Abstract
We have previously demonstrated that the induction of heme oxygenase-1 (HO-1) (EC 1.14.99.3) plays a protective role against oxidative stress in leaves and nodules of soybean plants subjected to cadmium, UV-B radiation, and salt stress. Here, we investigated HO-1, localization and their relationship with oxidative stress in different growth stages of soybean plants roots inoculated with Bradyrhizobium japonicum (3, 5, 7, 10, and 20 days post-inoculation) and nodules.
After 7 days of inoculation, we observed a 70% increase in thiobarbituric acid-reactive substances that correlates with an enhancement in the gene expression of HO-1, catalase, and superoxide dismutase. Furthermore, the inhibition of HO-1 activity by Zn-protoporphyrin IX produced an increase in lipid peroxidation and a decrease in glutathione content suggesting that, in this symbiotic process, HO-1 may act as a signal molecule that protects the root against oxidative stress.
We determined, for the first time, the tissular localization of HO-1 in nodules by electron-microscope examination. These results undoubtedly demonstrated that this enzyme is localized only in the plant tissue and its overexpression may play an important role as antioxidant defense in the plant. Moreover, we demonstrate that, in roots, HO-1 is induced by oxidative stress produced by inoculation of B. japonicum and exerts an antioxidant response against it.
Introduction
Nodules are unique, specialized organs that are the result of a symbiotic association between plants of the family Leguminosae and soil bacteria of the genera Rhizobium. The interaction of rhizobia and legumes begins with signal exchange and recognition of the symbiotic partners, followed by attachment of the rhizobia to the plant root hairs. The root hair deforms, and the bacteria invade the plant by a newly formed infection thread growing through it. Simultaneously, cortical cells are mitotically activated, giving rise to the nodule primordium. lnfection threads grow toward the primordium, and the bacteria are then released into the cytoplasm of the host cells, surrounded by a plant-derived peribacteroid membrane (PBM). The nodule primordium thereupon develops into a mature nodule, while the bacteria differentiate into their endosymbiotic form, which is known as the bacteroid. Bacteroids, together with the surrounding PBMs, are called symbiosomes. At this stage, bacteria synthesize nitrogenase, which enables them to fix atmospheric dinitrogen, thus allowing the plant host to grow without external reduced nitrogen.Citation1–Citation5
Oxidative stress may be defined as an increment of reactive oxygen species (ROS) and/or a depletion of antioxidant defenses. ROS are superoxide anion (O2−.), hydrogen peroxide (H2O2), and hydroxyl radical.Citation6 In plants, this ROS generation is enhanced by different stressors, including Cd, UV-B, and salinity.Citation7–Citation10 However, classic anti-oxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) protect plant against this deleterious production. In another group of enzymes, the non-classic antioxidant enzymes, Heme oxygenases (HO EC 1.14.99.3), an enzyme that catalyzes the oxidative conversion of heme to biliverdin IX alpha with the concomitant release of carbon monoxide and iron, plays a key role as an important defense against oxidative stress.Citation11 However, reports from our laboratory have shown the presence in soybean leaves and nodules of one HO closely related to the heme oxygenase-1 (HO-1) of mammalian cells on the basis of its induction by prooxidants and its antioxidant behavior under Cd, UV-B, and salt stresses, indicating that HO-1 may play a protective role against oxidative cell damage in soybean plants.Citation12–Citation15
In this study, we investigate if the inoculation with Bradyrhizobium japonicum induces any oxidative stress insult and overexpression of HO-1 in roots of soybean plants and, as a second aim, to elucidate the localization of HO-1 in the symbiosome.
Materials and methods
Plant material and treatments
Surface-sterilized soybean seeds (Glycine max L.) (A6445RG) were germinated directly in plastic pots containing vermiculite in controlled environmental chambers, with a photoperiod of 16 h, photon flux density of 175 µmol/m2/s, and a day/night regime of 25/20°C and were simultaneously inoculated with B. japonicum strain E 109 (INTA, Castelar), inoculated (I), not inoculated (NI) plants were used as controls. They were watered daily with N-free nutrient solutionCitation16 during 30 days and then roots were isolated (3, 5, 7, 10, and 20 days post-inoculation) and used for the determinations. For the determination of HO-1 behavior, we used as control NI soybean roots treated with H2O2 (2.4 mM) or N,N′-dimethyl-4,4′-bipyridinium dichloride (paraquat) (5 µM) or Zn-protoporphyrin IX (ZnPPIX) (20 µM) during 72 hours). On the other hand, another group of plants were performed to study HO-1 localization. In this way, after 4 weeks, inoculated plants were treated with nutrient solution devoid of salt (control) or containing 100 mM of NaCl. After 10 days of treatment, nodules were isolated and used for the immunohistochemical assays.
