Abstract
Oxidative stress results from a disparity between the generation of reactive oxygen species and the antioxidant ability of the organism. The alteration of the oxidant–antioxidant system brings in adults an effective state of imbalance, which may influence the pathogenesis of many diseases. Oxidative stress also plays a pivotal role in the progression of various pathologies in childhood, through a manipulation of regulatory proteins. In fact, several studies have demonstrated that an unbalanced oxidant–antioxidant status is able to determine toxic effects even during infancy. Therefore, the aim of this review was to summarize current knowledge about the dynamic relationship between oxidative stress and systemic diseases during childhood. In order to better understand these complex mechanisms, a comprehensive review of the literature was done, focusing mainly on pre-pubertal children. In fact, this age-group offers a unique opportunity to exclude confounding factors, especially those related to the metabolic effects induced by puberty. Early identification of these very young patients should be aimed at minimizing the degree of oxidative damage. Only by achieving early diagnosis, will it be possible to identify those children who could benefit from specific therapeutic approaches targeting oxidative stress.
Introduction
Oxidative stress results from a disparity between reactive oxygen species (ROS) generation and antioxidant ability. Unbalanced oxidant–antioxidant status is emerging as a prominent health concern in both adults and children. Previously published data have clearly emphasized the key role of unbalanced oxidant–antioxidant status in many biological complications, with a high risk of developing cardiovascular and metabolic diseases, certain cancers, neurodegenerative disorders, and aging.Citation1,Citation2 Interestingly, the impaired oxidant–antioxidant status contributes to toxic effects even during infancy, when the body is extremely vulnerable. Several studies have revealed a direct correlation between oxidative stress and the risk of developing complications in young subjects affected by systemic diseases, such as diabetes, obesity, non-alcoholic fatty liver disease (NAFLD), low and high birth weight-related alterations, growth hormone deficiency (GHD), asthma, and juvenile idiopathic arthritis (JIA).Citation3–Citation9 Careful follow-up of oxidant status alterations is therefore necessary throughout infancy to prevent associated damage. In order to better understand the pathophysiological mechanisms involved, the aim of the present review was to summarize current knowledge about the dynamic relationship between oxidative stress and systemic diseases during infancy. The literature review was focused mainly on pre-pubertal children. This age group offers a unique opportunity to exclude confounding factors, especially those related to the metabolic effects induced by puberty, which influence the oxidant–antioxidant balance.
Oxidant−antioxidant status: a dynamic system
Oxygen represents the most important chemical element for human life as it moderates the oxidation–reduction reactions that are indispensable for the energy metabolism of cells. The majority of inhaled oxygen is utilized by mitochondria to produce energy, while just a small percentage is transformed into ROS. Although the major sources of reactive species are mitochondria, their generation occurs also in non-phagocytic cells. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase (XO), and nitric oxide (NO) synthase represent the main enzymes involved in this process. ROS induce beneficial effects as they modulate a number of cellular signaling pathways and play a critical role in protection from pathogens. Usually, ROS are neutralized by the antioxidant defense system, while during pathological conditions they can dramatically increase and provoke severe damage. The main reactive molecules are hydroxyl radical (HO), superoxide (O2−), singlet oxygen (Citation1O2), hydrogen peroxide (H2O2), and NO. Effects triggered by these molecules include: alterations of cell membranes, inflammation, failed enzyme activity, inhibited protein synthesis, damage/mutation of DNA, and apoptosis. In particular, ROS determine toxic organic effects through the oxidation of lipids, amino acids, nucleic acids, and carbohydrates.
