Abstract
Background
Experimental liver fibrosis induced by carbon tetrachloride (CCl4) is associated with oxidative stress, lipid peroxidation, and inflammation. This work was focused on elucidating the anti-inflammatory and antioxidant effects of ethylenediaminetetraacetic acid (EDTA) in this model of hepatotoxicity
Methods
Wistar male rats were treated with CCl4 and EDTA (60, 120, or 240 mg/kg). Morphometric analyses were carried out in Masson's stained liver sections to determine fibrosis index. Coagulation tests prothrombin time (PT) and partial thromboplastin time (PTT) were also determined. Gene expression for transforming growth factor beta (TGF-beta1), alpha1(I) procollagen gene (alpha1 Col I), tumor necrosis factor alpha (TNF-alpha), interleukin-6 (IL-6), and superoxide dismutase (SOD) was monitored by real-time PCR. Antioxidant effect of EDTA was measured by its effects on lipid peroxidation; biological activity of ceruloplasmin (Cp), SOD, and catalase (Cat) were analyzed by zymography assays.
Results
Animals with CCl4-hepatic injury that received EDTA showed a decrement in fibrosis (20%) and lipid peroxidation (22%). The mRNA expression for TNF-alpha (55%), TGF-beta1 (50%), IL-6 (52%), and alpha1 Col I (60%) was also decreased. This group of animals showed increased Cp (62%) and SOD (25%) biological activities. Coagulation blood tests, Cat activity, and gene expression for SOD were not modified by EDTA treatment.
Conclusion
This study demonstrates that EDTA treatment induces the activity of antioxidant enzymes, decreases lipid peroxidation, hepatic inflammation, and fibrosis in experimental liver fibrosis induced by CCl4.
Introduction
Hepatic cirrhosis is considered a dynamic and highly integrated cellular response to chronic liver injury. Liver function can be detrimentally altered by acute or chronic exposure to toxins (i.e. pro-oxidants) which initiate lipid peroxidation that stimulate hepatic stellate cells (HSCs), major producers of extracellular matrix (ECM) in liver injury.Citation1 A correlation between the presence of reactive oxygen intermediates (ROI) adducts and collagen gene expression by HSCs has been well established.Citation2,Citation3 Activation of HSCs is produced by the generation of free radicals and is blocked by several antioxidants.Citation4 Several cellular antioxidant systems have been described among them are those which are enzymes of the glutathione family, ceruloplasmin (Cp), superoxide dismutase (SOD), and catalase (Cat).Citation5 The glutathione system eliminates H2O2 in a reaction catalyzed by GSH peroxidase (2GSH + H2O2 → GSSG + 2H2O). SOD removes O2− by catalyzing the dismutation reaction: 2O2− + 2H+ → H2O2 + O2, and plays an important role in the pathogenesis of toxin-induced hepatitis.Citation6 Cat protects the cells by direct decomposition of H2O2 into H2O and O2. Cp is a plasma glycoprotein that is synthesized by the liver and secreted into the blood. Cp is thought to play an essential role in iron metabolism, but it also has antioxidant properties.Citation6–Citation10 The ferroxidase activity of Cp allows it to inhibit lipid peroxidation and hydroxyl radicals (HO*) production.Citation11 Absence or dysfunction of these defense systems renders the cell vulnerable to oxidative damage.Citation12,Citation13
Ethylenediaminetetraacetic acid (EDTA) chelation therapy in combination with vitamins and minerals is proposed to have antioxidant properties and considered a complementary treatment for cardiovascular diseases and diabetes. In previous studies, several authors demonstrated that multiple sessions of intravenous EDTA chelation therapy with or without vitamin C decrease the oxidative DNA damage in total human blood and lipid peroxidation in human plasma.Citation14,Citation15 Based on the association of oxidative stress and liver fibrosis, we explored the antioxidant effect of EDTA and its impact in gene expression and the activity of antioxidant enzymes, as well as its effect on the regression of exacerbated ECM accumulation and mRNA expression of pro-inflammatory cytokines in CCl4-induced liver fibrosis.
Methods
Animals
Male Wistar rats were rendered cirrhotic by chronic administration of CCl4 during 8 weeks, in an animal model that closely resembles human hepatic cirrhosis induced by alcohol abuse or chronic infection with hepatitis C virus. Briefly, animals weighing 80 g received three intraperitoneal (i.p.) doses per week of CCl4 and mineral oil mix (200 µl) in a ratio of 1:6 for the first week, 1:5 for the second week, 1:4 for the third week, and 1:3 for the fourth through 8 weeks as was reported previouslyCitation16 (A). Normal rats were pair-fed and injected with vehicle only. All animal studies were performed in accordance with University of Guadalajara's animal guidelines and technical specifications for the production, care, and use of laboratory animals NOM-062-ZOO-1999.Citation17 Five male rats were used for each group.