Thiobarbituric acid-reactive substances (TBARS) determination
Lipid peroxidation was measured as the amount of TBARS determined by the TBA reaction as described by Heath and Packer.Citation17 Roots (0.3 g) were homogenized in 3 ml of 20% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 3500 × g for 20 minutes. To 1 ml of the aliquot of the supernatant, 1 ml of 20% TCA containing 0.5% (w/v) TBA and 4% butylated hydroxytoluene (BHT) in ethanol was added. The mixture was heated at 95°C for 30 minutes and then quickly cooled on ice. The contents were centrifuged at 10 000 × g for 15 minutes and the absorbance was measured at 532 nm. Value for non-specific absorption at 600 nm was subtracted. The concentration of TBARS was calculated using an extinction coefficient of 155/mM/cm.
Glutathione (GSH) determination
Non-protein thiols were extracted by homogenizing 0.3 g of roots in 3.0 ml of 0.1 N HCl (pH 2.0), and 1 g PVP. After centrifugation at 10 000 g for 30 minutes at 4°C, the supernatants were used for analysis. Total glutathione (GSH plus GSSG) was determined in the homogenates spectrophotometrically at 412 nm. GSH content was calculated from the difference between total GSH and GSSG.Citation18
Classical antioxidant enzymes
Extracts for determination of SOD and CAT activities were prepared from 0.3 g of roots homogenized under ice-cold conditions in 3 ml of extraction buffer, containing 50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 g PVP, and 0.5% (v/v) Triton X-100 at 4°C. The homogenates were centrifuged at 10 000 × g for 20 minutes and the supernatant fraction was used for the assays.
CAT activity was determined in the homogenates by measuring the decrease in absorption at 240 nm in a reaction medium containing 50 mM potassium phosphate buffer (pH 7.2) and 2 mM H2O2. The pseudo-first-order reaction constant (k′ = k(CAT)) of the decrease in H2O2 absorption was determined and the CAT content in pmol/mg protein was calculated using k = 4.7 × 107/M/s.Citation19
Total SOD activity was assayed by the inhibition of the photochemical reduction of NBT, as described by Becana et al.Citation20 The reaction mixture consisted of 50–150 µl of enzyme extract and 3.5 ml O2−. generating solution that contained 14.3 mM methionine, 82.5 µM NBT, and 2.2 µM riboflavin. Extracts were brought to a final volume of 0.3 ml with 50 mM K-phosphate (pH 7.8) and 0.1 mM Na2EDTA. Test tubes were shaken and placed 30 cm from a light bank consisting of six 15 W fluorescent lamps. The reaction was allowed to run for 10 minutes and stopped by switching the light off. The reduction in NBT was followed by reading absorbance at 560 nm. Blanks and controls were run the same way but without illumination and enzyme, respectively. One unit of SOD was defined as the amount of enzyme that produced a 50% inhibition of NBT reduction under the assay conditions.Citation20,Citation21
Histochemical analysis
Histochemical detection of lipid peroxidation was performed as described by Yamamoto.Citation22 Roots I and NI were stained with Schiff's reagent for 30 minutes, which detects aldehydes that originate from lipid peroxides. After staining, roots were rinsed with a sulfite solution (0.5% (w/v) K2S2O5 in 0.05 M HCl).
The localization of the loss of plasma membrane integrity was detected by staining roots with Evans Blue solution (0.025% (w/v) Evans Blue in 100 µM CaCl2, (pH 5.6) for 10 minutes. Then, roots were rinsed three times with 100 µM CaCl2 (pH 5.6).Citation22
In order to analyze H2O2 generation, roots were excised and immersed in a 1% solution of 3,3′-Diaminobenzidine (DAB) in Tris-HCl buffer (pH 6.5), vacuum-infiltrated for 5 minutes and then incubated at room temperature for 16 hours in the absence of light. Roots were illuminated until the appearance of brown colors characteristic of the reaction of DAB with H2O2.
