Abstract
Reactive oxidative compounds including superoxide anions and nitric oxide are believed to play a central role in many blinding eye diseases. In the present study, we examine the effect of ischemia on human retinal pigment epithelial (RPE) cells in an unusual clinical case. We show that ischemia leads to extensive nitrotyrosine deposition in the RPE and choroid, thus indicating NO-dependent oxidative stress. We also show for the first time the in vivo translocation of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) to the nuclei of RPE cells. This enzyme's nuclear translocation has previously been demonstrated in vitro where it is a marker of apoptosis. Furthermore, nitrotyrosine deposition and GAPDH translocation have been duplicated in vitro using human RPE cells. Thus, nitrotyrosine formation and GAPDH trafficking to the nucleus may be observed during ischemic conditions.
Keywords:
Introduction
Reactive oxygen species and reactive nitrogen species contribute to tissue damage in a broad variety of clinical conditions including atherosclerosis, diabetes, neurodegenerative diseases, and cancer. In addition, oxidative stress is believed to be a key participant in numerous eye diseases, including age-related macular degeneration, glaucoma, and diabetic retinopathy.Citation1 Among eye diseases, the apoptotic death of retinal pigment epithelial (RPE) cells is believed to be a key underlying mechanism.Citation2 To better understand ischemia-related RPE cell death, we have studied oxidative cell damage in human eye tissue. We also show, for the first time, that nuclear translocation of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), a biomarker of apoptotic oxidative stress, is associated with ischemia in human RPE cells.
Report of the case
These studies were conducted with the approval of the University of Michigan IRB. Written informed consent was obtained prior to sample collection.
A 70-year-old woman developed rapid, progressive left proptosis; restriction of ocular motility; and eyelid edema over the course of 1 month. Magnetic resonance imaging showed a left superotemporal orbital mass requiring biopsy by anterior orbitotomy. Immediately postoperatively, she developed severe hypertension that resulted in an extensive orbital hemorrhage and total blindness due to ischemia in the distribution of the ophthalmic artery. Because frozen sections of the biopsy revealed unequivocal adenoid cystic carcinoma agreed upon by four pathologists, no immediate intervention was performed. One week later, after staging revealed no metastasis, a left orbital exenteration was performed.
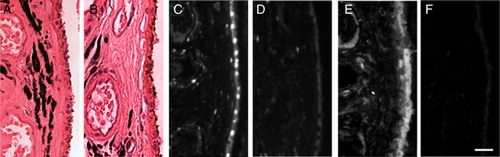
Histopathological examination confirmed the tumor diagnosis. The eye revealed arteriosclerosis and infarction of the optic nerve and surrounding portions of the choroid and outer retina as well as adjacent ischemia, all consistent with occlusion of short posterior ciliary artery perfusion (A). The inner retina did not show evidence of vascular compromise.
Figure 1. In vivo human RPE and choroidal GAPDH and nitrotyrosine. Ischemic choroid and RPE (A) demonstrate GAPDH nuclear translocation in RPE and vascular endothelial cells (C) as well as considerable immunoreactive nitrotyrosine (E). RPE and choroid of control eye show only cytoplasmic GAPDH and minimal nitrotyrosine (B, D, F). Bar = 25 µm.
Materials and methods
Immunohistochemistry was performed using standard methods. Paraffin-embedded human eye tissue was used. The tissue was fixed in 3.7% buffered formaldehyde and cut into 5 µm thick sections. Prior to labeling, sections were deparaffinized and gradually rehydrated by sequential incubation in ethanol 100, 95, 80, and 70%. After rehydration in phosphate buffer saline (PBS), sections were subjected to heat-mediated antigen retrieval in 10 mM citric acid buffer, pH 6.0. Sections were blocked using Invitrogen's Endogenous Biotin Blocking kit for 30 minutes (Carlsbad, CA, USA), and then blocked with blocking solution (10% normal goat serum, 6% BSA in PBS) for 1.5 hours at room temperature. After blocking procedures, sections were incubated with 2 µg/ml mouse monoclonal anti-nitrotyrosine antibody (1:50; Millipore, Billerica, MA, USA), monoclonal anti-GAPDH antibody (1:50; Millipore), or isotype control immunoglobulin G (IgG) diluted in 1% bovine cerum albumin (BSA) in PBS overnight at 4°C. The sections were washed with PBS and then incubated with 10 µg/ml biotin-XX goat anti-mouse IgG diluted in 1% BSA in PBS for 2 hours at room temperature. After incubation, the sections were washed with PBS. Finally, the sections were incubated with quantum dot (Qdot800; Invitrogen) nanoparticle–streptavidin conjugates for 1.5 hours, washed with PBS, then mounted in 90% glycerol in PBS, as previously described.Citation3
Results and discussion
To investigate the pathophysiologic phenomena associated with choroidal and outer retinal ischemia, immunofluorescence microscopy was performed using quantum dots. Using quantum dots absorbing in the ultraviolet and emitting in the infrared, we minimize the contribution of endogenous fluorescent molecules, such as lipofuscin, in the measured fluorescence emission. Because ischemia has been associated with biomarkers of oxidative stress,Citation4 we hypothesized that choroidal and outer retinal ischemia are associated with nitrotyrosine accumulation and apoptotic changes. Hence, retinal sections were stained with rabbit polyclonal anti-nitrotyrosine antibody as a marker for reactive nitrogen intermediate activity. In addition, monoclonal anti-GAPDH antibody was used as an apoptosis marker.Citation5,Citation6
Immunohistochemical analyses revealed GAPDH nuclear translocation in the RPE cells and the vascular endothelial cells of the choroid (C) in the regions of ischemia, but not in regions of RPE and choroid without histopathologically visible ischemia or in the inner retina. Oxidative reaction products were visualized by staining with anti-nitrotyrosine antibody. Regions of extensive nitrotyrosine reactivity correlated with regions of apoptotic changes (C and E). Three enucleation specimens without evidence of ischemia were used as controls. In all three specimens, normal RPE and choroid demonstrated GAPDH only in the cytoplasm of RPE, choroidal endothelial cells, and other cell types while little nitrotyrosine staining was observed (B, D, and F).