Figure 1. (A) Experimental design. Male Wistar rats were rendered cirrhotic by chronic intraperitoneal administration of CCl4. Animals weighing 80 g received three doses i.p. per week of CCl4 and mineral oil in a ratio of 1:6 for the first week, 1:5 for the second week, 1:4 for the third week, and 1:3 for the fourth to the 8th week. Fibrotic animals were treated with three different concentrations of EDTA to determine the lethal dose from 9th–11th week (dose/response curve). Additionally, two treatment protocols were performed: (1) preventive; where EDTA and CCl4 were administered for 11 weeks; and (2) therapeutic; where EDTA and CCl4 were administered for 3 weeks after liver fibrosis was established (detailed information is provided in the methods section). (B) The therapeutic dose of EDTA was determined by administering 60, 120, and 240 mg EDTA/kg in deionized water. Lethal doses (120 and 240 mg/kg) and therapeutic dose (60 mg/kg) were determined.
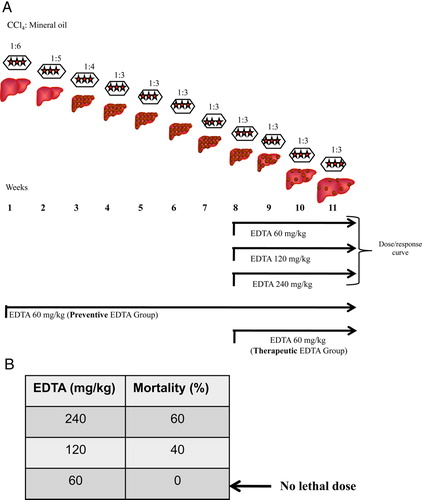
EDTA administration
First, a dose–response curve was carried out to find out the therapeutic and lethal dose of EDTA. Liver fibrosis was established in rats using CCl4 during 8 weeks. Then, EDTA at 60, 120, and 240 mg/kg (B) was administered via i.p.Citation18 three times per week during 3 weeks. During this period, CCl4 administration continued. EDTA and CCl4 were administered on alternate days. The therapeutic dose was evaluated by determining reversion of liver fibrosis and mortality at the end of the experiment. EDTA at 60 mg/kg was found to be non-toxic in fibrotic rats and improved liver histology as observed in sections stained with Masson trichromic stain. For the next experiments, the therapeutic EDTA dosage (60 mg/kg) was selected for treatment groups. Two protocols were performed: (1) preventive, where EDTA and CCl4 were administered during 11 weeks, three times per week on alternate days (preventive EDTA group); and (2) therapeutic, where EDTA and CCl4 were administered for 3 weeks, three times per week on alternate days, after liver fibrosis was established through 8 weeks of treatment with intraperitoneal CCl4 (therapeutic EDTA group A). Evaluated parameters in serum of all groups were: coagulation tests, lipid peroxidation, and Cp activity. SOD and Cat activities as well as mRNA expression of transforming growth factor beta (TGF-beta1), alpha1(I) procollagen gene (alpha1 Col I), tumor necrosis factor alpha (TNF-alpha), Interleukin-6 (IL-6), and SOD were analyzed in liver extracts.
Histopathological analysis
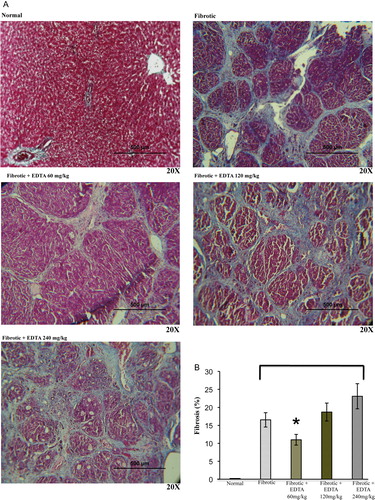
Hepatic sections were randomly taken from the right, median, and left lobes of rat livers in each experimental group and immediately fixed by immersion in 4% paraformaldehyde diluted in phosphate saline buffer, dehydrated in graded ethyl alcohol, and embedded in paraffin. Sections (5 mm thick) were stained with Masson's trichrome stain technique. Then, 20 random fields from each liver section were analyzed with light-microscopy (×20) using a computer-assisted morphometric analyzer (Image Proplus, Bethesda, MD, USA). The percentage of liver tissue affected by fibrosis was determined calculating the ratio of connective tissue to the whole area of the liver.