In the same way to show O2− production, roots were excised and immersed in a 0.1% solution of NBT in K-phosphate buffer (pH 6.4), containing 10 mM Na-azide, and were vacuum-infiltrated for 5 minutes and illuminated until the appearance of dark spots, characteristic of blue formazan precipitate.
The stained roots were photographed using Q Color 5 Olimpus 5 mp.
Isolation of RNA and RT-PCR analysis
Total RNA was isolated using Trizol reagent (Gibco BRL, Grand Island, NY 14072-2028), treated with RNase-free DNase I (Promega, Madison, WI 53711, USA), and reverse transcribed into cDNA using random hexamers and M-MLV Superscript II RT (Gibco BRL). PCR reactions were carried out using G. max L. HO-1-, CAT-, SOD-, and 18S-specific primers, as previously described.Citation23 Each primer set was amplified using an optimized number of PCR cycles to ensure the linearity requirement for semi-quantitative RT-PCR analysis. Ethidium bromide-stained gels were scanned (Photodyne Incorporated, WI, USA) and analyzed using Gel-Pro Analyzer 3.1 software (Media Cybernetics, MD, USA). The ratio of gene transcript to 18S mRNA was quantified.
Inmunogold localization of HO
Soybean nodules were fixed in 4% (w/v) formaldehyde and 1% (v/v) glutaraldehyde. Tissues were embedded in medium-grade LR White acrylic resin (London Resin, Basinstoke, UK) as described by Vanden Bosch.Citation24 Ultra-thin sections, 90–150 nm thick, were picked up on 300 mesh Ni grids. Grids were preincubated on drops of TBS containing 3% (w/v) BSA and 0.1% (v/v) Tween-20 for 1 hour at room temperature. After blocking the grids were incubated overnight, at room temperature, with rabbit antibody Arabdopsis thaliana HO-1Citation25 (diluted 1:100 in TBS containing 1% (w/v) BSA). After this, treatment grids were incubated for 1 hour, at room temperature, in rabbit anti-goat IgG conjugated to 10 nm colloidal gold (Sigma Chemical, St. Louis, Missouri 63178, USA). Grids were rinsed with water and sections were post-stained with 2% (w/v) aqueous uranyl acetate and with Reynolds solution for 10 seconds each. The sections were observed in Zeiss EM C10 transmission electron microscope at 80 k and photographed with 35 mm Kodak electron film.
Protein determination
Protein concentration was evaluated by the method of BradfordCitation26 using bovine serum albumin as a standard.
Statistic
All values reported in this work represent the means of four independent experiments. The mean values ± standard deviation (SD) is given in the tables and figures. A one-way analysis of variance test was used to confirm the significance of the data. Comparison with control and treatments was performed using the Tukey test.
Results
Oxidative stress generation
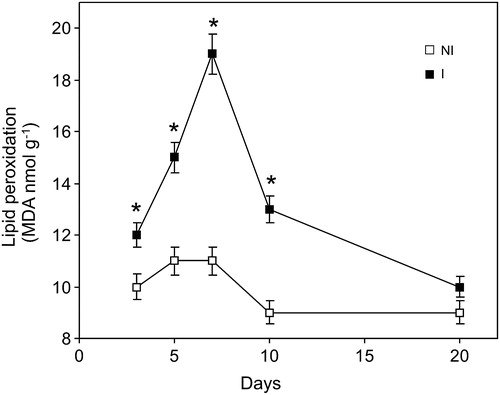
In order to evaluate the oxidative stress generated by B. japonicum inoculation, TBARS formation was determined in soybean roots during 3, 5, 7, 10, and 20 days post-inoculation. We found an increase at 3 days post-inoculation reaching a maximum (70%) in roots after 7 days of inoculation, compared to not-inoculated plants ().
Figure 1. Time course of lipid peroxidation caused by inoculation with B. japonicum on soybean roots. Data are mean ± SD, n = 4. P < 0.01 as assessed by Tukey's t-test. NI, not inoculated; I, inoculated plants.
