracture toughness/energy may be a more discriminating testCitation205,Citation209 as shown below.
It has been suggested that the ratio of the elastic shear modulus to the bulk modulus G/K can be utilised to predict the ductile or brittle behaviour of solids. For instance, the fracture energy (toughness) of Fe-based BMG alloys was correlated with changes in the G/K ratio as well as the Poisson’s ratio by Lewandowski et al.Citation205 Through a broad survey of the literature, it was shownCitation209,Citation210 that the critical value of G/K differentiating a brittle BMG from a ductile BMG appears to be in the range of 0·41–0·43. Bulk metallic glass alloys with a G/K value of over 0·41–0·43 are brittle. For example, the Fe50Mn10Mo14Cr4C16B6 BMG alloy with the G/K ratio of 0·423 has been shown to be brittle, when compared with the Zr-based BMG alloys. A recent mechanics-based model also predicts the importance of elastic constant ratio on the toughness of metallic glasses.Citation211
The ductility of conventional crystalline metals has been shown to increase by the introduction of thin metallic glassy ribbons into them.Citation212 Therefore, researchers have utilised this concept to increase the ductility of BMG alloys and have achieved some amount of success.Citation213–Citation215 But majority of these investigations have been concerned mostly with Zr-based BMG alloys and others. There have been very few investigations on Fe-based BMG alloys. For example, Shen et al.Citation117 produced a composite alloy by adding 0·25 at-%Cu to an (Fe0·5Co0·5)75B20Si5 BMG alloy. The microstructure consisted of ∼13·6 vol.-% of precipitates of α-(Fe,Co) and (Fe,Co)23B6 in a glassy matrix. While the fully glassy alloy (without any Cu in it) had a yield strength of 3700 MPa with no ductility, the composite alloy (with 0·25 at-%Cu in it) exhibited a fracture strength of 4500 MPa and also a plastic strain of 0·6%. Thus, it appears worthwhile to further explore this approach to increase the ductility of Fe-based BMGs. Similarly, the cast glassy rods in the alloy series of (Fe1−xCox)48Cr15Mo14C15B6Tm2 exhibit high fracture strength of >4000 MPa over the whole Co content range and the strength level of 4050–4150 MPa, which is almost independent of the Co content.Citation191 In addition, all the BMG alloys with a diameter of 2 mm did not fracture within the elastic elongation limit and exhibited nearly a constant fracture strength as well as the same elastic elongation limit of 2%. This strength value and the fracture behaviour indicate that these Fe-based BMG alloys also possess a relatively good ductile nature.
The important term in the literature on mechanical properties, and more specifically with reference to ductility, is ‘intrinsic’, and this term, along with the other term ‘extrinsic’, suggests new ways of approaching the field of BMG alloys. For instance, Yavari et al.Citation216 pointed out that the current intense interest in the mechanical response of glassy alloys is due to intrinsic and extrinsic factors, and that these factors explain remarkably well the extensive plastic deformation during compression or bending, serrations in the stress–strain curves, shear softening, sharp temperature rise around shear bands and resultant growth of nanocrystals that block the propagation of shear bands. Besides, Weibull modulus of Fe-based BMG alloys was investigated by paying attention to the intrinsic and extrinsic effects.Citation206 It was reported that extrinsic factors such as the presence of processing defects including inclusions or porosity are responsible for the large scatter in the toughness of some samples. In addition to toughness, a number of research papers have also dealt with the basic concepts of brittleness and plasticity. For example, Xi et al.Citation217 established a clear correlation between the fracture toughness and the length scale of the plastic process zone for various brittle and tough BMGs. They suggested that the fracture surfaces in brittle BMGs (e.g. those based on Fe and Mg) also revealed dimple structures, suggesting that the existence of damage microvoids does not depend on the chemical composition of the BMGs and that the nucleation of microvoids is related to the glassy structure, which contains free volume and inherent atomic density fluctuations at the nanometre level.Citation23 But the dimple structures in the relatively brittle BMGs is on the nanometre level, indicative of the activation of plastic flow processes, possibly as a result of the local softening mechanism. In fact, Xi et al.Citation217 have demonstrated a linear relationship between the square of the ratio of fracture toughness to yield strength and the measured plastic zone size; the more ductile BMGs showed a larger measured plastic zone. Varying degrees of compressive plasticity were also recently observed in Ti-based BMGs by changing the sample sizes and stress states.Citation218
The relationship between mechanical properties and the glass transition temperature (and GFA) was reported in some publications. For instance, the relationship between ductility and glass transition of metallic glasses was analysed by first-principle calculations for Fe–Cr–Mo–P–C–B BMG alloys containing up to 27 at-% metalloids, leading to atomic bonding and connectivity in the amorphous network.Citation219 The elastic moduli of Fe-based BMG alloys were studied in FeCrMoCBErMe (Me = Al, Be, In, Nb, Ni and Pb) amorphous steels with high Fe content (>58 at-%).Citation220 The authors showed that the elastic moduli of the alloys are much larger than those of Zr- and Cu-based BMGs, and can be simply described approximately by a sum of elastic constants and the atomic percentage of components. These alloys also show a low Poisson’s ratio similar to that of Mg-based BMGs, indicating that they belong to the brittle BMG family.
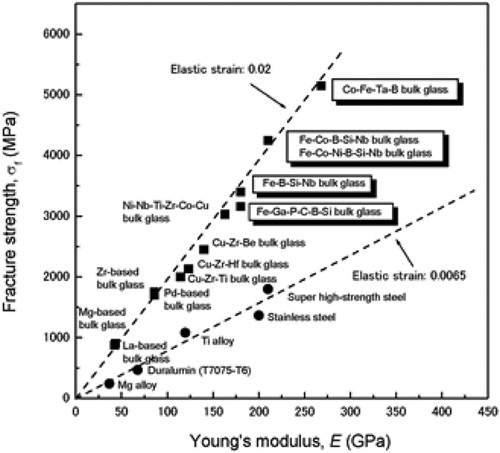
shows the relationship between the fracture strength and Young’s modulus for Fe-based BMG alloys, together with the data for other BMG alloys and conventional crystalline alloys.Citation23 The Fe-based BMG alloys have very high fracture strength of ∼3300 MPa for the Fe–TM–(P,C,B,Si) system and ∼4200 MPa for the Fe–Co–B–Si–Nb system. It is also noticed that there is a linear relationship between the fracture strength and the Young’s modulus. As seen in the figure, the slope of the linear relation corresponding to the elastic strain limit is about three times larger than that for crystalline alloys. Thus, it is suggested that Fe-based BMG alloys have a much higher fracture strength in conjunction with much larger elastic strain as compared with conventional crystalline alloys.
Figure 7. Relationship between fracture strength and Young’s modulus for Fe-based BMG alloys. The data of other BMG alloys and conventional crystalline alloys are also included for comparisonCitation23
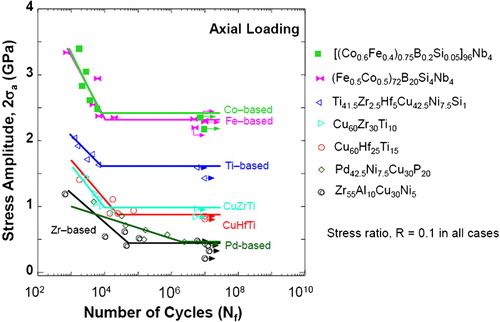
In addition to the static mechanical properties, the fatigue behaviour of the (Fe0·5Co0·5)72B20Si4Nb4 BMG alloy was examined as one of the dynamic mechanical properties. The fatigue test was performed under a tension–tension stress condition with a stress ratio of 0·1 and a frequency of 10 Hz for the rod specimen with a maximum diameter of 1·85 mm. The fatigue strength limit defined as the ratio of stress amplitude to fracture strength after 10Citation7 cycles was 0·55 for the Fe–Co-based BMG alloy which is slightly higher than that (0·49–0·54) for conventional alloy steels (Cr–Mo steels, SCM435 and alloy tool steel, SKD61).Citation192 Corresponding to the very high fracture strength of the Fe–Co-based bulk glassy alloy, the stress amplitude after 107 cycles shows very high value of 2310 MPa for the Fe–Co-based alloy which is much higher than those (900–1000) for SCM435 and SKD61 steels, as shown in . The fatigue crack always initiated at a defect site located on the outer surface of the specimen and then propagated into the inner region, accompanying the distinct striation pattern. This behaviour was independent of the type of the BMG. Similar behaviour was noted in Fe-, Co-, Ti- and Cu-based BMG alloys.Citation221 A fatigue ratio of 0·16–0·17 was reported in an Fe48Cr15Mo14Er2C15B6 glassy steel using a four-point bend testing method.Citation222 The fatigue fracture behaviour of BMGs suggests that elimination of defect sites on the outer surface could lead to an increase in the fatigue strength. It should be, however, mentioned that results on the fatigue behaviour of BMGs have not been conclusive; very widely differing results have been reported. The reader is advised to consult Ref. 7 for full details of the present situation.
Figure 8. Relationship between fatigue stress amplitude and cycles to failure (S–N curves) for Fe-based BMG alloys. The data for other BMG alloys and some conventional crystalline alloys are also shown for comparisonCitation7
Corrosion behaviour
For an effective use of BMGs, it is necessary to fully characterise them for their chemical behaviour also. The corrosion behaviour of BMGs becomes important when these materials need to be used in aggressive and hostile environments (high temperatures, oxidising atmospheres and corrosive media). Knowledge of the corrosion behaviour becomes critical when the BMGs are considered for biomedical applications and for decorative applications, or when surface appearance becomes important. The corrosion behaviour of metallic glassy alloy ribbons (about 20–50 μm in thickness) produced by RSP methods was evaluated starting from 1974.Citation223,Citation224 It was reported that Cr-containing Fe-based Fe80−xCrxP13C7 glassy ribbons exhibited much higher corrosion resistance than the crystalline Fe–Cr alloys. While the crystalline Fe–Cr alloys corroded at a rate of about 0·5–1 mm/year, the glassy Fe–Cr–P–C alloy did not show any measurable corrosion rate under identical conditions of exposure in 1 N NaCl solution at 30°C. Another important observation made was that the minimum amount of Cr required to achieve this corrosion resistance was only 8 at-%, much less in the glassy state than that required (>12 at-%) in the crystalline state. Furthermore, the glassy alloy did not exhibit any measurable weight change with the concentration of HCl (from 0·01 to 1 N) on exposure for 1 week at 30°C. On the other hand, the corrosion rate of the crystalline 18-8 austenitic stainless steel [(Fe–18Cr–8Ni (wt-%)] increased from 10−3 mm/year in 0·01 N HCl to over 10 mm/year in 1 N HCl solution; severe pitting corrosion occurred in the range of 0·5–1 N HCl in the crystalline alloy. A number of investigations were also carried out on Fe-based BMGs as well and an overview of the corrosion behaviour of BMG alloys is presented in Ref. 225.
Fe-based BMG alloys investigated for their corrosion behaviour generally contained Cr, Mo, C and B in varying proportions. Cr has been shown to be essential in forming a passive layer and this is further facilitated by Mo addition. Both carbon and boron (together to the extent of ∼20 at-%) were found to be necessary to achieve glass formation. A typical Fe-based glassy alloy composition appears to be Fe45Cr16Mo16C18B5. In some of the investigations, either additional metalloid elements, especially P, have been added or Mo has been partially replaced with Ta or Nb.