Coagulation tests
Blood was collected via heart puncture in animals from each group under ether anesthesia as scheduled in test tubes containing 0.129 M buffered sodium citrate solution (VACUTAINER Systems, Becton Dickinson, Franklin Lakes, NJ, USA). The samples were centrifuged at 1500× g for 10 minutes to obtain plasma. Prothrombin time (PT) and thromboplastin partial time (PTT) were measured using a coagulometer ST4 (Diagnostica Stago, Asnieres Sur Seine, France).
Lipid peroxidation assay
Lipid peroxidation was determined according to the modified method of Callaway.Citation19 Hundred milligrams of hepatic tissue from each experimental group were homogenized in 1 ml of buffer solution (150 mM KCl, 5 mM Tris, pH 7.4); 100 µl of supernatants were added to 10 nM ferrous sulfate and were incubated at 37°C for 10 minutes. Following that, 100 ml of 20 mM sodium dodecyl sulfate (SDS) and 750 ml of 20% (w/v) trichloroacetic acid were added and centrifuged at 10 000× g for 15 minutes at 4°C. The protein-free supernatant (500 µl) was removed from each tube and 0.5 ml aliquots of 0.8% (w/v) thiobarbituric acid were added, and incubated for 30 minutes at 95°C. After cooling (5 minutes), thiobarbituric acid reactive substance (TBARS) complexes were read at 532 nm. The TBARS concentration was calculated from a standard curve using a molar extinction coefficient of malondialdehyde (MDA). The final result is presented as nmol of MDA/mg of protein.
Zymography for biological activity of Cp, SOD, and Cat
Liver tissue (400 mg) was minced in 700 µl of protein extraction buffer, containing 96 mM Tris-HCl, pH 6.8, and 13.6 v/v glycerol. The homogenate was centrifuged at 12 000× g for 5 minutes at 4°C in a microtube to remove particulate matter and the supernatant was transferred to a clean tube and stored at −70°C. The protein concentration of each extract was measured according to Bradford.Citation20
Cp activity was detected using 500 µg of total protein from serum, which then was loaded onto a 9.54% sodium dodecyl sulpfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. After electrophoresis, SDS was removed from the gel by incubation in 2.5% (v/v) Triton X-100 at 4°C with gentle shaking. The gel was washed with distilled water to remove detergent and incubated at 37°C for 12 hours in a developing buffer containing 100 ml of 40 mM acetate buffer, pH 5.5, and 100 mg of O-dianisidine. Activity was detected as stained brown bands on a clear background.Citation21
SOD activity was analyzed using 500 µg of total protein applied to a 13.5% SDS-PAGE gel and used as a developing buffer, a mixture containing 10 mM potassium phosphate buffer, pH 7.5; 0.1 mM riboflavin, and 2 mM O-dianisidine. Incubation took place in the dark for 30 minutes at room temperature with agitation. Following incubation, the gels were rinsed with deionized water and exposed to daylight for 5–10 minutes at 25°C.Citation22,Citation23 Stained brown bands are representative areas detecting antioxidant activity. Activities in the gel slabs were quantified (relative units area). Cat activity was determined using 150 µg of total protein which were electrophoresed on 9.5% SDS-PAGE and developed using buffer containing a mixture of 15 ml 60 mM Na-thiosulfate, 35 ml 3% H2O2 (Sol. A), and 50 ml 90 mM potassium iodide and 250 ml of glacial acetic acid (Sol. B). Sol. A was quickly mixed before pouring it onto the gel; this was then incubated for 30 seconds. After that, Sol. A was drained, and Sol. B added. Following incubation, the gels were rinsed and placed in deionized water. This is a negative staining assay; hence, Cat activity results in achromatic spots on a dark background.Citation21 Activity in the gel slabs was quantified (all tests of zymography were measured and represented in relative units area using an image analyzer system Kodak 1D 3.5 image analyzer).