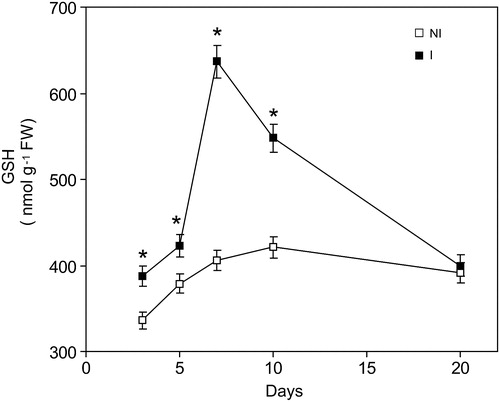
GSH is one of the most important soluble non-enzymatic defenses against oxidative stress. As can be see in , there is a correlation between GSH and TBARS. At 7 days, GSH content increased 90% () with respect to the not-inoculated plants group.
Figure 2. Time course changes of GSH levels caused by inoculation with Bradyrhizobium japonica on soybean roots. Data are mean ± SD, n = 4. P < 0.01 as assessed by Tukey's t-test. NI, not inoculated; I, inoculated plants.
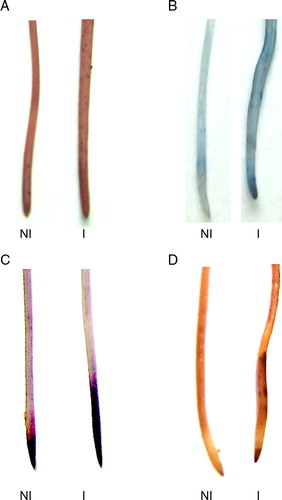
In the same way, the distribution of histochemical staining patterns of lipid peroxidation and the loss plasma membrane integrity, as well H2O2 and O2− production, were observed in root of 7 days post-inoculated plants ().
Figure 3. Histochemical detection of lipid peroxidation and the loss membrane integrity caused by inoculation. The roots were stained with Schiff's reagent (A), Evans blue (B), Nitroblue tetrazolium (C) and DAB (D) in soybean root as described in Materials and methods. NI, not inoculated; I, inoculated plants.
Classical antioxidant enzymes
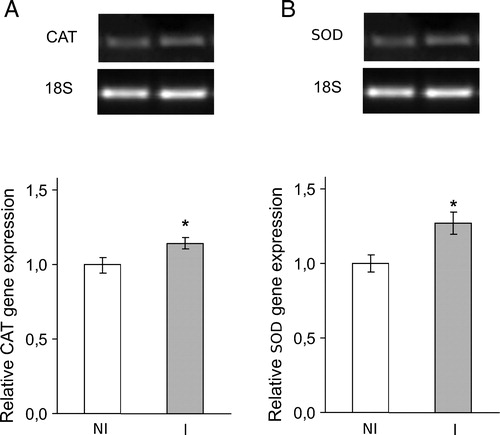
As expected, SOD and CAT activities showed significant increases (68 and 30%, respectively) with respect to the not-inoculated plants group (data no shown). To confirm these results, we performed RT-PCR with specific primers. SOD and CAT gene expressions increased in root at 7 days post-inoculation ().
Figure 4. Classical antioxidant enzymes SOD and CAT gene expression at seventh day post-inoculation with B. japonicum on soybeans roots. CAT and SOD mRNA expressions were analyzed by semiquantitative RT-PCR as described in Materials and methods. The 18S amplification band is shown to confirm equal loading of RNA and RT efficiency. Values are the mean of four independent experiments and bars indicate SD. *Significant differences (P < 0.01) with respect to not inoculated plants, according to Tukey's multiple range test. NI, not inoculated; I, inoculated plants.
Effect of inoculation with B. japonicum on HO-1 gene expression
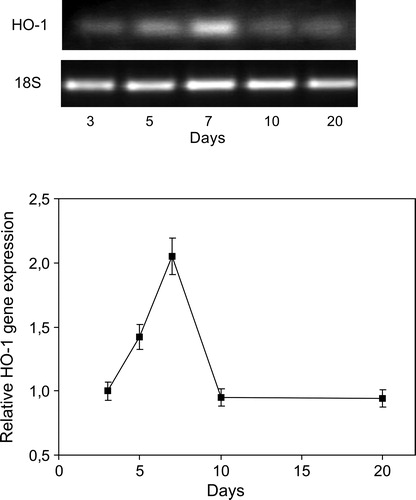
Because our previous works involved HO as part of the antioxidant enzymatic defense system in soybean plants,Citation12,Citation13 we subsequently explored the behavior of this enzyme in post-inoculation conditions (gene expression). At 7 days, semi-quantitative RT-PCR revealed that post-inoculation increase 2-fold HO-1 gene transcript in roots (). The increase in HO-1 gene expression correlates with the raise in lipid peroxidation (), GSH content (), and the activities and gene expression of antioxidant enzymes SOD and CAT (). Therefore, the HO increase could contribute of counteract oxidative stress generation.