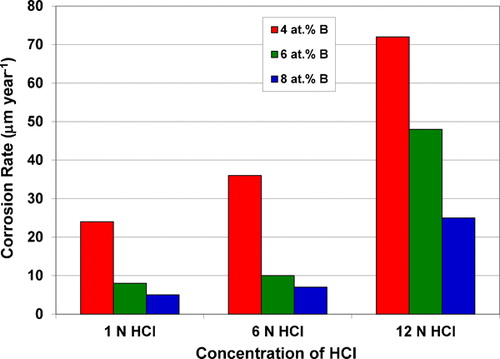
All the glassy alloys investigated exhibited good corrosion resistance in concentrated HCl with the measured corrosion rates of as low as 1–10 μm/year. The corrosion rate of the Fe-based glassy Fe50−xCr16Mo16C18Bx alloys was found to decrease with increasing B content in the alloy.Citation106,Citation226 A similar decrease in corrosion rate with increasing Cr content was also noted.Citation116,Citation227 shows the decreasing corrosion rate in the Fe50−xCr16Mo16C18Bx glassy alloys with B content and in the {[(Fe0·6Co0·4)0·75B0·2Si0·05]0·96Nb0·04}100−xCrx glassy alloy with Cr content respectively. It may be noted that the corrosion rate decreased from 700 to 1·6 μm/year as the Cr content increased from 0 to 4 at-%. Addition of Mo to Fe-based alloys has also been reported to improve their corrosion resistance in HCl solution, since it prevents dissolution of Cr during passivation.Citation228 However, it has been noted that when the dissolution rate of Fe-based alloys is very high as in the active region, Mo selectively remains in the alloy because the dissolution rate of Mo is slower than that of other constituents. Furthermore, Mo has not been able to form its own passive film in the passive region of the alloys. Mo is also known to dissolve even at lower potentials in the passive region of the alloys, indicating a lower stability of the passive film of Mo in comparison with passive hydrated chromium or iron oxyhydroxide film. Consequently, excessive amounts of Mo addition to replace Fe have been reported to be detrimental for the corrosion resistance of Fe-based glassy alloys.Citation229
Figure 9. a Decreasing corrosion rate with increasing B content in an Fe50−xCr16Mo16C18Bx BMG alloyCitation7 and b Decreasing corrosion rate with increasing Cr content in 0·5 N NaCl solution at 298 K open to air for 168 h for the {[(Fe0·6Co0·4)0·75B0·2Si0·05]0·96Nb0·04}100−xCrx alloyCitation7
![Figure 9. a Decreasing corrosion rate with increasing B content in an Fe50−xCr16Mo16C18Bx BMG alloyCitation7 and b Decreasing corrosion rate with increasing Cr content in 0·5 N NaCl solution at 298 K open to air for 168 h for the {[(Fe0·6Co0·4)0·75B0·2Si0·05]0·96Nb0·04}100−xCrx alloyCitation7](/cms/asset/0181195c-0da7-436d-bde7-2f43e78971fb/yimr_a_11673359_f0009_b.jpg)
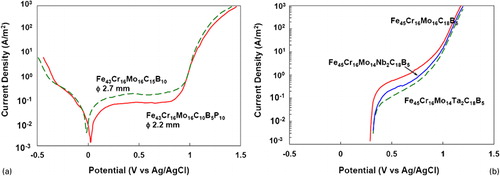
The corrosion resistance of the BMG alloys has been shown to be higher due to the presence of P in the alloy. shows the potentiodynamic polarisation curves of Fe43Cr16Mo16C15B10 and Fe43Cr16Mo16C10B5P10 bulk glassy alloys in 1 N HCl at 298 K.Citation230 From these curves, it may be noted that both the alloys passivate spontaneously. However, the passive current density is ∼10−1 A m−2 for the Fe43Cr16Mo16C15B10 alloy, whereas it is approximately half (5×10−2 A m−2) for the Fe43Cr16Mo16C10B5P10 alloy. The lower passive current density in the P-containing alloy clearly demonstrates that this alloy has a better corrosion resistance. shows that a similar improvement in the corrosion resistance of Fe45Cr16Mo16C18B5 and Fe45Cr16Mo14M2C18B5 (where M = Nb or Ta) BMG alloys in 6 N HCl solution has also been obtained by substituting Mo with Nb or TaCitation105 and also by addition of Nb to Cu-based BMG alloys.Citation231
Figure 10. a Potentiodynamic polarisation curves of Fe43Cr16Mo16C15B10 and Fe43Cr16Mo16C10B5P10 bulk glassy alloys in 1 N HCl solution open to air at 298 K showing that the passive current density for the P-containing alloy is lowerCitation230 and b Anodic polarisation curves of Fe45Cr16Mo16C18B5 and Fe45Cr16Mo14M2C18B5 (where M = Nb or Ta) BMG alloys in 6 N HCl solution open to air at 298 K. Note the lower passive current density when Nb or Ta is present in the alloyCitation105
The effect of test environment and structural changes on the corrosion behaviour of Fe-based BMGs was also evaluated.Citation106 It was shown that the corrosion rate increased with increasing concentration of the corrosive medium, irrespective of the B content in Fe50−xCr16Mo16C18Bx BMG alloys, even though the corrosion resistance increased with increasing B content for a given concentration of the corrosive medium (). It was also shown that pitting occurred on the surface of the alloy after immersion for 1 week in 12 N HCl at room temperature, especially when the B content was only ∼4 at-%; pitting did not occur at higher B levels. Such a phenomenon of pitting did not occur in 1 and 6 N HCl solutions; instead they passivated spontaneously.
Figure 11. Increasing corrosion rate with increasing concentration of HCl in an Fe50−xCr16Mo16C18Bx BMG alloyCitation7
The influence of structurally relaxing or crystallising the BMG alloys on the structure and corrosion behaviour of Fe-based BMG alloys has also been investigated. Pardo et al.Citation232,Citation233 studied the effect of Cr content on the corrosion behaviour of Fe73·5Si13·5B9Nb3Cu1 BMG alloys in different concentrations of H2SO4 (1, 3 and 5 N). They investigated the corrosion behaviour of this alloy in three different conditions: in the as-solidified fully glassy condition, by annealing it for 1 h at 813 K to obtain a nanocrystalline structure (10–15 nm grain size) and by fully crystallising the samples (to achieve a grain size of 0·1–1 μm) through annealing at 973 K for 1 h. The corrosion resistance was higher with increasing Cr content in the range studied (0–8 at-%). However, a minimum Cr concentration of 8 at-% was found necessary to generate a stable passive layer. Among all the conditions studied, the glassy structure showed the best corrosion resistance, followed by that in the nanocrystalline state. The fully crystallised alloy showed the least corrosion resistance.
Magnetic properties
Magnetic properties of materials are of fundamental importance for several applications in the electrical and electronic industries. A very large number of studies have also been conducted on Fe-based melt-spun ribbons starting from the pioneering investigation of Duwez and Lin on the Fe–C–P system in 1967.Citation234 Since the most important application to which the melt-spun glassy ribbons have been put to is in transformer core laminations based on the excellent soft magnetic properties of these alloys, a significant amount of effort has also been spent on investigating the magnetic properties of Fe-based BMG alloys. However, an important difference between the investigations on melt-spun ribbons and BMGs is that while both metal–metalloid and metal–metal type alloys have been investigated in the thin film category, only metal–metalloid type of alloys have been studied in the BMG group. Studies on the magnetic properties of BMG alloys in the metal–metal type category are conspicuous by their absence.
The nature of magnetic investigations in BMG alloys has followed trends very similar to what were done in the case of melt-spun glassy ribbons. And, in fact, even for BMG compositions, several researchers have been studying the magnetic behaviour using melt-spun ribbons. Some minor differences were noted in the magnetic properties of melt-spun ribbons and bulk rods, especially in those properties that are affected by structural relaxation, e.g. magnetostriction and coercivity. For example, it has been shown that the saturation magnetisation of the alloys is not any different whether measured on the melt-spun ribbon form or powderCitation235 or between the melt-spun ribbon and bulk rods of different diameters.Citation101 These are illustrated in the hysteresis loops presented in for Fe65Co10Ga5P12C4B4 and Fe62·8Co10B13·5Si10Nb3Cu0·7 alloys respectively.
Figure 12. a Hysteresis loops for the glassy a Fe65Co10Ga5P12C4B4 alloy in the melt-spun ribbon and gas-atomised powder conditions,Citation235 and b Fe62·8Co10B13·5Si10Nb3Cu0·7 alloy in the melt-spun ribbon of 20 μm thickness and bulk rod of 1·5 mm diameterCitation101
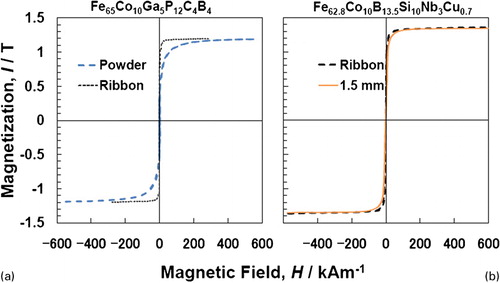
The saturation magnetisation of the Fe–M–(P,C,B,Si) (where M = Ga or Mo) BMG alloys with optimum alloy components in each alloy system was in the range of 1·53–1·10 T, depending on the Fe content and the type of metalloid element. The coercivity was <3 A m−1 and the effective permeability at 1 kHz was in the range of about 10 000–50 000. Besides, rather good high-frequency permeability with the level of 20 000 at 100 kHz was achieved for Fe–Ga–P–C–B–Si, Fe–Mo–P–C–B–Si, Fe–Co–Ga–P–C–B–Si and Fe–Co–Mo–P–C–B–Si BMG alloys. The soft magnetic BMG alloys in the Fe–(Cr,Mo)–(P,C,B,Si) system have been used in various application fields as discussed later.
and show the compositional dependence of saturation magnetisation Is and coercivity Hc respectively, for [(Fe1−x−yCoxNiy)0·75B0·20Si0·05]96Nb4 glassy alloys.Citation7 These soft magnetic data were obtained from the melt-spun ribbon samples with a cross-section of 0·02×10 mm and the samples were also subjected to annealing treatment for 5 min at a temperature which is 50 K lower than the glass transition temperature. As shown in , Is shows high values of >1·3 T for Fe-rich Fe–Co–B–Si–Nb alloys and decreases gradually with increasing Ni and Co contents. On the other hand, the Hc shows low values of <2·5 A m−1 over the whole composition range and decreases gradually with increasing Co content (). It is noticed that the Co-rich Co–Fe–B–Si–Nb glassy alloys exhibit very low Hc values (<1 A m−1), presumably because of very low saturation magnetostriction of 5×10−7.Citation236 Reflecting the very low Hc as well as the nearly zero saturated magnetostriction, the [(Co0·9Fe0·1)0·75B0·020Si0·05]96Nb4 glassy alloy exhibits excellent high-frequency permeability of 20 000 at 100 kHz.
Figure 13. Compositional dependence of saturation magnetisation in the [(Fe1−x−yCoxNiy)0·75B0·20Si0·05]96Nb4 BMG alloysCitation7
![Figure 13. Compositional dependence of saturation magnetisation in the [(Fe1−x−yCoxNiy)0·75B0·20Si0·05]96Nb4 BMG alloysCitation7](/cms/asset/8dcd58d3-2492-44e5-94fa-e3a964340562/yimr_a_11673359_f0013_b.jpg)
Figure 14. Effect of composition on the coercivity in [(Fe1−x−yCoxNiy)0·75B0·20Si0·05]96Nb4 BMG alloysCitation7
![Figure 14. Effect of composition on the coercivity in [(Fe1−x−yCoxNiy)0·75B0·20Si0·05]96Nb4 BMG alloysCitation7](/cms/asset/1a1a6f93-0890-4659-96d6-04e875e0d3fa/yimr_a_11673359_f0014_b.jpg)
The important features of the soft magnetic properties for Fe- and Co-based glassy alloys in the Fe–TM–(P,C,B,Si) and (Fe,Co)–B–Si–Nb systems are summarised below. shows the relationship between coercivity and electrical resistivity for Fe- and Co-based glassy alloys.Citation7 The data for conventional amorphous and nanocrystalline soft magnetic alloys are also presented for comparison. It is clear that the Fe- and Co-based BMG alloys have unique combination of very low coercivity and high electrical resistivity, a combination that cannot be obtained for any other kind of soft magnetic metallic material including amorphous and nanocrystalline alloys. Based on detailed studies on glassy alloys produced by RSP techniques, it was suggested earlierCitation237 that a clear relationship exists between coercivity and internal stresses; a low coercivity is obtained when the internal stresses are low. Such a situation of low internal stresses can be achieved in BMG alloys produced at relatively low cooling rates due to the formation of a more homogenised disordered structure consisting of unique network-like atomic configurations. Consequently, BMG alloys exhibit a low coercivity.
Figure 15. Relationship between coercivity and electrical resistivity for Fe- and Co-based glassy alloys. The data for nanocrystalline alloys and conventional amorphous alloys are also included for comparisonCitation7
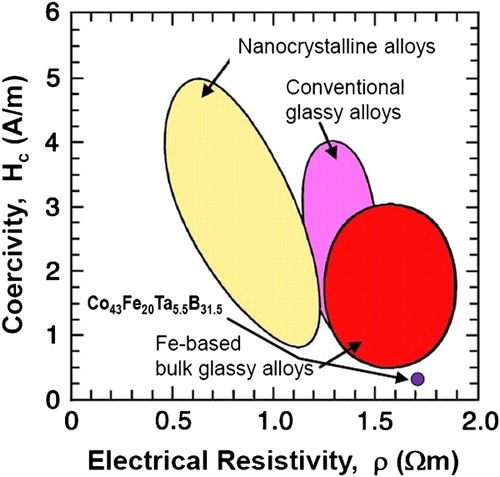
Results obtained to date show that in order to achieve a high saturation magnetisation, it is necessary to have as high an Fe content as possible and decrease the other metal and metalloid contents to the minimum value that is necessary to obtain the glassy phase. Investigating on these lines, Makino et al.Citation238 selected the Fe94−xNb6Bx alloy system to arrive at the optimum composition to achieve the best soft magnetic properties. They noted that a fully glassy phase was obtained only when the B content was a minimum of 10·5 at-%. The saturation magnetisation of >1·6 T was obtained in an alloy with x⩽9, where the alloys have a mixed glassy+α-Fe composite structure. The highest permeability μe of 28 000 and the smallest grain size of 10·5 nm were obtained at x = 11. Thus, a single composition was not going to offer the best soft magnetic properties. Therefore, these investigators have limited the Nb content in the alloy to 6 at-% and small amounts of Cu and P were added to this alloy to optimise the composition at Fe84·9Nb6B8P1Cu0·1. This alloy, fabricated by melt spinning in air, contained the α-Fe phase with an average grain size of 10 nm dispersed in a glassy matrix and showed excellent soft magnetic properties with a saturation magnetisation of 1·61 T, coercivity of 4·7 A m−1 and permeability of 41 000. The core losses for this alloy were very low at 0·11 W kg−1.