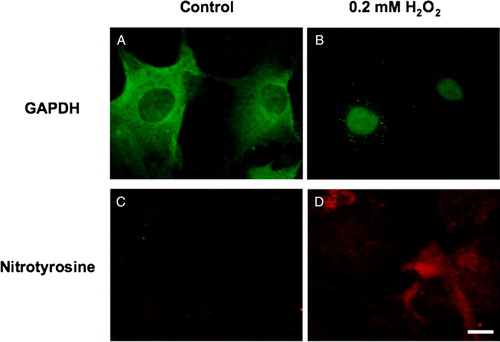
As the human studies described above are necessarily correlative, we sought to more fully develop the linkage between oxidant exposure and subsequent nitrotyrosine formation and GAPDH nuclear translocation. To do this, we employed in vitro cultures of human RPE cells to investigate the effect of oxidative stress on GAPDH nuclear translocation and the generation of nitrotyrosine under apoptotic conditions. Human RPE cells were isolated from donor eyes as previously described,Citation7 then exposed to control medium or medium containing 200 µM hydrogen peroxide for 24 hours. Immunocytochemical analysis revealed oxidative stress-induced GAPDH nuclear translocation and positive staining for nitrotyrosine (), thus mimicking the in vivo findings. However, RPE cells exposed to the identical culture conditions in the absence of hydrogen peroxide demonstrated no nitrotyrosine labeling and no GAPDH translocation to the nucleus. Hence, human RPE cells in vitro demonstrate nitrotyrosine deposition and GAPDH trafficking to the nucleus in response to oxidative stress.
Figure 2. In vitro human RPE GAPDH and nitrotyrosine. Cultured human RPE cells (second passage) were exposed to control medium (A, C) or medium containing 200 µM hydrogen peroxide (B, D) for 24 hours. Immunocytochemistry for GAPDH (A, B) and nitrotyrosine (C, D) was performed. Bar = 10 µm.
The RPE is particularly susceptible to oxidative stress because of its high consumption of oxygen, its high proportion of polyunsaturated fatty acids, and its exposure to visible light.Citation2 In this report, we demonstrated that GAPDH nuclear translocation in RPE cells is accompanied by nitrotyrosine accumulation, an indicator of irreversible oxidative cellular protein damage. GAPDH overexpression and nuclear translocation are considered to be important steps in apoptosis, and are often seen in neurodegenerative diseases.Citation5 As GAPDH is also a uracil DNA glycosylase, its translocation to the nucleus is thought to promote DNA repair. To our knowledge, this is the first description of GAPDH nuclear translocation in human RPE cells in vivo and in vitro, and the first direct evidence for oxidative reaction products in situ. This study provides fresh insights into the mechanism of human RPE apoptosis.
Acknowledgements
This work was supported by the Midwest Eye Bank and NIH grant # EY 019986 (HRP) and NIH grant # EY 09441 (VME). We also acknowledge NEI core grant # P30 EY007003. Dr Elner is a Senior Scientific Investigator Research Award recipient from Research to Prevent Blindness.
References
- Zierhut M, Cadenas E, Rao NA. Free radicals in ophthalmic disorders. New York, NY: Informa Healthcare; 2008. 217pp.
- Cai J, Nelson KC, Wu M, Sternberg P, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res 2000;19:205–21
- Petty HR, Elner VM, Kawaji T, Clark A, Thompson D, Yang DL. A facile method for immunofluorescence microscopy of human retinal sections with minimal interference from endogenous fluorescent species. J Neurosci Meth 2010;191:222–6.
- Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 2001;281:F948–57.
- Chuang DM, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu Rev Pharmacol Toxicol 2005;45:269–90.
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol 1996;271:1424–37.
- Yang D, Elner SG, Bian ZM, Till GO, Petty HR, Elner VM. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res 2007;85:462–72.