Figure 5. Time course of HO-1 gene expression caused by inoculation with B. japonicum on soybeans roots. HO-1 mRNA expression was analyzed by semiquantitative RT-PCR as described in Materials and methods. The 18S amplification band is shown to confirm equal loading of RNA and RT efficiency. Values are the mean of four independent experiments and bars indicate SD, with respect to control according to Tukey's multiple range test.
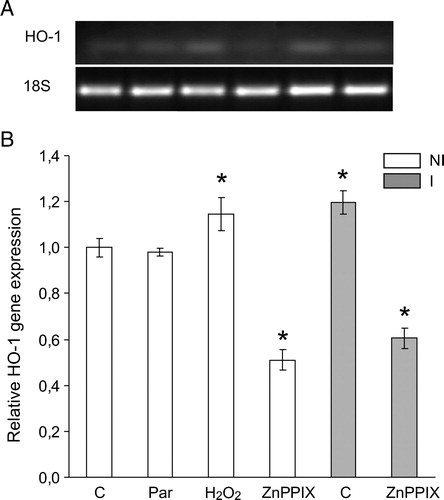
Effect of HO-1 blockade in roots of inoculated plants
To study the role of HO-1 in the symbiotic association, we performed a set of experiments with the specific inhibitor of HO-1, ZnPPIX. Interestingly, HO-1 inhibition generated an increase in TBARS and prevents the raise in GSH levels (data not shown) demonstrating that, HO-1 is involved as a key protein against oxidative stress in the symbiotic association between soybean plants and B. japonicum. In addition, and to gain a better understanding of the involvement of oxidative stress generation in inoculated soybean plants, we incubated not-inoculated soybean roots with H2O2 (a powerful oxidant) or paraquat (a well-known O2−. generator), and surprisingly we found that only H2O2 but not O2−. produced the HO-1 gene induction ().
Figure 6. HO-1 gene expression in soybean roots exposed to H2O2 (2.4 mM), paraquat (Par) (5 µM) or ZnPPIX (20 µM) during 24 hours. HO-1 mRNA expression was analyzed by semiquantitative RT-PCR as described in Materials and methods. The 18S amplification band is shown to confirm equal loading of RNA and RT efficiency. Values are the mean of four independent experiments and bars indicate SD. *Significant differences (P < 0.01) with respect to not inoculated control according to Tukey's multiple range test. NI, not inoculated; I, inoculated plants.
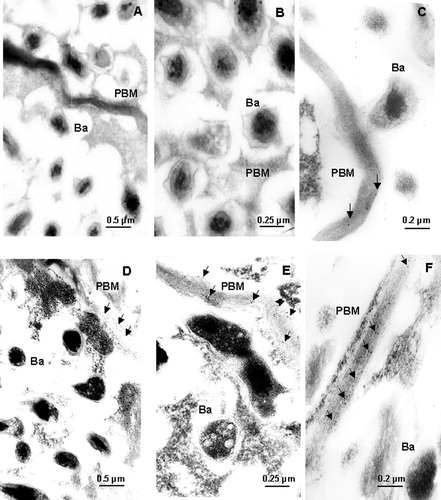
Electron microscopy of soybean nodules
Electron-microscope examination of immunogold-labeled sections of nodules showed that HO-1 antigen was present on the PBM (). While the presence of HO in salt-treated nodules was visualized in PMB at all magnifications assayed, in control nodules it can only be detected at a higher resolution (50 000×). These experiments clearly demonstrate not only the specific HO-1 localization but also the relationship between HO-1 protein expression and antigen detection.
Figure 7. Immunogold localization of HO-1 in mature nodules. Controls (A, B, and C) and salt-treated (D, E, and F) nodules were incubated and labeled as described in Materials and methods. Ba, bacteroid; PBM, peribacteroid membrane. Arrows indicate HO spots. Magnification: 20 000× (A and D), 40 000× (B and E), 50 000× (C and F).
Discussion
Oxidative stress is the result of an excessive production of oxidant species and/or depletion of intracellular antioxidant defenses, leading to an imbalance in the redox status of the cell.