The best soft magnetic properties are achieved by crystallising the glassy phase to produce a uniform nanostructure,Citation239 referred to as FINEMET alloys. And in FINEMET and other alloys, the volume fraction of the nanocrystalline phase is very large, reaching a value of almost 90%. Since a glassy precursor is a pre-requisite to achieve this microstructure, one needs to have a minimum amount of different metals and/or metalloids to first produce the glassy phase. Furthermore, to achieve the large volume fraction of the nanocrystalline phase, the presence of metals like Cu, Nb, Mo, W and Ta is required. However, the presence of these non-magnetic metals can significantly reduce the saturation magnetisation. Furthermore, these metallic elements are quite expensive. To overcome these difficulties and the requirement of metallic elements and also to achieve a very high saturation magnetisation, Makino et al.Citation240–Citation242 have developed novel Fe-based alloys that contain only metalloids. The generic composition of their alloys is Fe83·3–84·3Si4B8P3–4Cu0·7 and is based on the Fe82Si9B9 alloy that proved most promising among the Fe-based melt-spun magnetic alloys. In the modified composition, P substitutes for B and Cu for Fe. (The presence of a small amount of Cu is necessary to achieve nanocrystallisation). The large concentration of Fe provides a high saturation magnetisation and the absence of the metallic elements (other than Fe) ensures that the alloys are not expensive.
Applications
Bulk metallic glass alloys exhibit very high strength (both in tension and compression), large elastic elongation limit, very high hardness, excellent corrosion resistance and a good combination of soft magnetic properties. Recent rapid progress in the development of BMG alloys has made it possible to exploit these novel materials in a variety of application fields. For instance, Zr-, Ti-, Fe-, Co-, Ni- and Cu-based BMG alloys are already in use for magnetic sensing, chemical and structural applications. Specifically, it was suggestedCitation7,Citation243 that typical industrial applications of BMG alloys include: magnetic applications such as linear actuators, magnetic cores, choke coils and high-frequency magnetic-shielding sheets; chemical applications such as fuel-cell separators; and structural applications such as sporting goods, precision optical parts, precision gears for micromotors, diaphragms for pressure sensors, tubes for Coriolis mass flowmeters, aircraft parts, automobile valve springs, etc. On the basis of these current trends of applications of BMG alloys, this section focuses on the applications of Fe-based BMG alloys. This will be based on the two important attributes of the Fe-based BMG alloys, namely good soft magnetic properties and high strength for use as soft magnetic and structural materials respectively.
Soft magnetic materials
Soft magnetic glassy alloys in the Fe–TM–P–C–B–Si system have been commercialised under the trade name of Liqualloy,Citation244,Citation245 whose magnetic properties are summarised in ,Citation246 together with those of Mn–Zn ferrite and Fe–Al–Si alloy (Sendust) for comparison. The application fields of the soft magnetic powder cores ‘Liqualloy cores’ have been in choke coils of AC–DC converters, DC–DC converters, noise suppression sheets, etc. The Liqualloy magnetic powder cores exhibit good soft magnetic properties, e.g. nearly constant relative permeability in a wide frequency range up to several MHz; good linear relation with small degradation slope in the relation between permeability and DC bias field, i.e. good DC-superposed characteristics; and much lower core losses as compared with Ni–Fe–Mo permalloy and Sendust. The excellent core loss characteristics are due to the reduction in eddy current loss resulting from much higher electrical resistivity (168 μΩ cm) of Liqualloy than that of Sendust (82 μΩ cm), as shown in . In addition, as shown in , the Liqualloy powder core shows much better DC-superposed characteristics than those of Mn–Zn ferrite, in addition to nearly the same low core losses between Liqualloy and Mn–Zn ferrite.Citation246 Furthermore, in comparison with Mn–Zn ferrite, the Liqualloy power inductor shows better DC-superposed characteristics in the elevated temperature range up to 393 K. Through these advantages, the Liqualloy powder core has been used as the power inductor in lap-top type personal computers because of the advantages of higher efficiency and much smaller heat generation than those for commercial power inductors, as shown in .
Figure 16. Direct current-superposed characteristics and core losses of Liqualloy powder core. The data for Mn–Zn ferrite are also shown for comparisonCitation246
Figure 17. Application example of Liqualloy powder core to power inductor in lap-top type personal computerCitation246
Table 6. Magnetic properties of Liqualloy*Citation246
It may be noted that the shape of the Liqualloy powder produced by water atomisation can be changed from the original spherical shape to flake shape with a small thicknesses of about 1–3 μm by bead milling treatment. The resulting Liqualloy sheet consisting of the flaky powder and resin was found to exhibit good electromagnetic noise suppression characteristics because of high conversion ability from electromagnetic noise to heat.Citation246 The use of Liqualloy noise suppression sheet has resulted in significant reduction in noise level and hence the sheet has been used in digital still camera as exemplified in . Furthermore, the Liqualloy electro-magnetic sheet was found to exhibit good radio frequency identification characteristics. As shown in , the Liqualloy sheet shows better function as magnetic deflection yokes. One can obtain much longer transmission distance as compared with the case of loop antenna/metal parts. These better characteristics are attributed to the fact that high real and low imaginary parts of permeability can be achieved at a high carrier frequency of 13·56 MHz. As a result, the Liqualloy electromagnetic sheet for radio frequency identification system has been used in higher functional cell phones.
Figure 18. Characteristics of Liqualloy noise suppression sheet and its application example to digital still camera
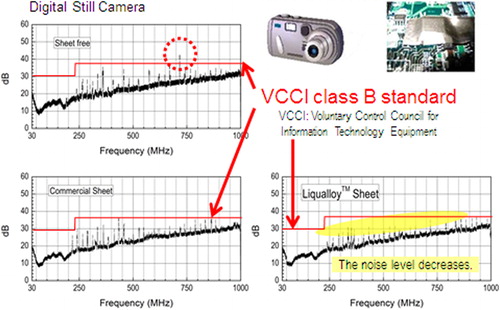
Figure 19. Functional characteristics of Liqualloy magnetic sheet for radio frequency identification system. The data of loop antenna and loop antenna/metal parts modes are also shown for comparison
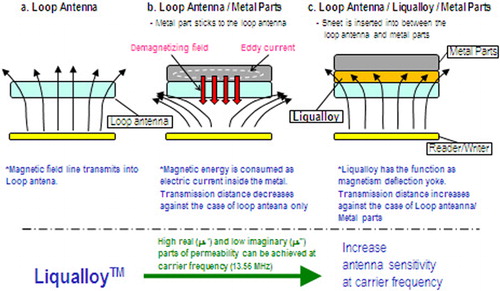
In addition, another type of soft magnetic powder core has been produced in the Fe–Nb–B–Si and Fe–Nb–Cr–P–B–Si systemsCitation247 with a high saturation magnetisation of 1·3 T. The cores were also produced by a procedure similar to that for Liqualloy, i.e. mass production of spherical glassy alloy powder by water atomisation, followed by cold consolidation of the mixture of glassy alloy powder and epoxy resin and then annealing. The new magnetic powder cores have been named as ‘SENNTIX-I’ and ‘SENNTIX-II’ for the Fe–Nb–B–Si and Fe–Nb–Cr–P–B–Si systems respectively, and can be characterised as exhibiting the lowest core losses among all kinds of magnetic powder cores developed to date. Besides, the higher saturation magnetisation has enabled use of the cores in a higher current range. The fundamental characteristics of SENNTIX are summarised in ,Citation247 together with other Fe–Si–B–M (M = alloying additions) systems named as FINEMETCitation248 and other Fe-based alloys.
Table 7. Specific temperatures, GFA and magnetic properties of Liqualloy*Citation247,Citation248
In addition to power inductor and electromagnetic sheet applications, soft magnetic BMG alloys have been tested for applications to position sensor, antennas for radio-controlled watch, solenoid valve, magnetic sensor, etc.Citation249 For instance, by using the sensing method with saturable coil, a Co–Fe–B–Si–Nb magnetic sensor has been identified to show sharper transient signal, higher output voltage and higher mechanical strength than those for conventional magnetic sensors. Such a high performance is expected to cause improved sensitivity, high S/N ratio, miniaturisation and easy handling and operation. Besides, an Fe–Co–B–Si–Nb position sensor was confirmed to detect a higher impedance change by movement of core output voltage which resulted in improved sensitivity and miniaturisation.
Applications to structural materials
The high yield (or fracture) strength, low Young’s modulus, large elastic strain limit and easy formability in the supercooled liquid region are the main attributes of BMGs that make them attractive for structural applications. The large supercooled liquid region in BMG alloys offers an excellent opportunity to form complex shapes easily. This is mainly because the plastic flow of the material in this temperature regime is Newtonian in nature (i.e. the strain rate is proportional to the applied stress). This attribute of BMG alloys has been extensively exploited to produce different types of parts with complex shapes such as gears, coiled springs and other complex parts. The parts using BMG alloys have complex shapes and the sizes of these parts are much smaller than what have been achieved using conventional crystalline alloys. But in the context of this review, it should be made clear that the applications that have been described in the literature are not just based on Fe-based BMG alloys, but for many different types of BMG alloys. However, these have been included for the sake of completion and also to bring awareness to the readers of the potential structural applications of BMG alloys.
Sporting goods
Bulk metallic glasses have first found widespread application in sporting goods due to their desirable mechanical properties, namely high strength and large elastic elongation limit. The excellent mechanical properties of Zr-based BMGs were exploited commercially in golf clubs, followed by tennis rackets, baseball and softball bats, skis and snowboards, bicycle parts, scuba gear, fishing equipment and marine applications.Citation250
The modulus of resilience U calculated as the area under the elastic portion of the stress–strain curve works out to be(3) where σy and ϵy are the yield stress and elastic strain limit respectively, and E is the Young’s modulus. Since the ϵy value for BMGs is at least twice that in a crystalline material, the modulus of resilience is at least four times that of a crystalline material. It is suggested that 99% of the impact energy from a BMG golf club head is transferred to the ball. This value should be compared with 70% for a titanium head. Liquidmetal Technologies calls this high energy transfer as ‘pure energy transfer’. This company has started marketing baseball bats, tennis rackets and other sporting goods as well. The HEAD Radical Liquidmetal tennis rackets seem to offer large sweetspots, plenty of control and impressive feel, with very little vibration.
Precision gears
Complicated structures can be easily formed from BMGs in their supercooled liquid region and this offers many advantages including: formation of highly homogeneous features, even on a nanoscale, due to the absence of crystalline features such as grains in the glassy material; very little solidification shrinkage during superplastic forming of BMGs in the supercooled liquid region, due to the absence of first-order phase transformations which introduce significant shrinkage into the casting, reduced degree of porosity in the formed part; and superior surface imprintability and net-shape forming capability, which have been found to be very attractive in the field of micro-machines.Citation251 Because of the excellent filling characteristics of BMG alloys, it has been possible to produce extremely small parts of complex design using BMGs. A sun-carrier made with a Ni53Nb20Zr8Ti10Co6Cu3 BMG alloy consisting of a micro-gear, carrier plate and three pins has been fabricated. The outer diameter of the gear is 0·65 mm, it has 14 teeth with a module of 0·04 and the micro-gear is seated on a carrier plate 1·7 mm in diameter. Three pins with a diameter of 0·30 mm and a length of 0·45 mm are located at the bottom of the carrier plate for rotating planetary gears.Citation252
Motors
Micro-geared motors with high torque have been used in different engineering fields. With advancing technology and the need for miniaturisation, the size of the motors has been constantly decreasing. Bulk metallic glasses appear to be particularly suited for this application. The wear resistance behaviour of a 2·4 mm diameter gear was evaluated using sliding-wear and rolling-wear tests.Citation253 It was reported that the wear loss (volume) of the Ni-based BMG was larger than that of the carbon steel under sliding-wear conditions, but smaller under rolling-wear conditions. It was noted that the gear teeth of the carbon steel are worn off and heavily damaged even after just 8 h of use, while the teeth of the BMG alloy are in very good condition even after 2500 h of use. The wear life of the gear was 1·6 times higher than an all-steel gear when one of the gears was replaced by a BMG alloy gear. However, the wear life increased by seven times when more gears were replaced with BMG alloys. But in the case of all BMG alloy gears, the wear life was 313 times higher than all-steel gears.Citation254
Encouraged by these results, Inoue et al.Citation255 have fabricated the world’s smallest size micro-geared motor (1·5 mm in diameter and 9·4 mm in length) using a high-strength Ni-based BMG alloy. The components of this geared motor cannot be made by any mechanical machining methods. It consists of a sun-carrier, an output shaft and six pieces of planetary gear, all made out of the Ni-based BMG with the composition Ni53Nb20Zr8Ti10Co6Cu3. It was confirmed that this micro-geared motor had high rotating torques of 0·1 mN m at two stages stacked gear-ratio reduction system and 0·6 mN m at three stages which were 6–20 times higher than the vibration force for conventional geared motor with a diameter of 4·5 mm in mobile telephones. These micro-geared motors are expected to be used in advanced medical equipment such as endoscopes, micropumps, rotablator and catheter for thrombus removal, precision optics, micro-industries, micro-factory, etc.