Antioxidants are substances able to counteract or reduce molecular and cellular ROS injury. These defense systems have been categorized as cellular, membrane, and extracellular antioxidants. Cellular antioxidants are composed of several enzymes such as superoxide dismutase (SOD), catalase and glutathione peroxidases (GPX). SOD catalyzes O2− dismutation into H2O2 and oxygen, while catalase converts H2O2 into oxygen and water. GPX reduces H2O2 producing glutathione (GSH). GSH has a key role in antioxidant intracellular defense, including removal of ROS and discharge of lipid peroxides, and in nutrient metabolism, such as regulation of cysteine bioavailability and its intracellular transport. Furthermore, GSH modulates important pathways, including glutathionylation of proteins and regulation of the intracellular redox status.Citation10 Membrane antioxidants include Coenzyme Q10 (CoQ10), and vitamin A, vitamin C (ascorbate), and vitamin E (alpha-tocopherol). Vitamin A is placed in lipophilic membranes, while vitamin C is the main water-soluble antioxidant. Vitamin E is a proficient ROS scavenger in the lipid core of cell membranes and blocks lipid peroxidation. There is an important relationship between intracellular and membrane antioxidants by the self-regenerating ascorbate-alpha-tocopherol-GSH system. In physiological conditions this system defends the human cells and tissues against oxidative stress, preventing above all lipid peroxidation. More specifically, alpha-tocopherol is regenerated from tocopheroxy radicals by ascorbate or GSH. In turn, ascorbyl radicals are cyclically reduced back at the expense of GSH or NADPH. Oxidized GSH is recycled back into the original form by GSH reductase in the presence of NADPH.Citation11 Finally, the extracellular antioxidants encompass several ligands including haptoglobin, hemopexin, and albumin as well as lactoferrin, transferrin, and ceruloplasmin.
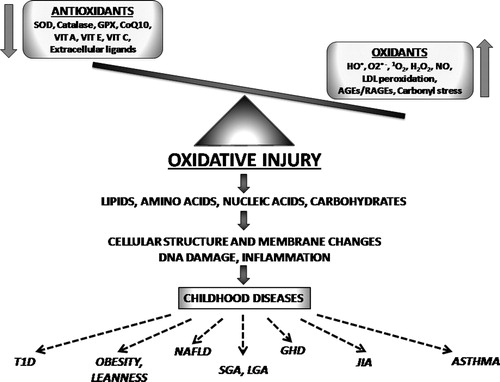
The oxidant–antioxidant mechanisms and their effects in childhood are shown in .
Figure 1. The oxidant–antioxidant mechanisms and their effects in childhood. SOD, superoxide dismutase; GPX, glutathione peroxidases; CoQ10, coenzyme Q10; VIT A, vitamin A; VIT E, vitamin E; VIT C, vitamin C; HO, hydroxyl radical; O2−, superoxide; 1O2, singlet oxygen; H2O2, hydrogen peroxide; NO, nitric oxide; LDL, low-density lipoproteins; AGEs, advanced glycation endproducts; RAGEs, receptors for advanced glycation endproducts; T1D, type 1 diabetes; NAFLD, non-alcoholic fatty liver disease; SGA, small for gestational age; LGA, large for gestational age; GHD, growth hormone deficiency; JIA, juvenile idiopathic arthritis.
Pathophysiology of oxidant-related organic damage in pediatric diseases
The main oxidant pathways involved in the pathophysiology of pediatric diseases are lipid peroxidation, advanced glycation endproducts (AGEs)/receptors for advanced glycation endproducts (RAGEs) pathway, NADPH oxidase and XO, NO, and carbonyl stress.
Lipid peroxidation represents the mechanism through which lipids are degraded, leading to the generation of peroxidized lipids and aldehydes. When redox regulation is impaired, lipids rich in polyunsatured fatty acids (FAs) undergo the subtraction of hydrogen, and react with oxygen to produce H2O2. This molecule interacts with further lipids creating a persistent process of auto-oxidation and causing cellular damage. In particular, the relationship between oxidative stress and childhood disease originates in oxidative changes of low-density lipoproteins (LDL).Citation12,Citation13 Among the numerous markers of oxidative stress, malonyldialdehyde (MDA), which reflects the lipid peroxide content of LDL, lag phase, an indicator of susceptibility of LDL to in vitro oxidation, and isoprostanes, a new class of molecule indicating both in vivo and ex vivo lipid peroxidation, represent reliable methodologies.