However, it has been established that ROS are directly sensed by key regulatory molecules that activate the expression of genes encoding antioxidant enzymes at transcription level that protects from oxidative stress.Citation27,Citation28 One of the major cellular defense enzymes in mammals and plants is SOD, an enzyme that catalyzes the dismutation of O2−. to O2 and H2O2. Then, CAT converts this harmful H2O2 to water.Citation29 The results presented in this study showed that SOD and CAT activities were induced by inoculation with B. japonicum on the seventh day. In the same way; this result correlates with an increase in lipid peroxidation, ROS generation (NBT and DAB histochemical analysis), and GSH content. We may conclude that this induction of the antioxidant defense system was an attempt to protect the cells root against the oxidative stress produced by the inoculation with Bradyrhizobium Japonicum in an early stage. Also, we found a greater intensity of color in the inoculated, both Schiff's reagent and Evans blue. Therefore, histochemical observations suggest that lipid peroxidation and the loss plasma membrane integrity are caused directly by the interaction of B. japonicum with the root at 7 days post-inoculation. Thus, taking into account this result and the fact that, in previous works, we demonstrated that oxidative stress produced by cadmiun or saline stress correlates with an important increase in HO-1 activity and protein expression,Citation8,Citation13 we decided to evaluate the HO-1 gene expression and its relationship with the symbiotic association between plant host and bacteria. Here, we found that HO-1 induction occurs as a result of inoculation with B. japonicum and this effect is followed by an augmentation in the production of lipid peroxidation. That induction was observed at 3 and 5, while at 7 days post-inoculation the HO-1 induction reach a maximum, just when an increase in lipid peroxidation and GSH content were observed. It is important to note that the expression of HO-1 gene, in the NI group, was produced by H2O2 but not with paraquat (or its product O2−.). Thus, we may infer that the expression of HO-1 in roots inoculated with B. japonicum was mediated by an increase in the H2O2 level. In agreement with our results, the involvement of H2O2 as a leading ROS in Cd- and UV-B-induced oxidative stress generation has been reported.Citation23,Citation30 Besides, H2O2 has been shown to act directly or indirectly in signal transduction of defense responses.Citation31
Furthermore, the inhibition of HO-1 gene expression by ZnPPIX produced an increase in lipid peroxidation and a decrease in GSH content 7 days post-inoculation suggesting that, in this symbiotic process, HO-1 may act as a signal molecule that protects the root against oxidative stress.
In a previous report,Citation7,Citation8,Citation13 we carried out HO-1 determination in extracts from whole, unfractionated nodules; consequently, it was not possible to determine whether the enzyme activity was associated with the plant host or the bacterial symbiont. Recently, we tested that HO-1 antibody did not recognize any antigen in extracts from free-living B. japonicum (data not shown). Considering that leghemoglobin, the major heme protein in nodules, is synthesized in plant tissue and not into the symbiotic bacteria,Citation1 it seems logical to suppose that the enzyme involved in heme degradation is also localized in the same place. Besides, it has been postulated that HO-1 is involved in leghemoglobin metabolism in the alfalfa mature nodules.Citation32 In this work we demonstrate through the use of electronic microscopy that HO-1 is only present in PBM and, it is more abundant in salt-stressed than in control nodules. According to the data reported, lets us assume that HO-1 induction could be associated with the plant host.
To sum up, findings reported here demonstrate, by the first time, that HO is localized on the PBM, thus indicating that this enzyme is synthesized in the plant tissue and it is not present in the bacteroid. Moreover, we demonstrate that, in the root of soybean plants, HO-1 is induced by oxidative stress produced by inoculation of B. japonicum and exerts an antioxidant response against it, corroborating our previous results with cadmium, UV-B radiation, and salt.Citation12–Citation15
Acknowledgements
We thank Dr T. Kohchi for kindly providing the Arabidopsis HO-1 antibodies. This work was supported by grants from the Universidad de Buenos Aires (Argentina) and from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (Argentina). M.L.T., A.H.P., and K.B.B. are career investigators from CONICET.
References
- Santana MA, Pihakaski-Maunsbach K, Sandal N, Marcker KA, Smith G. Evidence that the plant host synthesizes the heme moiety of leghemoglobin in root nodules. Plant Physiol 1998;116:1259–69.
- Stougaard J. Genetics and genomics of root symbiosis. Curr Opin Plant Biol 2001;4:328–35.