Shot peening balls
By utilising the good GFA, high mechanical strength, large elastic elongation limit, high corrosion resistance and good surface smoothness of Fe-based BMG alloys, commercialisation of Fe-based BMG alloy powders with particle sizes of 0·05–1 mm has been accomplished as shot peening particles.Citation256 The high GFA has made it possible to produce glassy alloy powders over a wide particle size range by conventional water atomisation methods, leading to very good cost advantage. The much higher yield strength and large elastic elongation limit also permit to generate much deeper compressive residual stress region as well as much higher level of compressive residual stress. In addition, Fe-based glassy alloy shot balls have much longer endurance times which are about 10 times longer than those for cast alloy steel and high speed steel shot balls.
Similarly, BMG alloys have also been used as automobile valve springs,Citation257 diaphragms for pressure sensors,Citation249 pipes for a Coriolis mass flowmeter,Citation249,Citation258 optical mirror devicesCitation259 and structural parts for aircraft.Citation260 Reasons for the choice of these materials and the details of applications may be found in Ref. 7.
Coating applications
Here, special attention should be paid to a new high velocity oxygen fuel (HVOF) spray coating technique because of the advantages of very high moving velocity of atomised powders, relatively low spray flame temperature, very compact equipment size and rather easy operation. This technique was applied to the formation of spray-coated glassy alloy layer of Fe-based glassy alloy powders on various alloy substances.Citation261 shows the cross-section of spray-coated glassy alloy layer of Fe50Cr15Mo15C14B6 alloy on stainless steel substrate, together with the XRD patterns of the original alloy powder and the coated surface layer. The spray-coated layer with a thickness of ∼0·2 mm has a rather high relative density of over 99% and retains the glassy structure without any crystalline phase being present and thus the glassy structure of the original powder is retained. Here, it is important to point out that a similar spray-coated layer was formed on various metallic substrates such as carbon steel, aluminium alloy and magnesium alloy and the deposition tendency of the sprayed powder was independent of the kind of substrate material. In addition, it has been confirmed that the maximum thickness for the formation of the Fe–Cr–Mo–C–B glassy alloy layer reaches at least 0·2 mm in the case of the stainless steel substrate. However, when the crystalline alloy powder, obtained by crystallisation of the atomised Fe–Cr–Mo–C–B glassy alloy powder, was used as the powder for the HVOF spray technique, the coated layer consisted of mostly crystalline phases including a small amount of glassy phase. This result indicates two important points: the heating temperature of the powder during HVOF spray coating is not high enough to obtain a fully melted state and the incomplete melted state prevents formation of the glassy alloy coated layer; and the heating temperature reaches the (solid+liquid) two-phase region and that the formation of a small amount of glassy phase is the result of rapid solidification of the partially melted liquid region. This result also suggests that the cooling rate of the HVOF spray technique is high enough to result in the formation of a glassy phase through suppression of precipitation of a crystalline phase for individual powder.
Figure 20. Optical micrograph and XRD patterns taken from the cross-section of Fe50Cr15Mo15C14B6 glassy alloy coated layer on SUS 304 steel substrate produced by an HVOF spraying techniqueCitation261
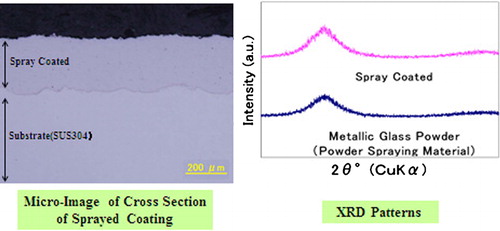
shows the corrosion resistance and Vickers hardness of spray-coated Fe–Cr–Mo–C–B glassy alloy layer shown in , in comparison with the data of SUS 304 steel and hard Cr-coated plate.Citation262 The spray-coated glassy alloy layer exhibited much better corrosion resistance as revealed by the much lower corrosion current density and much higher anodic potential than those for SUS 304 steel in the anodic polarisation curve. These tests were conducted in 1 N H2SO4 at 298 K. In addition, the Vickers hardness of the spray-coated glassy alloy layer is about 1000–1100 which is considerably higher than that (800–900) for the hard-coated chromium plate. Furthermore, the wear resistance of the coated glassy alloy surface layer is demonstrated in , which shows SEM images of the coated Fe–Cr–Mo–C–B glassy alloy layer subjected to the ring-on-disc friction wear test,Citation263 together with the data for cast iron (FC) and alloy tool steel (SKD) materials tested under similar conditions. The SEM images reveal that the surface of the glassy alloy layer keeps much better smoothness as compared with those for the conventional steel materials. In addition, the wear resistance is better for the coated glassy alloy layer made of the finer alloy powder rather than the coarse alloy powder presumably because of the achievement of higher relative density for the coated surface layer and the higher hardness of the finer alloy powder.
Figure 21. Corrosion behaviour and Vickers hardness values of spray-coated Fe50Cr15Mo15C14B6 glassy alloy coated layer in comparison with the data for crystalline stainless steel and hard Cr-coated plateCitation262
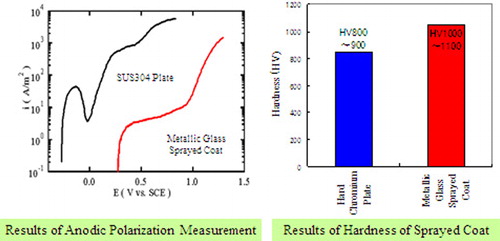
Figure 22. Images (SEM) of spray-coated Fe50Cr15Mo15C14B6 glassy alloy layer after the ring-on-disc friction wear test. The data of the FC and SKD materials tested under the same conditions were also shown for comparisonCitation263
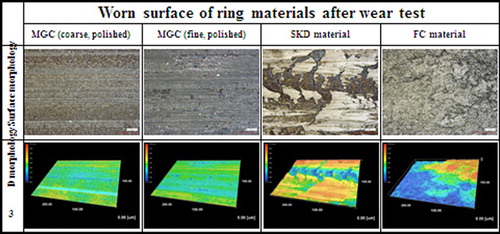
By exploiting the above-described advantages, an appropriate application field for practical use is now under consideration. A coating was applied to the inside surface of a lead-free soldering vessel with a diameter of 50 cm and height of 34–40 cm. The coating was found to be effective even after continuous use of the vessel for melting. As shown in , one cannot recognise any detectable damage caused by solder melt attack even after use for 6 months. This is in stark contrast to serious erosive damage of SUS 304 stainless steel vessel used only for 1 month.Citation263
Figure 23. Surface appearance of spray-coated Fe50Cr15Mo15C14B6 glassy alloy surface layer inside the lead-free soldering vessel of 50 cm in diameter and 40 cm in height which was used for 6 monthsCitation263

Concluding remarks
Fe-based BMG alloys are relatively new materials with interesting combination of properties such as high strength and good soft magnetic properties. Starting with the synthesis of Fe80B20 high strength glassy alloys produced in the form of thin ribbons in 1970s, there has been continuous progress in the synthesis of a number of alloy compositions with increasing section thickness. A maximum section thickness of ∼16 mm is now achieved in (Fe0·8Co0·2)48Cr15Mo14C15B6Tm2 and Fe41Co7Cr15Mo14C15B6Y2 BMG alloys, through additions of rare-earth elements such as Y and Tm. Several reports are available discussing the recent developments in the mechanical and magnetic properties and corrosion resistance of these BMG alloys. In contrast to the developments in thin film ribbons, non-ferromagnetic Fe-based BMG alloys with an Fe content <50 at-% have also been developed. Both the ferromagnetic and non-ferromagnetic types of Fe-based BMG alloys have been developed by taking advantage of their unique characteristics such as high mechanical strength and good corrosion resistance. The achievements of the ferromagnetic Fe-based BMG alloys led to practical applications utilising the magnetic properties, while the superior GFA of the non-ferromagnetic Fe-based BMG alloys makes it possible to produce them with maximum diameter of Fe-based BMG alloys reaching >1 cm. Thus, the two types of Fe-based BMG alloys have been developed separately by utilising their unique characteristics. A number of applications have been developed for these magnetic Fe-based BMG alloys.
The authors are highly thankful to Dr Akira Takeuchi of Institute of Materials Research, Tohoku University, Sendai, for many valuable discussions and help with the preparation of the manuscript.
References
- Bloor D, Brook RJ, Flemings MC, Mahajan (eds.) S: ‘The encyclopedia of advanced materials’, xi; 1994, Oxford, Pergamon/Elsevier.
- Suryanarayana C, ed (ed.): ‘Non-equilibrium processing of materials’; 1999, Oxford, Pergamon/Elsevier.
- Luborsky F E, ed (ed.): ‘Amorphous metallic alloys’; 1983, London, Butterworths.
- Anantharaman T R, ed (ed.): ‘Metallic glasses’; 1984, Zurich, Trans Tech Publications.
- Liebermann H H, ed (ed.): ‘Rapidly solidified alloys: processes, structures, properties, applications’; 1993, New York, Marcel Dekker.
- Miller MK, Liaw (eds.) PK: ‘Bulk metallic glasses’; 2008, New York, Springer.
- Suryanarayana C, Inoue A: ‘Bulk metallic glasses’; 2011, Boca Raton, FL, CRC Press.
- Suryanarayana C, Jones H: ‘Formation and characteristics of quasicrystalline phases: a review’, Int. J. Rapid Solidif., 1988, 3, 253–293.
- Trebin H R, ed (ed.): ‘Quasicrystals: structure and physical properties’; 2003, Weinheim, Wiley-VCH.
- Gleiter H: ‘Nanocrystalline materials’, Prog. Mater. Sci., 1989, 33, 223–315.
- Suryanarayana C: ‘Nanocrystalline materials’, Int. Mater. Rev., 1995, 40, 41–64.
- Gleiter H: ‘Nanostructured materials: basic concepts and microstructure’, Acta Mater., 2000, 48, 1–29.
- Suryanarayana C: ‘Recent developments in nanostructured materials’, Adv. Eng. Mater., 2005, 7, 983–992.
- Klement W, Willens RH, Duwez P: ‘Non-crystalline structure in solidified gold-silicon alloys’, Nature, 1960, 187, 869–870.
- Suryanarayana C: ‘Rapidly quenched metals. A bibliography 1973–1979’; 1980, New York, IFI Plenum Press.
- Anantharaman TR, Suryanarayana C: ‘Review: a decade of quenching from the melt’, J. Mater. Sci., 1971, 6, 1111–1135.
- Jones H, Suryanarayana C: ‘Rapid quenching from the melt: an annotated bibliography 1958–72’, J. Mater. Sci., 1973, 8, 705–753.
- Chen HS: ‘Glassy metals’, Rep. Prog. Phys., 1980, 43, 353–432.
- Anantharaman TR, Suryanarayana C: ‘Rapidly solidified metals’; 1987, Aedermannsdorf, Trans Tech Publications.
- Smith CH: ‘Applications of rapidly solidified soft magnetic alloys’, in ‘Rapidly solidified alloys: processes, structures, properties, applications’, (ed. , Liebermann H H, ed), 617–663; 1993, New York, Marcel Dekker.
- Inoue A: ‘High strength bulk amorphous alloys with low critical cooling rates’, Mater. Trans., JIM, 1995, 36, 866–875.
- Johnson WL: ‘Bulk glass-forming metallic alloys: science and technology’, MRS Bull., 1999, 24, (10), 42–56.
- Inoue A: ‘Stabilization of metallic supercooled liquid and bulk amorphous alloys’, Acta Mater., 2000, 48, 279–306.
- Inoue A: ‘Bulk glassy and nonequilibrium crystalline alloys by stabilization of supercooled liquid: fabrication, functional properties and applications’, Proc. Jpn Acad. B, 2005, 81B, 156–188.
- Koshiba H, Inoue A: ‘Preparation and magnetic properties of Co-based bulk glassy alloys’, Mater. Trans., 2001, 42, 2572–2575.
- Inoue A, Zhang W, Zhang T, Kurosaka K: ‘High-strength Cu-based bulk glassy alloys in Cu-Zr-Ti and Cu-Hf-Ti ternary systems’, Acta Mater., 2001, 49, 2645–2652.
- Inoue A, Gook JS: ‘Fe-based ferromagnetic glassy alloys with wide supercooled liquid region’, Mater. Trans. JIM, 1995, 36, 1180–1183.