In children with type 1 diabetes (T1D) persistent hyperglycemia induces an impaired oxidant–antioxidant status, leading to precocious endothelial injury. LDL peroxidation stimulates H2O2 production, impairs GSH and NO bioavailability, and promotes the expression of pro-atherogenic molecules by the activation of oxidized LDL-receptors in vessels. In addition, oxidized LDL stimulate endothelial cells, smooth muscle cells, and monocytes to produce growth and chemotactic factors promoting acceleration of atherosclerosis.Citation14,Citation15 High MDA levels have been demonstrated in T1D children with scarce glycemic control,Citation16 while no difference was detected between pre-pubertal children with well-controlled diabetes and healthy peers. These data show that a high-quality metabolic control represents an important factor that is able to suppress the production of ROS.Citation17 Unfortunately, oxidative stress seems to take place even during the initial phases of diabetes. In fact, markers of endothelial alterations have already been detected in children with T1D without complications, thus demonstrating the role of an early intervention in restoring oxidative status in this high-risk group of children.Citation18
An impaired oxidant–antioxidant status has also been demonstrated in childhood obesity. Adipose tissue represents one of the main sources of ROS,Citation19 which are critical for the pathogenesis of early coronary-wall alterations, even from infancy. These oxidant molecules induce LDL peroxidation, which is a well-known key determinant of the development of foam cells and fatty streaks.Citation12 Moreover, insulin resistance (IR), which is associated with adipose tissue, is able to intensify ROS generation.Citation20 In fact, both the higher degree of IR and increased levels of isoprostanes, detected in obese pre-pubertal children in comparison with healthy subjects, have been associated with increased carotid intima medial thickness.Citation21 Notably, oxidative stress has been shown to be significantly reduced by a 6-month dietary restriction, demonstrating the possibility of reversing the obese-related oxidant–antioxidant alteration in young subjects.Citation4 In addition to the adverse effects of increased adipose tissue on oxidative stress, constitutional leanness has been similarly characterized by an impaired oxidant–antioxidant status. Oxidative stress has recently been demonstrated in a group of pre-pubertal constitutional lean children in comparison with healthy peers. It is notable that in lean children no significant difference was documented in oxidative status when compared with obese children,Citation22 revealing an important role of adipose tissue depletion in regulating the oxidant–antioxidant balance.
Oxidative stress is also an important risk factor in the development of NAFLD, one of the most significant liver complications related to childhood obesity.Citation23 Although the pathogenesis of fatty liver disease is not completely clear, the interrelationship between oxidative stress and IR mechanisms plays a pivotal role in the progression from liver steatosis to steatohepatitis.Citation24 Fat accumulation in the liver is likely to result from IR, which alters lipolysis and lipid storage leading to an increased flux of free FAs to the hepatocytes.Citation24 This hepatic overloading of FAs increases mitochondrial ROS production by depletion of n-3 long-chain polyunsaturated FAsCitation25 and exacerbation of IR, through the phosphorylation of insulin receptor.Citation26 The progression of liver steatosis to steatohepatitis is related to an exacerbation of oxidative stress, due to upregulation of cytochrome P450-family 2-subfamily E-polypeptide 1, mitochondrial dysfunction and increased activity of NADPH oxidase.Citation27–Citation29 The redox upregulation of pro-inflammatory mediators (tumor necrosis factor-alpha and interleukin-1alpha),Citation26 through activation of the redox-sensitive transcription factors (nuclear factor-kappa B (NF-kB) and activator protein-1), reinforces the initial mechanism of IR and ROS.Citation29 A recent study has shown that the link between oxidative stress and the severity of non-alcholic steatohepatitis is identifiable from childhood, as demonstrated by increased circulating immunoglobulin (Ig)G against MDA-adducted human serum albumin in children with scores for lobular inflammation significantly higher than patients with IgG in the normal range.Citation30
The crucial role of lipid peroxidation has widely been recognized in children born small for gestational age (SGA) as well as in children born large for gestational age (LGA). In fact, an impaired oxidant–antioxidant status has been documented in pre-pubertal normal-weight SGA and LGA children. These data clearly suggest a background of oxidative derangement, most likely due to an adverse fetal environment with adaptive reactions and pathophysiological modifications.Citation31 An additional impaired oxidant–antioxidant status has been detected in a group of obese pre-pubertal SGA and LGA children.Citation6 These data show that the background oxidative derangement related to birth weight can be exacerbated by excessive fat mass accumulation.