Gene expression of antioxidant enzymes and pro-inflammatory cytokines
RNA was isolated from liver homogenates of different rat groups with Trizol reagent (Invitrogen, Carlsbad, CA, USA). Two micrograms of total RNA were incubated with 1 U of ribonuclease-free deoxyribonuclease I at 37°C for 30 minutes, and the reaction was terminated by the addition of 50 mmol/L EDTA and heating at 75°C for 10 minutes. Samples were incubated with 0.5 mg of oligo (dT) primer at 70°C for 10 minutes and subjected to 100 U of Moloney murine leukemia virus reverse transcriptase (RT) for 50 minutes at 37°C. After incubation, samples were stored at −70°C. Two microliters of cDNA were subjected to real-time PCR using a Rotor Gene Thermocycler RG-3000-instrument (Corbett Research, Sydney, Australia) under the following conditions: 1 cycle of 50°C (2 minutes), 1 cycle of 95°C (10 minutes), and 40 cycles of 95°C (30 seconds) and 60°C (40 seconds). The total reaction was made in 10 µl containing 2 µl of cDNA, 1× of Universal PCR Master Mix (Applied Biosystems, Carlsbad, NJ, USA) and 1× of final concentration of primers and TaqMan probes. Specific probes designed to align with TNF-alpha, IL-6 TGF-beta1, and alpha1 Col I rat mRNAs were acquired from Applied Biosystems, NJ, USA. Gene amplification was normalized against 18S rRNA expression. Relative quantification by the 2−ΔΔCT method was carried out comparing to the control group as internal calibrator.Citation24,Citation25
Statistical analysis
Normally distributed data were analyzed using one-way analysis of variance (ANOVA). Then, Dunnett's post hoc test was used for determination of statistical significance. Significance was defined as a P value <0.05. Results are shown as the mean ± standard deviation of the mean. For real-time PCR experiments, results are shown as the 2−ΔΔCT value ± SD, but this standard deviation was calculated as: s= √s(18S)Citation2 + s(Targetgene)Citation2, according to the Applied Biosystems user bulletin.
Results
Standardization of dose/response curve for EDTA
Fibrotic animals were treated with three different concentrations of EDTA in order to determine the lethal dose following international guidelines for C-7 risk assessment of toxicity.Citation26 At the same time, all surviving animals were evaluated for liver fibrosis staging by using a computer-assisted image analyzer. To this purpose, animals reaching 8 weeks of CCl4 treatment were administered 60, 120, and 240 mg/kg EDTA from the 9th to the 11th week along with three weekly injections of CCl4 on alternate days. As indicated in B, animals undergoing the 120 and 240 mg/kg EDTA dosing showed a significant degree of mortality. On the other hand, all rats given 60 mg/kg survived and had a substantial fibrosis regression (20%) as compared with fibrotic controls (A, B). This therapeutic dose correlated with previous evidence reported in clinical protocols.Citation27 These results highlight the relevance of EDTA in inducing regression of liver fibrosis.
Figure 2. (A) Fibrosis stage determination. Representative images (×20) of liver sections stained with Masson trichrome from normal, fibrotic, and EDTA-treated rats at different concentrations (60, 120, and 240 mg/kg) (B) Morphometric analysis of liver section photographs using a computerized image analyzer showed that EDTA treatment at 60 mg/kg reduced by 20% the accumulation of ECM proteins. EDTA therapy with doses of 120 and 240 mg/kg increased ECM accumulation. Values are the mean ± standard deviation of five rats per group. Asterisks indicate values significantly different (*P ≤ 0.05).
Coagulation tests
A shows PTT and PT for normal rats and for the different groups of fibrotic animals treated or not with EDTA. It is clear that there were no significant differences between fibrotic and EDTA-treated animals.
Figure 3. Coagulation times, lipid oxidation (MDA), and ceruloplasmin curve. (A) Coagulation times of different treated groups. PTT and PT were analyzed. There were no significant differences between fibrotic and EDTA-treated animals. (B) Lipid peroxidation measurement by the MDA technique. Significant decrease in lipid peroxidation was observed in fibrotic animals treated with EDTA (60 mg/kg) in the preventive and therapeutic EDTA groups with respect to fibrotic control. (C) Activity of Cp at 2, 6, and 11 weeks of CCl4 treatment. Cp activity diminished gradually in serum concomitantly with time of CCl4 insult. (D) Activity of Cp at 2 weeks of CCl4 intoxication with or without concomitant administration of EDTA (60 mg/kg). EDTA treatment groups maintained Cp activity even in the presence of a continued administration of CCl4. (E) Activity of Cp at 6 weeks of CCl4 intoxication with or without concomitant administration of EDTA (60 mg/kg). As the time of intoxication advanced, Cp activity increased in the EDTA-treated group (62%). (F) Cp activity in therapeutic and preventive groups. EDTA treatment is associated with increased Cp activity. The Cp activity in the normal EDTA group was not statistically different with respect to the normal group in all assays (data not shown). Cp activity was determined by zymography assay. Activities in the gel slabs were quantified (relative units area) using an image analyzer system (Kodak 1D 3.5 image analyzer). Values are the mean ± standard deviation of the mean of five rats per group. Asterisks indicate values significantly different (*P ≤ 0.05).