- Inoue A, Zhang T, Masumoto T: ‘Al-La-Ni amorphous alloys with a wide supercooled liquid region’, Mater. Trans. JIM, 1989, 30, 965–972.
- Inoue A, Kohinata M, Tsai AP, Masumoto T: ‘Mg-Ni-La amorphous alloys with a wide supercooled liquid region’, Mater. Trans. JIM, 1989, 30, 378–381.
- Zhang T, Inoue A: ‘New bulk glassy Ni-based alloys with high strength of 3000 MPa’, Mater. Trans., 2002, 43, 708–711.
- Inoue A, Nishiyama N, Matsuda T: ‘Preparation of bulk glassy Pd40Ni10Cu30P20 alloy of 40 mm in diameter by water quenching’, Mater. Trans. JIM, 1996, 37, 181–184.
- Zhang T, Inoue A: ‘Bulk glassy alloys with low liquidus temperature in Pt-Cu-P system’, Mater. Trans., 2003, 44, 1143–1146.
- Inoue A: ‘Synthesis and properties of Ti-based bulk amorphous alloys with a large supercooled liquid region’, Mater. Sci. Forum, 1999, 312–314, 307–314.
- Inoue A, Zhang T, Masumoto T: ‘Zr-Al-Ni amorphous alloys with high glass transition temperature and significant supercooled liquid region’, Mater. Trans. JIM, 1990, 31, 177–183.
- Peker A, Johnson WL: ‘A highly processable metallic glass: Zr41·2Ti13·8Cu12·5Ni10·0Be22·5’, Appl. Phys. Lett., 1993, 63, 2342–2344.
- Inoue A, ed (ed.): ‘Materials science and engineering of bulk metallic glasses’; 2008, Tokyo, Shiemshi Pub. Corp.
- Schroers J, Lohwongwatana B, Johnson WL, Peker A: ‘Gold based bulk metallic glass’, Appl. Phys. Lett., 2005, 87, 061912-1–061912-3.
- Guo FQ, Poon SJ, Shiflet GJ: ‘CaAl-based bulk metallic glasses with high thermal stability’, Appl. Phys. Lett., 2004, 84, 37–39.
- Zhang B, Pan MX, Zhao DQ, Wang WH: ‘“Soft” bulk metallic glasses based on cerium’, Appl. Phys. Lett., 2004, 85, 61–63.
- Gu X, Xing LQ, Hufnagel TC: ‘Glass-forming ability and crystallization of bulk metallic glass (HfxZr1−x)52·5Cu17·9Ni14·6Al10Ti5’, J. Non-Cryst. Solids, 2002, 311, 77–82.
- He Y, Price CE, Poon SJ, Shiflet GJ: ‘Formation of bulk metallic glasses in neodymium-based alloys’, Phil. Mag. Lett., 1994, 70, 371–377.
- Zhao ZF, Zhang Z, Wen P, Pan MX, Zhao DQ, Wang WH, Wang WL: ‘A highly glass-forming alloy with low glass transition temperature’, Appl. Phys. Lett., 2003, 82, 4699–4701.
- Wu J, Wang Q, Qiang JB, Chen F, Dong C, Wang YM, Shek CH: ‘Sm-based Sm–Al–Ni ternary bulk metallic glasses’, J. Mater. Res., 2007, 22, 573–577.
- Guo FQ, Poon SJ, Shiflet GJ: ‘Metallic glass ingots based on yttrium’, Appl. Phys. Lett., 2003, 83, 2575–2577.
- Zhang W, Inoue A: ‘Formation and mechanical strength of new Cu-based bulk glassy alloys with large supercooled liquid region’, Mater. Trans., 2004, 45, 1210–1213.
- Li R, Pang S, Ma C, Zhang T: ‘Influence of similar atom substitution on glass formation in (La–Ce)–Al–Co bulk metallic glasses’, Acta Mater., 2007, 55, 3719–3726.
- Zheng Q, Xu J, Ma E: ‘High glass-forming ability correlated with fragility of Mg–Cu(Ag)–Gd alloys’, J. Appl. Phys., 2007, 102, 113519-1–113519-5.
- Zeng YQ, Nishiyama N, Yamamoto T, Inoue A: ‘Ni-Rich bulk metallic glasses with high glass-forming ability and good metallic properties’, Mater. Trans., 2009, 50, 2441–2445.
- Inoue A, Nishiyama N, Kimura HM: ‘Preparation and thermal stability of bulk amorphous Pd40Cu30Ni10P20 alloy cylinder of 72 mm in diameter’, Mater. Trans. JIM, 1997, 38, 179–183.
- Schroers J, Johnson WL: ‘Highly processable bulk metallic glass-forming alloys in the Pt-Co-Ni-Cu-P system’, Appl. Phys. Lett., 2004, 84, 3666–3668.
- Inoue A, Zhang T: ‘Fabrication of bulk glassy Zr55Al10Ni5Cu30 alloy of 30 mm in diameter by a suction casting method’, Mater. Trans. JIM, 1996, 37, 185–187.
- Yokoyama Y, Mund E, Inoue A, Schultz L: ‘Production of Zr55Cu30Ni5Al10 glassy alloy rod of 30 mm in diameter by a cap-cast technique’, Mater. Trans., 2007, 48, 3190–3192.
- Inoue A, Shen BL: ‘A new Fe-based bulk glassy alloy with outstanding mechanical properties’, Adv. Mater., 2004, 16, 2189–2192.
- Inoue A, Takeuchi A, Zhang T: ‘Ferromagnetic bulk amorphous alloys’, Metall. Mater. Trans. A, 1998, 29A, 1779–1793.
- Inoue A, Shinohara Y, Gook JS: ‘Thermal and magnetic properties of bulk Fe-based glassy alloys prepared by copper mold casting’, Mater. Trans. JIM, 1995, 36, 1427–1433.
- Shen J, Chen QJ, Sun JF, Fan HB, Wang G: ‘Exceptionally high glass-forming ability of an FeCoCrMoCBY alloy’, Appl. Phys. Lett., 2005, 86, 151907-1–151907-3.
- Lu ZP, Liu CT, Porter WD: ‘Role of yttrium in glass formation of Fe-based bulk metallic glasses’, Appl. Phys. Lett., 2003, 83, 2581–2583.
- Liu DY, Sun WS, Wang AM, Zhang HF, Hu ZQ: ‘Preparation, thermal stability, and magnetic properties of Fe–Co–Zr–Mo–W–B bulk metallic glass’, J. Alloys Compd, 2004, 370, 249–253.
- Pawlik P, Davies HA: ‘The bulk glass forming abilities and mechanical and magnetic properties of Fe–Co–Zr–Mo–W–B alloys’, J. Non-Cryst. Solids, 2003, 329, 17–21.
- Guo SF, Wu ZY, Liu L: ‘Preparation and magnetic properties of FeCoHfMoBY bulk metallic glasses’, J. Alloys Compd, 2009, 468, 54–57.
- Hu Y, Liu L, Chan KC, Pan MX, Wang WH: ‘The effect of crystallization on microstructure and magnetic properties of Fe61Co7Zr9·5Mo5W2B15·5 bulk metallic glass’, Mater. Lett., 2006, 60, 1080–1084.
- Makino A, Men H, Kubota T, Yubuta K, Inoue A: ‘New Fe-metalloids based nanocrystalline alloys with high Bs of 1·9 T and excellent magnetic softness’, J. Appl. Phys., 2009, 105, 07A308-1–07A308-3.
- Inoue A, Park RE: ‘Soft magnetic properties and wide supercooled liquid region of Fe–P–B–Si base amorphous alloys’, Mater. Trans. JIM, 1996, 37, 1715–1721.
- Shen BL, Akiba M, Inoue A: ‘Excellent soft-ferromagnetic bulk glassy alloys with high saturation magnetization’, Appl. Phys. Lett., 2006, 88, 131907-1–131907-3.
- Makino A, Kubota T, Chang CT, Makabe M, Inoue A: ‘FeSiBP bulk metallic glasses with unusual combination of high magnetization and high glass-forming ability’, Mater. Trans., 2007, 48, 3024–3027.
- Shen BL, Koshiba H, Mizushima T, Inoue A: ‘Bulk amorphous Fe–Ga–P–B–C alloys with a large supercooled liquid region’, Mater. Trans. JIM, 2000, 41, 873–876.
- Shen BL, Inoue A: ‘Bulk glassy Fe–Ga–P–C–B–Si alloys with high glass-forming ability, high saturation magnetization and good soft magnetic properties’, Mater. Trans., 2002, 43, 1235–1239.
- Shen BL, Akiba M, Inoue A: ‘Effects of Si and Mo additions on glass-forming in FeGaPCB bulk glassy alloys with high saturation magnetization’, Phys. Rev. B, 2006, 73B, 10424-1–10424-6.
- Shen BL, Akiba M, Inoue A: ‘Effect of Cr addition on the glass-forming ability, magnetic properties, and corrosion resistance in FeMoGaPCBSi bulk glassy alloys’, J. Appl. Phys., 2006, 100, 043523-1–043523-5.
- Shen BL, Chang CT, Inoue A: ‘Formation, ductile deformation behavior and soft-magnetic properties of (Fe,Co,Ni)–B–Si–Nb bulk glassy alloys’, Intermetallics, 2007, 15, 9–16.
- Shen BL, Kimura HM, Inoue A, Mizushima T: ‘Bulk glassy Fe–Co–Ga–P–C–B alloys with high glass-forming ability, high saturation magnetization and good soft magnetic properties’, Mater. Trans., 2000, 41, 1675–1678.
- Jiang JZ, Olsen JS, Gerward L, Abdali S, Eckert J, Schlorke-de Boer N, Schultz L, Truckenbrodt J, Shi PX: ‘Pressure effect on crystallization of metallic glass Fe72P11C6Al5B4Ga2 alloy with wide supercooled liquid region’, J. Appl. Phys., 2000, 87, 2664–2666.
- Shen BL, Kimura HM, Inoue A, Omori M, Okubo A: ‘Preparation of Fe65Co10Ga5P12C4B4 bulk glassy alloy with good soft magnetic properties by spark-plasma sintering of glassy powder’, Mater. Trans., 2002, 43, 1961–1965.
- Liu FJ, Yang QW, Pang SJ, Ma CL, Zhang T: ‘Ductile Fe-based BMGs with high glass forming ability and high strength’, Mater. Trans., 2008, 49, 231–234.
- Chueva TR, Dyakonova NP, Molokanov VV, Sviridova TA: ‘Bulk amorphous alloy Fe72Al5Ga2C6B4P10Si1 produced by mechanical alloying’, J. Alloys Compd, 2007, 434–435, 327–332.
- Mizushima T, Igarashi K, Yoshida S, Makino A, Inoue A: ‘Soft magnetic properties of ring shape bulk glassy Fe-Al-Ga-P-C-B-Si alloy prepared by copper mold casting’, Mater. Trans. JIM, 1999, 40, 1019–1022.
- Li F, Zhang T, Inoue A, Guan SK, Shen NF: ‘(Fe,Co)–Zr–Nd–B bulk amorphous alloys with good soft magnetic properties’, Intermetallics, 2004, 12, 1139–1142.
- Zhang W, Matsusita M, Li CF, Kimura HM, Inoue A: ‘Glass-forming ability, crystallized structure and magnetic properties of Fe67Co9·5Nd3Dy0·5B20 glassy alloy with large supercooled liquid region’, Mater. Trans., 2001, 42, 2059–2063.
- Zhang W, Inoue A: ‘Formation and magnetic properties of bulk glassy Fe–Co–Nd–Dy–B alloys with high boron concentrations’, Mater. Trans., 2000, 41, 1679–1682.
- Xu M, Quan MX, Hu ZQ, Cheng LZ, He KY: ‘Thermal stability and magnetic properties of amorphous Fe-based alloys with significant supercooled liquid region’, J. Alloys Compd, 2002, 334, 238–242.
- Inoue A, Koshiba H, Zhang T, Makino A: ‘Thermal and magnetic properties of Fe56Co7Ni7Zr10−xNbxB20 amorphous alloys with wide supercooled liquid range’, Mater. Trans. JIM, 1997, 38, 577–582.
- Chiriac H, Lupu N: ‘Design and preparation of new soft magnetic bulk amorphous alloys for applications’, Mater. Sci. Eng. A, 2004, A375–A377, 255–259.
- Pawlik P, Davies HA: ‘Glass formability of Fe–Co–Pr–Dy–Zr–B alloys and magnetic properties following devitrification’, Scr. Mater., 2003, 49, 755–760.
- Parida S, Ram S, Eckert J, Roth S, Loser W, Schultz L: ‘Bulk glass forming and thermal stability in Fe67·0Co9·5Nd3·0Dy0·5B20 alloy’, Mater. Lett., 2004, 58, 1844–1852.
- Pawlik P, Davies HA, Gibbs MRJ: ‘Magnetic properties and glass formability of Fe61Co10Zr5W4B20 bulk metallic glassy alloy’, Appl. Phys. Lett., 2003, 83, 2775–2777.