Oxidative stress has also been reported to be directly involved in GHD-related cardiovascular diseases.Citation32 Specifically, low insulin-like growth factor 1 (IGF-1) levels seem to be directly responsible for an increased generation and a reduced removal of ROS. A recent study has demonstrated high oxidative stress and low IGF-1 levels in pre-pubertal children with GHD, with a significant correlation between oxidative stress and IGF-1. However, these effects appear to be reversible. In fact, after a 1-year treatment with recombinant GH (rGH), children showed a significant increase of IGF-1 levels associated with a parallel improvement of oxidant–antioxidant status markers.Citation7
Moreover, oxidative stress plays a pivotal role in the pathogenesis and progression of JIA,Citation33 a chronic arthritis of unidentified cause starting in subjects younger than 16 years of age.Citation34 In fact, children with inflamed joints alternate activity with static periods, leading to sequences of hypoxia-reperfusion with a redox milieu and ROS generation.Citation35 High thiobarbituric acid-reactive substances in children with JIA were initially reported,Citation36 and have subsequently been demonstrated to be associated with high MDA and H2O2 levels.Citation37
Finally, a close link between asthma and oxidative stress has been demonstrated even during childhood. As a result of an imbalance between oxidants and antioxidants leading to hyper-responsiveness and bronchial inflammation, an elevated pulmonary amount of ROS has been found in asthmatic subjects.Citation38 Moreover, high MDA levels have been reported in children with asthma,Citation8 as well as high levels of isoprostanes, which appear to be related to asthma severity.Citation39
The AGEs/RAGEs pathway has recently been demonstrated to be involved in the pathogenesis of oxidative-related organic damage during infancy. AGEs–RAGEs interaction induces oxidative stress, modifying gene expression in numerous cells.Citation8,Citation40 More specifically, RAGE is a multiligand cell-surface receptor with three main splicing variants.Citation41 The full-length RAGE and the N-truncated variants stay in the plasma membrane, whereas the C-truncated variant, named endogenous secretory RAGE (esRAGE), lacks cytosolic and trans-membrane domains and is extracellularly secreted. The enzymatic cleavage of the full-length cell surface generates an additional type of extracellularly secreted RAGE, named soluble RAGE (sRAGE). esRAGE and sRAGE combine with the circulating ligands and counteract their effects. However, in the presence of a large amount of circulating ligands, the decoy receptors decrease drastically, revealing the system function.
In children with T1D, hyperglycemia can induce oxidative stress by glucose autoxidation and non-enzymatic protein glycation with production of glucose-derived AGEs.Citation42 Although glucose oxidation seems to be the principal ROS source,Citation43 the AGEs/RAGEs system is strongly engaged in the pathophysiology of diabetic complications.Citation42 The AGEs/RAGEs system leads to oxidative stress by impairing NO bioavailability, inducing heme oxygenase mRNA and activating NADPH oxidase and nuclear transcription factors, such as NF-kB. This activation increases the expression of adhesion molecules as well as pro-inflammatory and chemoattractant mediators which in turn provoke further endothelial damage through artery-wall infiltration.Citation44 A corresponding and notable correlation between AGEs and MDA has been detected in T1D children with scarce glycemic control.Citation16
Considerably reduced esRAGE and sRAGE levels with an independent correlation with steatosis have been reported in obese pre-pubertal children with NAFLD.Citation5 These data suggest that the AGEs/RAGEs pathway has an independent role in the progression of liver diseases, even during infancy.
NADPH oxidase and XO have commonly been defined as the two major enzymes involved in ROS generation. It has been widely demonstrated in children with T1D that hyperglycemia induces an impaired oxidant–antioxidant status throughout these two important enzymes.Citation20,Citation45 In T1D, hyperglycemia induces an over-expression of mitochondrial NADPH oxidase by the activation of protein kinase C, causing a decreased production of reduced GSH.Citation46 Furthermore, the role of IR in the amplification of ROS generation through NADPH oxidase has been described in obese children.Citation20
The NO molecule has widely been identified as an important endogenous vasodilator. Appropriate levels of this molecule are essential in protecting the human body from ischemia, while high levels of NO lead to toxic effects.