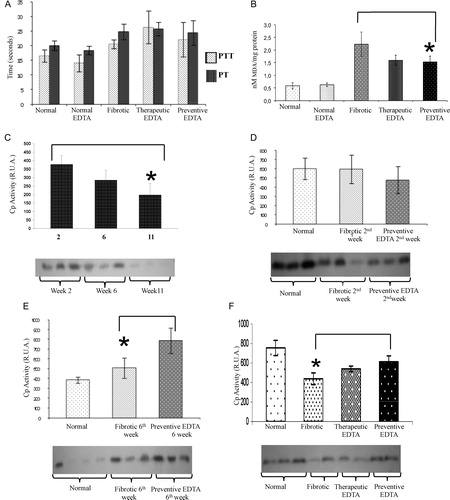
Lipid peroxidation
In B, an 18% decrease in lipid peroxidation was observed in fibrotic animals treated with EDTA (60 mg/kg) in the preventive EDTA group and a significant diminution of 22% in the therapeutic EDTA group with respect to fibrotic controls.
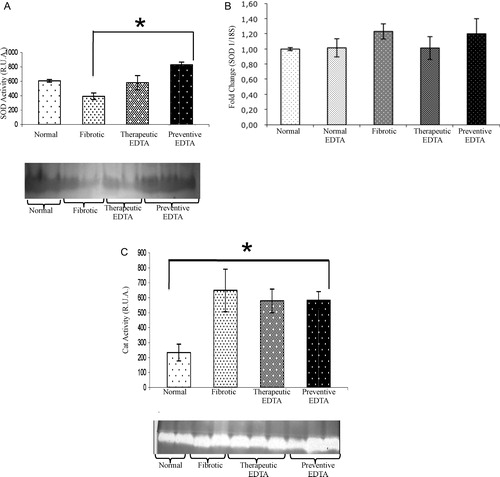
Determination of biological activity for Cp, SOD, and Cat
As described in previous studies, Cp is an acute phase protein present in high amounts in liver acute injury, which diminishes as CCl4 damage advances (C).Citation28 In this experimental assay, Cp did not show an important variation at the second week of CCl4 plus EDTA (60 mg/kg) treatment with respect to fibrotic and normal controls (D). Nevertheless, Cp functional activity was increased by 30% in the presence of EDTA treatment at the 6th week in comparison with the fibrotic group (E). In the same way, Cp activity was increased by 20% in preventive EDTA group in the 11th week (F). The therapeutic effect of EDTA treatment is evident in fibrotic animals that were treated with EDTA (60 mg/kg) in a preventive fashion (F). SOD activity increased significantly (50%) in the liver from animals of the preventive group in comparison with non-EDTA-treated animals at the end of the regimen (A). C depicts an increase in fibrotic animals in Cat activity as compared with normal rats, suggesting a compensation mechanism induced by CCl4 injury. EDTA treatment did not induce a further increase in Cat in fibrotic animals, but did not induce a down-regulation of this enzyme either.
Figure 4. Activity and gene expression of superoxide-dismutase and activity of catalase. Both analyzes were realized in liver tissues of each experimental group. In graphic (A) SOD and (C) Cat activities were analyzed by zymography. Bands of antioxidant activity and their densitometric analysis are presented. The SOD and Cat activities in the normal EDTA group were not statistically different from the normal group (data not shown). EDTA treatment did not modify Cat activity, while it increased the SOD activity. Graphic (B) represents the gene expression of SOD determined by real-time PCR. Gene expression analysis was realized by real-time PCR using TaqMan probes (Applied Biosystems). The housekeeping gene 18S was used to normalize the measurement. Activities in the gel slabs from zymographies were quantified (relative units area) using an image analyzer system (Kodak 1D 3.5 image analyzer). Values are the mean ± standard deviation of the mean of five rats per group. Asterisks indicate values significantly different (*P ≤ 0.05).