- Panda AK, GhoshChowdhury S, Mitra A, Nishiyama N, Inoue A: ‘Electron transport behaviour and soft magnetic properties of bulk amorphous Fe72Si4B20Nb4 alloy’, J. Phys. D, 2006, 39D, 3536–3542.
- Amiya K, Urata A, Nishiyama N, Inoue A: ‘Fe–B–Si–Nb bulk metallic glasses with high strength above 4000 MPa and distinct plastic elongation’, Mater. Trans., 2004, 45, 1214–1218.
- Inoue A, Shimizu T, Yamaura S, Fujita Y, Takagi S, Kimura HM: ‘Development of glassy alloy separators for a proton exchange membrane fuel cell (PEMFC)’, Mater. Trans., 2005, 46, 1706–1710.
- Amiya K, Urata A, Nishiyama N, Inoue A: ‘Magnetic properties of (Fe,Co)–B–Si–Nb bulk glassy alloys with high glass-forming ability’, J. Appl. Phys., 2005, 97, 10F913-1–10F913-3.
- Chang HW, Huang YC, Chang CW, Hsieh CC, Chang WC: ‘Soft magnetic properties and glass formability of Y–Fe–B–M bulk metals (M = Al, Hf, Nb, Ta, and Ti)’, J. Alloys Compd, 2009, 472, 166–170.
- Ponnambalam V, Poon SJ, Shiflet GJ, Keppens VM, Taylor R, Petculescu G: ‘Synthesis of bulk metallic glasses as nonferromagnetic amorphous steel alloys’, Appl. Phys. Lett., 2003, 83, 1131–1133.
- Yao JH, Yang H, Zhang J, Wang JQ, Li Y: ‘The influence of Nb and Zr on glass-formation ability in the ternary Fe–Nb–B and Fe–Zr–B and quaternary Fe–(Nb,Zr)–B alloy systems’, J. Mater. Res., 2008, 23, 392–401.
- Yao JH, Wang JQ, Li Y: ‘Ductile Fe–Nb–B bulk metallic glass with ultrahigh strength’, Appl. Phys. Lett., 2008, 98, 251906-1–251906-3.
- Makino A, Li X, Yubuta K, Chang CT, Kubota T, Inoue A: ‘The effect of Cu on the plasticity of Fe–Si–B–P-based bulk metallic glass’, Scr. Mater., 2009, 60, 277–280.
- Gu XJ, Poon SJ, Shiflet GJ: ‘Mechanical properties of iron-based bulk metallic glasses’, J. Mater. Res., 2007, 22, 344–351.
- Gu XJ, McDermott AG, Poon SJ, Shiflet GJ: ‘Critical Poisson’s ratio for plasticity in Fe–Mo–C–B–Ln bulk amorphous steel’, Appl. Phys. Lett., 2006, 88, 211905-1–211905-3.
- Gu XJ, Poon SJ, Shiflet GJ: ‘Effects of carbon content on the mechanical properties of amorphous steel alloys’, Scr. Mater., 2007, 57, 289–292.
- Amiya K, Inoue A: ‘Fe–(Cr,Mo)–(C,B)–Tm bulk metallic glasses with high strength and high glass-forming ability’, Mater. Trans., 2006, 47, 1615–1618.
- Ponnambalam V, Poon SJ, Shiflet GJ: ‘Fe-based bulk metallic glasses with diameter thickness larger than one centimeter’, J. Mater. Res., 2004, 19, 1320–1323.
- Jayaraj J, Kim KB, Ahn HS, Fleury E: ‘Corrosion mechanism of N-containing Fe–Cr–Mo–Y–C–B bulk amorphous alloys in highly concentrated HCl solution’, Mater. Sci. Eng. A, 2007, A449–A451, 517–520.
- Shen BL, Inoue A: ‘Soft magnetic properties of bulk nanocrystalline Fe–Co–B–Si–Nb–Cu alloy with high saturated magnetization of 1·35 T’, J. Mater. Res., 2004, 19, 2549–2552.
- Wang ZM, Zhang J, Chang XC, Hou WL, Wang JQ: ‘Susceptibility of minor alloying to corrosion behavior in yttrium-containing bulk amorphous steel’, Intermetallics, 2008, 16, 1036–1039.
- Shen J, Chen QJ, Sun JF, Fan HB, Wang G: ‘Exceptionally high glass-forming ability of an FeCoCrMoCBY alloy’, Appl. Phys. Lett., 2005, 86, 151907-1–151907-3.
- Shen TD, Schwarz RB: ‘Bulk ferromagnetic glasses prepared by flux melting and water quenching’, Appl. Phys. Lett., 1999, 75, 49–51.
- Pang SJ, Zhang T, Asami K, Inoue A: ‘New Fe–Cr–Mo–(Nb, Ta)–C–B glassy alloys with high glass-forming ability and good corrosion resistance’, Mater. Trans., 2001, 42, 376–379.
- Pang SJ, Zhang T, Asami K, Inoue A: ‘Bulk glassy Fe–Cr–Mo–C–B alloys with high corrosion resistance’, Corros. Sci., 2002, 44, 1847–1856.
- Inoue A, Shen BL: ‘Soft magnetic bulk glassy Fe–B–Si–Nb alloys with high saturation magnetization above 1·5 T’, Mater. Trans., 2002, 43, 766–769.
- Inoue A, Shen BL, Ohsuna T: ‘Soft magnetic properties of nanocrystalline Fe–Si–B–Nb–Cu rod alloys obtained by crystallization of cast amorphous phase’, Mater. Trans., 2002, 43, 2337–2341.
- Gu XJ, Poon SJ, Shiflet GJ, Widom M: ‘Ductility improvement of amorphous steels: roles of shear modulus and electronic structure’, Acta Mater., 2008, 56, 88–94.
- Kim DH, Park JM, Kim DH, Kim WT: ‘Development of quaternary Fe–B–Y–Nb bulk glassy alloys with high glass-forming ability’, J. Mater. Res., 2007, 22, 471–477.
- Inoue A, Shen BL, Chang CT: ‘Super-high strength of over 4000 MPa for Fe-based bulk glassy alloys in [(Fe1−xCox)0·75B0·2Si0·05]96Nb4 system’, Acta Mater., 2004, 52, 4093–4099.
- Inoue A, Shen BL, Chang CT: ‘Fe- and Co-based bulk glassy alloys with ultrahigh strength of over 4000 MPa’, Intermetallics, 2006, 14, 936–944.
- Chang CT, Shen BL, Inoue A: ‘FeNi-based bulk glassy alloys with superhigh mechanical strength and excellent soft-magnetic properties’, Appl. Phys. Lett., 2006, 89, 051912-1–051912-3.
- Long ZL, Chang CT, Ding YH, Shao Y, Zhang P, Shen BL, Inoue A: ‘Corrosion behavior of Fe-based ferromagnetic (Fe, Ni)–B–Si–Nb bulk glassy alloys in aqueous electrolytes’, J. Non-Cryst. Solids, 2008, 354, 4609–4613.
- Chang CT, Shen BL, Inoue A: ‘Synthesis of bulk glassy alloys in the (Fe,Co,Ni)–B–Si–Nb system’, Mater. Sci. Eng. A, 2007, A449–A451, 239–242.
- Long ZL, Shao Y, Deng XH, Zhang ZC, Jiang Y, Zhang P, Shen BL, Inoue A: ‘Cr effects on magnetic and corrosion properties of Fe–Co–Si–B–Nb–Cr bulk glassy alloys with high glass-forming ability’, Intermetallics, 2007, 15, 1453–1458.
- Shen BL, Men H, Inoue A: ‘Fe-based bulk glassy alloy composite containing in-situ formed α-(Fe,Co) and (Fe,Co)23B6 microcrystalline grains’, Appl. Phys. Lett., 2006, 89, 101915-1–101915-3.
- Inoue A, Shen BL: ‘New Fe-based bulk glassy alloys with high saturated magnetic flux density of 1·4–1·5 T’, Mater. Sci. Eng. A, 2004, A375–A377, 302–306.
- Lu ZP, Liu CT, Thompson JR, Porter WD: ‘Structural amorphous steels’, Phys. Rev. Lett., 2004, 92, 245503-1–245503-4.
- Pang SJ, Zhang T, Asami K, Inoue A: ‘Effects of chromium on the glass formation and corrosion behavior of bulk glassy Fe–Cr–Mo–C–B alloys’, Mater. Trans., 2002, 43, 2137–2142.
- Pang SJ, Zhang T, Asami K, Inoue A: ‘Formation of bulk glassy Fe75-x-yCrxMoyC15B10 alloys and their corrosion behavior’, J. Mater. Res., 2002, 17, 701–704.
- Li HX, Kim KB, Yi S: ‘Enhanced glass-forming ability of Fe-based bulk metallic glasses prepared using hot metal and commercial raw materials through the optimization of Mo content’, Scr. Mater., 2007, 56, 1035–1038.
- Inoue A, Zhang T, Zhang W, Takeuchi A: ‘Bulk Nd–Fe–Al amorphous alloys with hard magnetic properties’, Mater. Trans. JIM, 1996, 37, 99–108.
- Inoue A, Zhang T, Takeuchi A: ‘Preparation of bulk Pr–Fe–Al amorphous alloys and characterization of their hard magnetic properties’, Mater. Trans. JIM, 1996, 37, 1731–1740.
- Inoue A, Gook JS: ‘Effect of additional elements (M) on the thermal stability of supercooled liquid in Fe72-xAl5Ga2P11C6B4Mx glassy alloys’, Mater. Trans. JIM, 1996, 37, 32–38.
- A. Inoue, A. Makino and T. Mizushima: ‘Ferromagnetic bulk glassy alloys’, J. Mag. Mag. Mater., 2000, 215–216, 246–252.
- Inoue A, Zhang T, Takeuchi A: ‘Bulk amorphous alloys with high mechanical strength and good soft magnetic properties in Fe–TM–B (TM = IV–VIII group transition metal) system’, Appl. Phys. Lett., 1997, 71, 464–466.
- Inoue A, Zhang W: ‘New Fe-based amorphous alloys with large magnetostriction and wide supercooled liquid region before crystallization’, J. Appl. Phys., 1999, 85, 4491–4493.
- Shen BL, Koshiba H, Kimura HM, Inoue A: ‘High strength and good soft Magnetic properties of bulk glassy Fe–Mo–Ga–P–C–B alloys with high glass-forming ability’, Mater. Trans., 2000, 41, 1478–1481.
- Makino A, Kubota T, Makabe M, Chang CT, Inoue A: ‘FeSiBP metallic glasses with high glass-forming ability and excellent magnetic properties’, Mater. Sci. Eng. B, 2008, B148, 166–170.
- Turnbull D: ‘Under what conditions can a glass be formed?’, Contemp. Phys., 1969, 10, 473–488.
- Egami T, Waseda Y: ‘Atomic size effect on the formability of metallic glasses’, J. Non-Cryst. Solids, 1984, 64, 113–134.
- Egami T: ‘The atomic structure of aluminum-based metallic glasses and universal criterion for glass formation’, J. Non-Cryst. Solids, 1996, 205–207, 575–582.
- Yan ZJ, Li JF, He SR, Zhou YH: ‘Evaluation of the optimum solute concentration for good glass forming ability in multicomponent metallic glasses’, Mater. Res. Bull., 2003, 38, 681–689.
- Ueno S, Waseda Y: ‘Evaluation of the optimum solute concentration for good glass formability in multi-component alloys’, J. Mater. Eng., 1987, 9, 199–204.
- Miracle DB: ‘A structural model for metallic glasses’, Nat. Mater., 2004, 3, 697–702.
- Miracle DB: ‘The efficient cluster packing model – an atomic structural model for metallic glasses’, Acta Mater., 2006, 54, 4317–4336.
- Miracle DB, Senkov ON: ‘Topological criterion for metallic glass formation’, Mater. Sci. Eng. A, 2003, A347, 50–58.
- Senkov ON, Miracle DB: ‘A topological model for metallic glass formation’, J. Non-Cryst. Solids, 2003, 317, 34–39.
- Inoue A: ‘Recent progress of Zr-based bulk amorphous alloys’, Sci. Rep. Res. Inst. Tohoku Univ. A, 1996, A42, 1–11.
- Inoue A: ‘Stabilization of supercooled liquid and opening-up of bulk glassy alloys’, Proc. Jpn Acad. B, 1997, 73B, 19–24.
- Inoue A: ‘Bulk amorphous alloys: preparation and fundamental characteristics’; 1998, Uetikon-Zürich, Trans Tech Publications.
- Xia L, Ding D, Shan ST, Dong YD: ‘The glass forming ability of Cu-rich Cu–Hf binary alloys’, J. Phys.: Condens. Matter, 2006, 18, 3543–3548.
- Xu D, Lohwongwatana B, Duan G, Johnson WL, Garland C: ‘Bulk metallic glass formation in binary Cu-rich alloy series – Cu100-xZrx (x = 34, 36, 38·2, 40 at.%) and mechanical properties of bulk Cu64Zr36 glass’, Acta Mater., 2004, 52, 2621–2624.