- Limpens E, Bisseling T. Signaling in simbiosis. Curr Opin Plant Biol 2003;6:343–50.
- Whitehead LF, Day DA. The peribacteroid membrane. Physiol Plant 1997;100:30–44.
- Wan X, Hontelez J, Lillo A, Guarnerio C, van de Peut D, Fedorova E, et al. Medicago truncatula ENOD40-1 and ENOD40-2 are both involved in nodule initiation and bacteroid development. J Exp Bot 2007;58:2033–41.
- Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci 1985;17:617–31.
- Balestrasse KB, Yannarelli GG, Noriega GO, Batlle A, Tomaro ML. Heme oxygenase and catalase gene expression in nodules and roots of soybean plants subjected to cadmium stress. BioMetals 2008;21:433–41.
- Zilli CG, Balestrasse KB, Yannarelli GG, Polizio AH, Santa-Cruz DM, Tomaro ML. Heme oxygenase up-regulation under salt stress protects nitrogen metabolism in nodules of soybean plants. Environ Exp Bot 2008;64:83–9.
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ 2002;25:239–50.
- Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Safe 2005;60:324–49.
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 1968;61:748–55.
- Noriega GO, Balestrasse KB, Batlle A, Tomaro ML. Heme oxygenase exerts a protective role against oxidative stress in soybean leaves. Biochem Biophys Res Commun 2004;323:1003–8.
- Balestrasse KB, Noriega GO, Batlle A, Tomaro ML. Involvement of heme oxygenase as antioxidant defence in soybean nodules. Free Radic Res 2005;39:145–51.
- Santa-Cruz DM, Pacienza NA, Polizio AH, Balestrasse KB, Tomaro ML, Yannarelli GG. Nitric oxide synthase-like dependent NO production enhances heme oxygenase up-regulation in ultraviolet-B-irradiated soybean plants. Phytochemistry 2010;71:1700–7.
- Zilli CG, Santa-Cruz DM, Yannarelli GG, Noriega GO, Tomaro ML, Balestrasse KB. Heme oxygenase contributes to alleviate salinity damage in Glycine max L. leaves. Int J Cell Biol 2009; 2009:9 pages, article id 848516, doi:10.1155/2009/848516.
- Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Calif Agricul Exp Station Circular 1950;347:1–32.
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 1968;125:189–98.
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 1985;113:548–54.
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 1979;59:527–605.
- Becana M, Aparicio-Tejo P, Irigoyen JJ, Sanchez-Dıaz M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol 1986;82:1169–71.
- Giannopolitis CN, Ries SK. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 1997;59:309–14.
- Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by Aluminum, but not the primary cause of elongation inhibition in Pea roots. Plant Physiol 2001;125:199–208.
- Yannarelli GG, Noriega GO, Batlle A, Tomaro ML. Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta 1006;224:1154–62.
- Vanden Bosch KA, Sherrier DJ, Dreyer DA. Light microscopic applications of immunocytochemistry. In: , Gelvin SB, Schilperoort RA (eds.) Plant Molecular Biology Manual. Dordrecht: Kluwer Academic; 1995. p. 1–18.
- Muramoto T, Kohchi T, Yokota A, Hwang I, Goodman HM. The Arabidopsis photomorphogenic mutant HY1 is deficient in phytochrome chromophore biosynthesis as a result of a mutation in a plastid haeme oxygenase. Plant Cell 1999;11:335–48.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54.
- Storz G, Tartaglia LA, Ames BN. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 1990;248:189–94.
- Demple B, Amábile-Cuevas CF. Redox redux: the control of oxidative stress responses. Cell 1991;67:837–9.
- Bassham JA, Calvin M. Carbon dioxide fixation and carbohydrate synthesis. In: , Salisbury FB, Ross CW (eds.) Plant Physiology. 4th ed. New York: Wadsworth; 1992. p. 225–48.
- Olmos E, Martínez-Solano JR, Piqueras A, Hellín E. Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot 2003;54:291–301.
- Vranová E, Inzé D, Van Breusegem F. Signal transduction during oxidative stress. J Exp Bot 2002;53:1227–36.
- Baudouin E, Frendo P, Le Gleuher M, Puppo A. A Medicago sativa haem oxygenase gene is preferentially expressed in root nodules. J Exp Bot 2004;55:43–7.