Gene expression by real-time PCR
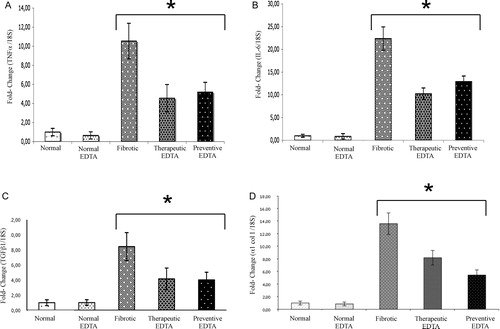
The effect of EDTA treatment on SOD gene expression was not evident (B). The mRNA for TNF-alpha decreased significantly in preventive and therapeutic group (55 and 58%, respectively A). Similarly, IL-6 mRNA decreased in therapeutic and in preventive EDTA groups with a decrement of 52 and 48%, respectively (B). Analysis of mRNA for an important profibrogenic protein (TGF-beta1) shows a 48% decrease in the therapeutic EDTA group. A similar decrement (52%) was found in preventive EDTA group (C). Regarding the expression of alpha1 Col I, a molecule of immediate response to TGF-beta1 action, a decrease was also found in both the therapeutic EDTA group (36%) and the preventive EDTA group (60%) (D).
Figure 5. Gene expression of TNF-alpha, IL-6, TGF-beta1, alpha1 (I) Col. The upper panels represent the anti-inflammatory activity of EDTA treatment, which showed a significant decrement in gene expression of (A) TNF-alpha and (B) IL-6 in the preventive and therapeutic EDTA groups. Similarly, the molecular analysis of the anti-fibrotic effect of EDTA, determined by gene expression of TGF-beta1 and alpha1 Col I (panels C and D, respectively), showed an important decrement of these proteins in the same groups. This analysis was realized by real-time PCR using TaqMan probes (Applied Biosystems). The housekeeping gene 18S was used to normalize the measurement. Values are the mean ± standard deviation of five rats per group. Asterisks indicate values significantly different (*P ≤ 0.05).
Discussion
Intraperitoneal administration of CCl4 to rats is associated with the generation of pro-oxidant agents (ROI), production of pro-inflammatory cytokines, and ECM accumulation in the liver parenchyma.Citation29 As we have shown, these characteristics of hepatic damage were reduced with EDTA therapy. The mechanism through which EDTA exerts its therapeutic effect is presumably through its antioxidant activity, as we demonstrated in this study. There is limited information about the antioxidant effect of EDTA in vivo. Recently, it has been described that intravenous EDTA chelation therapy is able to decrease the oxidative DNA damage in total human blood and lipid peroxidation in human plasma.Citation14,Citation15 Additionally, it has been demonstrated that EDTA shows a protective effect in platelets in which peroxidation of lipids was stimulated by UV light.Citation30 The reported protective effect of EDTA on lipid peroxidation was reproduced in the model of experimental liver fibrosis that we used; there was a diminution of 22% in lipid peroxidation in fibrotic animals treated with EDTA in relation to the cognate control groups (B).
Decrease in lipid peroxidation supposes an increased efficiency against damage, as reflected in reduced accumulation of ECM and in limited expression of pro-inflammatory and pro-fibrotic cytokines that is shown in and . Application of EDTA to CCl4 injured rats caused an important diminution of gene expression of pro-inflammatory molecules activated by reactive oxygen species (ROS) such as TNF-alpha and IL-6 (A), as well as profibrogenic molecules that respond to pro-oxidant stimuli, i.e TGF-beta1 and alpha1 Col I (C, D). The potential beneficial effects of reduced production of pro-inflammatory and pro-fibrotic cytokines and limited ROS generation include inhibition of hepatic stellate cell activation/collagen production, an effect that is reflected in limited liver fibrosis as we observed with a therapeutic dose of EDTA in our experiment (). Other antioxidants in addition to EDTA have also been shown to inhibit TNF-alpha, IL-6, and IL-8 in liver diseases.Citation31,Citation32
Further evidence of the diminution in liver damage with EDTA therapy is the change in Cp activity. It has been established that a diminution of its antioxidant activity is correlated with hepatic damage.Citation33 In the Cp activity curve that we observed, Cp activity diminished gradually until reaching hardly detectable serum levels concomitantly with damage and time of insult in CCl4-treated animals (C). Surprisingly, EDTA treatment groups maintained Cp levels even in the presence of continued CCl4 administration (D). As the time of intoxication advanced, Cp activity increased in the EDTA-treated group (62%) (E). This result suggests a stimulus of EDTA on Cp activity as a protective effect vs. oxidative stress, correlating with the previous reports,Citation34,Citation35 which demonstrated Cp's capacity to inhibit lipid peroxidation. In the same way, the Cp antioxidant activity during the last period of damage remained increased, suggesting a protective function during liver chronic damage (F). It has been previously demonstrated that antioxidants can prevent CCl4 toxicity, particularly hepatotoxicity, by inhibiting lipid peroxidation and increasing activities of antioxidant enzymes.Citation36,Citation37 In this scenario, the application of EDTA to CCl4 injured rats increases not only the antioxidant activity of Cp, but also the SOD enzymatic activity as shown in A–C. SOD activity transforms and eliminates pro-oxidative elements. EDTA can enhance the antioxidant activity of SOD independent of its gene expression, since the enzyme's mRNA did not change (B). In this metabolic order of elimination of the free radicals, Cat is responsible of transmutation of hydrogen peroxide to water and oxygen. Here, Cat responded to CCl4 insult in the intoxicated animal groups by an increased activity, and remained high in cirrhotic animals treated with EDTA (C). It seems that the increase in Cat activity is independent of EDTA. CAT is activated by free radicals and generation of H2O2 groups in such a way that its activity is enhanced after the onset of damage.Citation33 The findings in lipid peroxidation and antioxidant activity associated with the expression of pro-fibrotic and pro-inflammatory cytokines, together, suggest that the EDTA therapy promotes the cellular antioxidant systems and limits the generation of pro-oxidative elements and in consequence diminishes the liver injury.