- Xia L, Li WH, Fang SS, Wei BC, Dong YD: ‘Binary Ni–Nb bulk metallic glasses’, J. Appl. Phys., 2006, 99, 026103-1–026103-3.
- Yao KF, Ruan F: ‘Pd–Si binary bulk metallic glass prepared at low cooling rate’, Chin. Phys. Lett., 2005, 22, 1481–1483.
- Mondal K, Murty BS: ‘On the parameters to assess the glass forming ability of liquids’, J. Non-Cryst. Solids, 2005, 351, 1366–1371.
- Yuan Z.-Z, Bao S.-L, Lu Y, Zhang D.-P, Yao L: ‘A new criterion for evaluating the glass-forming ability of bulk glass forming alloys’, J. Alloys Compd, 2008, 459, 251–260.
- Lu ZP, Liu CT: ‘A new glass-forming ability criterion for bulk metallic glasses’, Acta Mater., 2002, 50, 3501–3512.
- Lu ZP, Liu CT: ‘Glass formation criterion for various glass-forming systems’, Phys. Rev. Lett., 2003, 91, 115505-1–115505-4.
- Du XH, Huang JC, Liu CT, Lu ZP: ‘New criterion for glass forming ability for bulk metallic glasses’, J. Appl. Phys., 2007, 101, 086108-1–086108-3.
- Chen QJ, Shen J, Zhang D, Fan HB, Sun JF, McCartney DG: ‘A new criterion for evaluating the glass-forming ability of bulk metallic glasses’, Mater. Sci. Eng.A , 2006, A433, 155–160.
- Hrubý A: ‘Evaluation of glass forming tendency by means of DTA’, Czech. J. Phys.B , 1972, 22B, 1187–1193.
- Fan GJ, Choo H, Liaw PK: ‘A new criterion for the glass-forming ability of liquids’, J. Non-Cryst. Solids, 2007, 353, 102–107.
- Kim JH, Park JS, Lim HK, Kim WT, Kim DH: ‘Heating and cooling rate dependence of the parameters representing the glass forming ability in bulk metallic glasses’, J. Non-Cryst. Solids, 2005, 351, 1433–1440.
- Long ZL, Wei HQ, Ding YH, Zhang P, Xie GQ, Inoue A: ‘A new criterion for predicting the glass-forming ability of bulk metallic glasses’, J. Alloys Compd, 2009, 475, 207–219.
- Long ZL, Xie GQ, Wei HQ, Su XP, Peng J, Zhang P, Inoue A: ‘On the new criterion to assess the glass-forming ability of metallic alloys’, Mater. Sci. Eng.A, 2009, A509, 23–30.
- Suryanarayana C, Seki I, Inoue A: ‘A critical analysis of the glass-forming ability of alloys’, J. Non-Cryst. Solids, 2009, 355, 355–360.
- Takeuchi A, Inoue A: ‘Calculations of mixing enthalpy and mismatch entropy for ternary amorphous alloys’, Mater. Trans. JIM, 2000, 41, 1372–1378.
- Takeuchi A, Inoue A: ‘Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element’, Mater. Trans., 2005, 46, 2817–2829.
- Takeuchi A, Inoue A: ‘Mixing enthalpy of liquid phase calculated by Miedema’s scheme and approximated with sub-regular solution model for assessing forming ability of amorphous and glassy alloys’, Intermetallics, 2010, 18, 1779–1789.
- Lu ZP, Liu CT, Dong YD: ‘Effects of atomic bonding nature and size mismatch on thermal stability and glass-forming ability of bulk metallic glasses’, J. Non-Cryst. Solids, 2004, 341, 93–100.
- Suryanarayana C: ‘Mechanical alloying and milling’, Prog. Mater. Sci., 2001, 46, 1–184.
- Suryanarayana C: ‘Mechanical alloying and milling’; 2004, New York, Marcel Dekker.
- Koch CC: ‘Mechanical alloying and milling’, in ‘Processing of metals and alloys’, (ed. , Cahn R W, ed), 193–245; 1991, Weinheim, VCH Verlagsgesellschaft mbH.
- Koch CC, Whittenberger JD: ‘Mechanical milling/alloying of intermetallics’, Intermetallics, 1996, 4, 339–355.
- Fecht HJ, Johnson WL: ‘Destabilization and vitrification of crystalline matter’, J. Non-Cryst. Solids, 1990, 117–118, 704–707.
- Sharma S, Vaidyanathan R, Suryanarayana C: ‘Criterion for predicting the glass-forming ability of alloys’, Appl. Phys. Lett., 2007, 90, 111915-1–111915-3.
- Suryanarayana C, Sharma S: ‘Glass formation in mechanically alloyed Fe-based systems’, Funct. Mater. Lett., 2009, 4, 147–155.
- Cho YS, Koch CC: ‘Mechanical milling of ordered intermetallic compounds: the role of defects in amorphization’, J. Alloys Compd, 1993, 194, 287–294.
- Froes FH, Suryanarayana C, Russell KC, Li CG: ‘Synthesis of intermetallics by mechanical alloying’, Mater. Sci. Eng. A, 1995, A192–A193, 612–623.
- Klassen T, Oehring M, Bormann R: ‘Microscopic mechanisms of metastable phase formation during ball milling of intermetallic TiAl phases’, Acta Mater., 1997, 45, 3935–3948.
- Stoloff NS, Davies RG: ‘The mechanical properties of ordered alloys’, Prog. Mater. Sci., 1968, 13, 1–84.
- Wong R, Merz MD: ‘Non-crystallinity and polymorphism in elemental solids’, Nature, 1976, 260, 35–36.
- Matsubara E, Sato S, Imafuku M, Nakamura T, Koshiba H, Inoue A, Waseda Y: ‘Structural study of amorphous Fe70M10B20 (M = Zr, Nb and Cr) alloys by X-ray diffraction’, Mater. Sci. Eng. A, 2001, A312, 136–144.
- Hanada T, Hirotsu Y, Ohkubo T: ‘Nanoscale phase separation in an Fe70Nb10B20 glass studied by advanced electron microscopy techniques’, Mater. Trans., 2004, 45, 1194–1198.
- Frank FC: ‘Supercooling of liquids’, Proc. R. Soc. Lond. A, 1952, 215A, 43–46.
- Köster U, Meinhardt J, Roos S, Liebertz H: ‘Formation of quasicrystals in bulk glass forming Zr–Cu–Ni–Al alloys’, Appl. Phys. Lett., 1996, 69, 179–181.
- Chen MW, Zhang T, Inoue A, Sakai A, Sakurai T: ‘Quasicrystals in a partially devitrified Zr65Al7·5Ni10Cu12·5Ag5 bulk metallic glass’, Appl. Phys. Lett., 1999, 75, 1697–1699.
- Murty BS, Ping DH, Hono K, Inoue A: ‘Direct evidence for oxygen stabilization of icosahedral phase during crystallization of Zr65Cu27·5Al5 metallic glass’, Appl. Phys. Lett., 2000, 76, 55–57.
- Hirata A, Hirotsu Y, Amiya K, Inoue A: ‘Nanoscale metastable state exhibiting pseudotenfold diffraction pattern in Fe-based bulk metallic glass’, Phys. Rev. B, 2009, 79B, 020205(R)-1–020205(R)-4.
- Hirata A, Hirotsu Y, Amiya K, Nishiyama N, Inoue A: ‘Fe23B6-type quasicrystal-like structures without icosahedral atomic arrangement in an Fe-based metallic glass’, Phys. Rev. B, 2009, 80B, 140201(R)-1–140201(R)-4.
- Kelly A, Nicholson RB: ‘Precipitation hardening’, Prog. Mater. Sci., 1963, 10, 151–391.
- Hirata A, Guan PF, Fujita T, Hirotsu Y, Inoue A, Yavari AR, Sakurai T, Chen MW: ‘Direct observation of local atomic order in a metallic glass’, Nat. Mater., 2011, 10, 28–33.
- Sheng HW, Luo WK, Alamgir FM, Ma E: ‘Atomic packing and short-to-medium-range order in metallic glasses’, Nature, 2006, 439, 419–425.
- Kazimirov VYu, Louca D, Widom M, Gu XJ, Poon SJ, Shiflet GJ: ‘Local organization and atomic clustering in multicomponent amorphous steels’, Phys. Rev. B, 2008, 78B, 054112-1–054112-5.
- Li F, Shen BL, Makino A, Inoue A: ‘Excellent soft-magnetic properties of (Fe,Co)–Mo–(P,C,B,Si) bulk glassy alloys with ductile deformation behavior’, Appl. Phys. Lett., 2007, 91, 234101-1–234101-3.
- Shen BL, Chang CT, Kubota T, Inoue A: ‘Superhigh strength and excellent soft-magnetic properties of [(Co1−xFex)0·75B0·2Si0·05]96Nb4 bulk glassy alloys’, J. Appl. Phys., 2006, 100, 013515-1–013515-5.
- Bitoh T, Makino A, Inoue A, Greer AL: ‘Large bulk soft magnetic [(Fe0·5Co0·5)0·75B0·20Si0·05]96Nb4 glassy alloy prepared by B2O3 flux melting and water quenching’, Appl. Phys. Lett., 2006, 88, 182510-1–182510-3.
- Makino A, Bitoh T, Inoue A, Greer AL: ‘Soft magnetic bulk glassy alloy synthesized by flux melting and water quenching’, Mater. Sci. Forum, 2007, 539–543, 1921–1925.
- Amiya K, Inoue A: ‘Fe–(Cr, Mo)–(C, B)–Tm bulk metallic glasses with high strength and high glass-forming ability’, Rev. Adv. Mater Sci., 2008, 18, 27–29.
- Inoue A: Unpublished results, Tohoku University, Sendai, Japan, 2009.
- Lin C.-Y, Tien H.-Y, Chin T.-S: ‘Soft magnetic ternary iron-boron-based bulk metallic glasses’, Appl. Phys. Lett., 2005, 86, 162501-1–162501-3.
- Park JM, Park JS, Kim DH, Kim J.-H, Fleury E: ‘Formation, and mechanical and magnetic properties of bulk ferromagnetic Fe–Nb–B–Y–(Zr, Co) alloys’, J. Mater. Res., 2006, 21, 1019–1024.
- Wang WH: ‘Roles of minor additions in formation and properties of bulk metallic glasses’, Prog. Mater. Sci., 2007, 52, 540–596.
- Lu ZP, Liu CT: ‘Role of minor alloying additions in formation of bulk metallic glasses: a review’, J. Mater. Sci., 2004, 39, 3965–3974.
- Inoue A, Zhang T, Koshiba H, Makino A: ‘New bulk amorphous Fe–(Co,Ni)–M–B (M = Zr,Hf,Nb,Ta,Mo,W) alloys with good soft magnetic properties’, J. Appl. Phys., 1998, 83, 6326–6328.
- Perepezko JH: ‘Nucleation-controlled reactions and metastable structures’, Prog. Mater. Sci., 2004, 49, 263–284.
- Nouri AS, Liu Y, Lewandowski JJ: ‘Effects of thermal exposure and test temperature on structure evolution and hardness/viscosity of an iron-based metallic glass’, Metall. Mater. Trans. A, 2009, 40A, 1314–1323.
- Schuh CA, Huffnagel T, Ramamurty U: ‘Mechanical behavior of amorphous alloys’, Acta Mater., 2007, 55, 4067–4109.
- Chen MW: ‘Mechanical behavior of metallic glasses: microscopic understanding of strength and ductility’, Annu. Rev. Mater. Res., 2008, 38, 445–469.
- Suryanarayana C, Inoue A: ‘Bulk metallic glasses’, 361–458; 2011, Boca Raton, FL, CRC Press.
- Davis LA, Ray R, Chou C.-P, O’Handley RC: ‘Mechanical and thermal properties of Fe80B20 glass’, Scr. Metall., 1976, 10, 541–546.
- Inoue A, Shen BL, Koshiba H, Kato H, Yavari AR: ‘Ultra-high strength above 5000 MPa and soft magnetic properties of Co–Fe–Ta–B bulk glassy alloys’, Acta Mater., 2004, 52, 1631–1637.
- Lewandowski JJ, Gu XJ, Nouri AS, Poon SJ, Shiflet GJ: ‘Tough Fe-based bulk metallic glasses’, Appl. Phys. Lett., 2008, 92, 091918-1–091918-3.
- Nouri AS, Gu XJ, Poon SJ, Shiflet GJ, Lewandowski JJ: ‘Chemistry (intrinsic) and inclusion (extrinsic) effects on the toughness and Weibull modulus of Fe-based bulk metallic glasses’, Philos. Mag. Lett., 2008, 88, 853–861.
- Chen HS, Krause JT, Coleman E: ‘Elastic constants, hardness, and their implications to flow properties of metallic glasses’, J. Non-Cryst. Solids, 1975, 18, 157–171.