Finally, a principal concern about EDTA therapy is that addition of a calcium chelator like EDTA might induce a decrement of procoagulation capacity. EDTA concentration in the extracellular space is sufficient for chelation of metallic-ionic elements, but does not reach the minimal concentrations within platelets necessary to affect their function, as we showed here (A). EDTA chelates the large pool of freely diffusible calcium ions in the plasma, and also chelates the free iron in the blood without affecting coagulation function.Citation38 Furthermore, EDTA has a short lifespan and is eliminated in 12–14 hours.Citation39
In conclusion, EDTA has been used in diverse clinical protocols in pathologies that present exacerbated production of free radicals, mainly cardiovascular diseases. We have presented the analysis of some aspects of pro-inflammatory and pro-oxidant molecules involved in the development of experimental liver fibrosis. According to this model, in vivo EDTA use suggests a modification of homeostatic systems through an antioxidant effect. Taken altogether, our results suggest an anti-inflammatory and antioxidant profile stimulated by the application of EDTA in vivo in experimental hepatic fibrosis.
References
- Slater TF. Free-radical mechanisms in tissue injury. Biochem J 1984;222(1):1–15.
- Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology 1997;26(1):135–42.
- Paradis V, Mathurin P, Kollinger M, Imbert-Bismut F, Charlotte F, Piton A, et al. In situ detection of lipid peroxidation in chronic hepatitis C: correlation with pathological features. J Clin Pathol 1997;50(5):401–6.
- Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest 1995;96(5):2461–8.
- Shuvalova EP, Antonova TV, Baranovskaia VB. The importance of the antioxidant protection systems of the blood in adaptation to the infectious process in viral hepatitis B. Ter Arkh 1991;63(11):47–9.
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989;320(14):915–24.
- Aksoy H, Taysi S, Altinkaynak K, Bakan E, Bakan N, Kumtepe Y. Antioxidant potential and transferrin, ceruloplasmin, and lipid peroxidation levels in women with preeclampsia. J Investig Med 2003;51(5):284–7.
- Taysi S, Gul M, Sari RA, Akcay F, Bakan N. Serum oxidant/antioxidant status of patients with systemic lupus erythematosus. Clin Chem Lab Med 2002;40(7):684–8.
- Cogalgil S, Taysi S. Levels of antioxidant proteins and soluble intercellular adhesion molecule-1 in serum of patients with rheumatoid arthritis. Ann Clin Lab Sci 2002;32(3):264–70.
- Zowczak M, Iskra M, Paszkowski J, Manczak M, Torlinski L, Wysocka E. Oxidase activity of ceruloplasmin and concentrations of copper and zinc in serum of cancer patients. J Trace Elem Med Biol 2001;15(2–3):193–6.
- Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 1990;186:1–85.
- Badwey JA, Karnovsky ML. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem 1980;49:695–726.
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol 1986;58:61–97.
- Hininger I, Waters R, Osman M, Garrel C, Fernholz K, Roussel AM, et al. Acute prooxidant effects of vitamin C in EDTA chelation therapy and long-term antioxidant benefits of therapy. Free Radic Biol Med 2005;38(12):1565–70.