- Sunny G, Lewandowski JJ, Prakash V: ‘Effects of annealing and specimen geometry on dynamic compression of a Zr-based bulk metallic glass’, J. Mater. Res., 2007, 22, 389–401.
- Lewandowski JJ, Wang WH, Greer AL: ‘Intrinsic plasticity or brittleness of metallic glasses’, Philos. Mag. Lett., 2005, 85, 77–87.
- Suryanarayana C, Inoue A: ‘Bulk metallic glasses’, 392; 2011, Boca Raton, FL, CRC Press.
- Hennan DL, Anand L: ‘A large strain isotropic elasticity model based on molecular dynamics simulations of a metallic glass’, J. Elast., 2011, 104, 281–302.
- Cytron SJ: ‘A metallic glass-metal matrix composite’, J. Mater. Sci. Lett., 1982, 1, 211–213.
- Conner RD, Dandliker RB, Johnson WL: ‘Mechanical properties of tungsten and steel fiber reinforced Zr41·25Ti13·75Cu12·5Ni10Be22·5 metallic glass matrix composites’, Acta Mater., 1998, 46, 6089–6102.
- Pan DG, Zhang HF, Wang AM, Hu ZQ: ‘Enhanced plasticity in Mg-based bulk metallic glass composite reinforced with ductile Nb particles’, Appl. Phys. Lett., 2006, 89, 261904-1–261904-3.
- Hoffmann DC, Suh JY, Wiest A, Duan G, Lind ML, Demetriou MD, Johnson WL: ‘Designing metallic glass matrix composites with high toughness and tensile ductility’, Nature, 2008, 451, 1085–1090.
- Yavari AR, Lewandowski JJ, Eckert J: ‘Mechanical properties of bulk metallic glasses’, MRS Bull., 2007, 32, (8), 635–638.
- Xi XK, Zhao DQ, Pan MX, Wang WH, Wu Y, Lewandowski JJ: ‘Fracture of brittle metallic glasses: brittleness or plasticity’, Phys. Rev. Lett., 2005, 94, 125510-1–125510-4.
- Gu XJ, Poon SJ, Shiflet GJ, Lewandoski JJ: ‘Compressive plasticity and toughness of a Ti-based bulk metallic glass’, Acta Mater., 2010, 58, 1708–1720.
- Gu XJ, Poon SJ, Shiflet GJ, Widom M: ‘Mechanical properties, glass transition temperature, and bond enthalpy trends of high metalloid Fe-based bulk metallic glasses’, Appl. Phys. Lett., 2008, 92, 161910-1–161910-3.
- Zhao YH, Luo CY, Xi XK, Zhao DQ, Pan MX, Wang WH: ‘Synthesis and elastic properties of amorphous steels with high Fe content’, Intermetallics, 2006, 14, 1107–1111.
- Fujita K, Hashimoto T, Zhang W, Nishiyama N, Ma C, Kimura HM, Inoue A: ‘Ultrahigh fatigue strength in Ti-based bulk metallic glass’, Rev. Adv. Mater. Sci., 2008, 18, 137–139.
- Qiao DC, Wang GY, Liaw PK, Ponnambalam V, Poon SJ, Shiflet GJ: ‘Fatigue behavior of an Fe48Cr15Mo14Er2C15B6 amorphous steel’, J. Mater. Res., 2007, 22, 544–550.
- Naka M, Hashimoto K, Masumoto T: ‘Corrosion resistivity of amorphous Fe alloys containing Cr’, J. Jpn Inst. Met., 1974, 38, 835–841 (in Japanese).
- Naka M, Hashimoto K, Masumoto T: ‘High corrosion resistance of Cr-bearing amorphous Fe alloys in neutral and acidic solutions containing chloride’, Corrosion, 1976, 32, 146–152.
- Scully JR, Gebert A, Payer JH: ‘Corrosion and related mechanical properties of bulk metallic glasses’, J. Mater. Res., 2007, 22, 302–313.
- K. Asami, S. J. Pang, T. Zhang and A. Inoue: ‘Preparation and corrosion resistance of Fe-Cr-Mo-C-B-P bulk glassy alloys’, J. Electrochem. Soc., 2002, 149, B366–B369.
- F. Gostin, U. Siegel, C. Mickel, S. Baunack, A. Gebert and L. Schultz: ‘Corrosion behavior of the bulk glassy (Fe44.3Cr5Co5Mo12.8Mn11.2C15.8B5.9)98.5Y1.5 alloy’, J. Mater. Res., 2009, 24, 1471–1479.
- Tan MW, Akiyama E, Habazaki H, Kawashima A, Asami K, Hashimoto K: ‘The role of chromium and molybdenum in passivation of amorphous Fe–Cr–Mo–P–C alloys in deaerated 1 M HCl’, Corros. Sci., 1996, 38, 2137–2154.
- Asami K, Naka M, Hashimoto K, Masumoto T: ‘Effect of molybdenum on the anodic behavior of amorphous Fe–Cr–Mo–B alloys in hydrochloric acid’, J. Electrochem. Soc., 1980, 127, 2130–2138.
- Pang SJ, Zhang T, Asami K, Inoue A: ‘Synthesis of Fe–Cr–Mo–C–B–P bulk metallic glasses with high corrosion resistance’, Acta Mater., 2002, 50, 489–497.
- C. L. Qin, W. Zhang, H. Kimura, K. Asami, N. Ohtsu and A. Inoue: ‘Glass formation, corrosion behavior and mechanical properties of bulk glassy Cu-Hf-Ti-Nb alloys’, Acta Mater., 2005, 53, 3903–3911.
- Pardo A, Merino MC, Otero E, López MD, M’hich A: ‘Influence of Cr additions on corrosion resistance of Fe- and Co-based metallic glasses and nanocrystals in H2SO4’, J. Non-Cryst. Solids, 2006, 352, 3179–3190.
- Pardo A, Otero E, Merino MC, López MD, Vázquez M, Agudo P: ‘The influence of Cr addition on the corrosion resistance of Fe73·5Si13·5B9Nb3Cu1 metallic glass in SO2 contaminated environments’, Corros. Sci., 2001, 43, 689–705.
- Duwez P, Lin SCH: ‘Amorphous ferromagnetic phase in iron–carbon–phosphorus alloys’, J. Appl. Phys., 1967, 38, 4096–4099.
- Shen BL, Inoue A: ‘Fabrication of large-size Fe-based glassy cores with good soft magnetic properties by spark plasma sintering’, J. Mater. Res., 2003, 18, 2115–2121.
- Shen BL, Zhou YJ, Chang CT, Inoue A: ‘Effect of B to Si concentration ratio on glass-forming ability and soft-magnetic properties in (Co0·705Fe0·045B0·25−xSix)96Nb4 glassy alloys’, J. Appl. Phys., 2007, 101, 09N101-1–09N101-3.
- Kronmüller H, Goll D: ‘Micromagnetic theory of the pinning of domain walls at phase boundaries’, Physica B: Condens. Matter, 2002, 319, 122–126.
- Makino A, Bitoh T, Inoue A, Masumoto T: ‘Nb-poor Fe–Nb–B nanocrystalline soft magnetic alloys with small amount of P and Cu prepared by melt spinning in air’, Scr. Mater., 2003, 48, 869–874.
- G. Herzer: ‘Soft magnetic nanocrystalline materials’, Scr. Metall. Mater., 1995, 33, 1741–1756.
- Makino A, Men H, Kubota T, Yubuta K, Inoue A: ‘FeSiBPCu nanocrystalline soft magnetic alloys with high Bs of 1·9 Tesla produced by crystallizing hetero-amorphous phase’, Mater. Trans., 2009, 50, 204–209.
- Makino A, Men H, Kubota T, Yubuta K, Inoue A: ‘New Fe-metalloids based nanocrystalline alloys with high Bs of 1·9 T and excellent magnetic softness’, J. Appl. Phys., 2009, 105, 07A308-1–07A308-3.
- Makino A, Men H, Kubota T, Yubuta K, Inoue A: ‘New excellent soft magnetic FeSiBPCu nanocrystallized alloys with high Bs of 1·9 T from nanohetero-amorphous phase’, IEEE Trans. Mag., 2009, 45, 4302–4305.
- Inoue A, Nishiyama N: ‘New bulk metallic glasses for applications as magnetic-sensing, chemical, and structural materials’, MRS Bull., 2007, 32, (8), 651–658.
- Mizushima T, Koshiba H, Naito Y, Inoue A: ‘Preparation of the Fe-based glassy alloy powder cores and their applications’, J. Jpn Soc. Powder Powder Metall., 2007, 54, 768–772 (in Japanese).
- Koshiba H, Naito Y, Mizushima T, Inoue A: ‘Development of Fe-based metallic glass LIQUALLOYTM and its application to dust cores’, Materia, 2008, 47, 39–41 (in Japanese).
- Mizushima T, Koshiba H, Naito Y, Inoue A: ‘Soft magnetic properties and applications of the Fe-based glassy alloy ‘LiqualloyTM’ powder core’, J. Jpn Soc. Powder Powder Metall., 2008, 55, 146–148 (in Japanese).
- Matsumoto H., Urata A., Yamada Y., Inoue A. ‘Novel Fe(97-x-y)PxByNb2Cr1 glassy alloys with high magnetization and low loss characteristics for inductor core materials’, IEEE Trans. Mag., 2010, 46, 373–376.
- Yoshizawa Y, Oguma S, Yamauchi K: ‘New Fe-based soft magnetic alloys composed of ultrafine grain structure’, J. Appl. Phys., 1988, 64, 6044–6048.
- Nishiyama N, Amiya K, Inoue A: ‘Novel applications of bulk metallic glass for industrial products’, J. Non-Cryst. Solids, 2007, 353, 3615–3621.
- Liquidmetal Technologies. http://www.liquidmetal.com
- Sharma P, Zhang W, Amiya K, Kimura HM, Inoue A: ‘Nanoscale patterning of Zr–Al–Cu–Ni metallic glass thin films deposited by magnetron sputtering’, J. Nanosci. Nanotech., 2005, 5, 416–420.
- Ishida M, Takeda H, Watanabe D, Amiya K, Nishiyama N, Kita K, Saotome Y, Inoue A: ‘Fillability and imprintability of high-strength Ni-based bulk metallic glass prepared by the precision die-casting technique’, Mater. Trans., 2004, 45, 1239–1244.
- Ishida M, Takeda H, Nishiyama N, Kita K, Shimizu Y, Saotome Y, Inoue A: ‘Wear resistivity of super-precision microgear made of Ni-based metallic glass’, Mater. Sci. Eng. A, 2007, A449–A451, 149–154.
- Inoue A, Shen BL, Takeuchi A: ‘Fabrication, properties and applications of bulk glassy alloys in late transition metal-based systems’, Mater. Sci. Eng.A, 2006, A441, 18–25.
- Inoue A, Shen BL, Takeuchi A: ‘Developments and applications of bulk glassy alloys in late transition metal base system’, Mater. Trans., 2006, 47, 1275–1285.
- Inoue A, Yoshii I, Kimura HM, Okumura K, Kurosaki J: ‘Enhanced shot peening effect for steels by using Fe-based glassy alloy shots’, Mater. Trans., 2003, 44, 2391–2395.
- Son K, Soejima H, Nishiyama N, Wang XM, Inoue A: ‘Process development of metallic glass wires by a groove quenching technique for production of coil springs’, Mater. Sci. Eng. A, 2007, A449–A451, 248–252.
- Ma CL, Nishiyama N, Inoue A: ‘Fabrication and characterization of Coriolis mass flowmeter made form Ti-based glass tubes’, Mater. Sci. Eng.A, 2005, A407, 201–206.
- Hata S, Yamada N, Saotome Y, Inoue A, Shimokohbe A: ‘Precision and micromachining of metallic glasses – formability of Zr-based metallic glasses in the supercooled liquid state’, Proc. China–Japan Bilateral Conf. on ‘Advanced manufacturing engineering’, Huangshan, China, October 1998, 81–86.
- Soejima H, Nishiyama N, Takehisa H, Shimanuki M, Inoue A: ‘Viscous flow forming of Zr-based bulk metallic glasses for industrial products’, J. Metastable Nanocryst. Mater., 2005, 24–25, 531–534.
- Sugiyama M, Igarashi T, Okano T, Kimura HM, Inoue A: ‘Glassy alloy coating by high velocity thermal powder deposition and its application to lead-free soldering vessel’, Materia, 2007, 46, 31–33 (in Japanese).
- Sugiyama M, Igarashi T, Fukumoto M, Kimura HM, Inoue A: ‘Formation of Fe-based metallic glass coating films produced by high velocity oxy-fuel spraying process and their applications’, J. Jpn Soc. Powder Powder Metall., 2007, 54, 784–789 (in Japanese).
- Kim HG, Nakata K, Tsumura T, Sugiyama M, Igarashi T, Fukumoto M, Kimura HM, Inoue A: ‘Microstructure and wear behavior of Fe-based metallic glass sprayed coatng by HVOF on aluminum alloy’, Proc. ATSC-2006 Conf., Gyeongju, Korea, November 2006, 98–99.