- Roussel AM, Hininger-Favier I, Waters RS, Osman M, Fernholz K, Anderson RA. EDTA chelation therapy, without added vitamin C, decreases oxidative DNA damage and lipid peroxidation. Altern Med Rev 2009;14(1):56–61.
- Armendariz-Borunda J, Seyer JM, Kang AH, Raghow R. Regulation of TGF beta gene expression in rat liver intoxicated with carbon tetrachloride. FASEB J 1990;4(2):215–21.
- Norma Oficial Mexicana NOM-062-ZOO-1999 [Internet]. Available from: http://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF
- Foreman H, Vier M, Magee M. The metabolism of C14-labeled ethylenediaminetetraacetic acid in the rat. J Biol Chem 1953;203(2):1045–53.
- Callaway JK, Beart PM, Jarrott B. A reliable procedure for comparison of antioxidants in rat brain homogenates. J Pharmacol Toxicol Methods 1998;39(3):155–62.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Manchenko GP. Handbook of detection of enzymes on electrophoretic gels Boca Raton: CRC Press LLC; 2003. p. 168–70.
- Burke JJ, Oliver MJ. Differential temperature sensitivity of pea superoxide dismutases. Plant Physiol 1992;100(3):1595–8.
- Misra HP, Fridovich I. Superoxide dismutase and peroxidase: a positive activity stain applicable to polyacrylamide gel electropherograms. Arch Biochem Biophys 1977;183(2):511–5.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25(4):402–8.
- Yuan JS, Reed A, Chen F, Stewart CN Statistical analysis of real-time PCR data. BMC Bioinformatics 2006;7:85.
- C7-Risk assessment. European Commission Health & Consumer Protection Directorate-General. Directorate C —Public Health and Risk Assessment [Internet]. Available from: http://ec.europa.eu/food/fs/sc/sct/out191_en.pdf
- Ernst E. Chelation therapy for coronary heart disease: an overview of all clinical investigations. Am Heart J 2000;140(1):139–41.
- Wachowicz B, Krajewski T, Zbikowska H. Protective effect of ceruloplasmin against lipid peroxidation in blood platelets. Acta Biochim Pol 1990;37(2):261–6.
- Rao KS, Recknagel RO. Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Exp Mol Pathol 1968;9(2):271–8.
- Stocks J, Gutteridge JM, Sharp RJ, Dormandy TL. The inhibition of lipid autoxidation by human serum and its relation to serum proteins and alpha-tocopherol. Clin Sci Mol Med 1974;47(3):223–33.
- Barollo M, D'Inca R, Scarpa M, Medici V, Cardin R, Bortolami M, et al. Effects of iron manipulation on trace elements level in a model of colitis in rats. World J Gastroenterol 2005;11(28):4396–9.
- Barve A, Khan R, Marsano L, Ravindra KV, McClain C. Treatment of alcoholic liver disease. Ann Hepatol 2008;7(1):5–15.
- Meilhac O, Zhou M, Santanam N, Parthasarathy S. Lipid peroxides induce expression of catalase in cultured vascular cells. J Lipid Res 2000;41(8):1205–13.
- Nakano H, Ogita K, Gutteridge JM, Nakano M. Inhibition by the protein ceruloplasmin of lipid peroxidation stimulated by an Fe3+-ADP-adriamycin complex. FEBS Lett 1984;166(2):232–6.
- Gutteridge JM. Inhibition of the Fenton reaction by the protein caeruloplasmin and other copper complexes. Assessment of ferroxidase and radical scavenging activities. Chem Biol Interact 1985;56(1):113–20.
- Teselkin YO, Babenkova IV, Kolhir VK, Baginskaya AI, Tjukavkina NA, Kolesnik YA, et al. Dihydroquercetin as a means of antioxidative defence in rats with tetrachloromethane hepatitis. Phytother Res 2000;14(3):160–2.
- Kumaravelu P, Dakshinamoorthy DP, Subramaniam S, Devaraj H, Devaraj NS. Effect of eugenol on drug-metabolizing enzymes of carbon tetrachloride-intoxicated rat liver. Biochem Pharmacol 1995;49(11):1703–7.
- Zhang M, Wong IG, Gin JB, Ansari NH. Assessment of methylsulfonylmethane as a permeability enhancer for regional EDTA chelation therapy. Drug Deliv 2009;16(5):243–8.
- Allain P, Mauras Y, Premel-Cabic A, Islam S, Herve JP, Cledes J. Effects of an EDTA infusion on the urinary elimination of several elements in healthy subjects. Br J Clin Pharmacol 1991;31(3):347–9.