A recent study reported that children with T1D showed high NO levels, leading to intrarenal hemodynamic alterations from early stages of nephropathy.Citation47 Chronic hyperglycemia has several effects: induces increased NO biosynthesis and actions; enhances blood flow and vascular permeability; and leads to endothelial alterations, glomerular hyperfiltration, and microalbuminuria.Citation48 High NO levels have been shown in children with JIA, highlighting the important role of nitrogen and reactive species in joint injury.Citation49
In asthmatic children exhaled NO (eNO) reflects bronchial inflammationCitation50 and has recently been identified as a new complementary tool in the follow-up of airways inflammation by using the eNO fractional concentration (FeNO).Citation51 Above all, children with increased FeNO seem to be at high risk of new-onset asthma,Citation52 while in asthmatic children antioxidant albumin concentrations have been demonstrated to be decreased and related to elevated FeNO.Citation53
Oxidative stress can produce carbonyl stress (reactive carbonyl complexes) from amino acids, lipids, and carbohydrates, leading to the production of AGEs.Citation54 Persistent exposure of proteins to ROS can provoke structural alterations, including the generation of carbonyl proteinsCitation55 which have cytotoxic properties on cell metabolism.
Increased levels of carbonyls have been detected in children with JIA, principally in those children with high-disease activity.Citation56 It has been reported, however, that protein carbonyls did not increase during a 1-year follow-up study, perhaps due to proteolysis during anti-inflammatory therapy.Citation57
Pathophysiology of antioxidant-related organic damage in pediatric diseases
The main antioxidant pathways involved in the pathophysiology of pediatric diseases include cellular and membrane systems.
With regard to cellular antioxidants, an increased activity of erythrocytic GPX has been detected in children with steatosis, suggesting that toxic damage stimulates antioxidant enzymes, even during the first phases of NAFLD.Citation58
Furthermore, an impaired activity of catalase has been documented in overweight LGA children in comparison with SGA children, exacerbating the risk of obesity-related complications.Citation59
A reduced SOD activityCitation36 has been detected in children with JIA, who also demonstrated a decreased GPX activity.Citation60 Conversely, another study reported increased SOD and GPX activity not only in the serum but also in the saliva of young JIA subjects.Citation61 These data have been supported by a further study showing a high antioxidant enzyme activity in the saliva of children with JIA.Citation9
In children with asthma significantly decreased concentrations of reduced GSH have been reported.Citation8 Specifically, increased oxidative stress has been found in the lung epithelial lining fluid of asthmatic children, with a large glutathione disulfide production and its transformation to the more oxidized form.Citation62 Oxidative stress can also modify the immune response of T helper 1/T helper 2 cells, which leads to the activation of NF-kB, a well-known inducer of pro-inflammatory genes.Citation63 In fact, a crucial point is represented by the genetic vulnerability to oxidative stress, because antioxidant enzyme polymorphisms are potential risk factors for bronchial inflammation. Glutathione S-transferase (GST) represents a candidate gene for its protection against oxidative stress and also because the GST-T1 null genotype has commonly been found in asthmatic subjects.Citation64 These genes may be crucial during intrauterine life as they could modify the respiratory outcome of fetal exposure to maternal smoke.Citation65
Regarding membrane antioxidants, evidence from several studies demonstrates reduced vitamin E levels in obese children, due to its sequestration by fat mass with alteration in assimilation and metabolism.Citation4,Citation66 Reduced concentrations of other lipophilic antioxidant vitamins, including lycopene, and alpha-tocopherol, have been found in obese children.Citation66 So, it can be presumed that an excessive food intake of poor nutritional quality may provoke excessive ROS production in young children.
Concerning NAFLD, the first therapeutic approach in children is to change lifestyle, including weight loss and physical activity. However, as these approaches are not easy to put into practice, antioxidants have been recognized as valid alternative treatments to prevent progression to cirrhosis.Citation67 In particular, vitamin E supplementation has been shown to normalize transaminase levels in obese children with NAFLD and non-alcoholic steatohepatitis.Citation68
Low levels of vitamin E have also been detected in children with GHD; however, after 1 year of rGH therapy there was a significant improvement in this antioxidant vitamin.Citation7 These data confirm that children with GHD have an adverse oxidant–antioxidant status, which can apparently be restored by rGH treatment.
Decreased vitamin E levels have been shown in normal-weight pre-pubertal SGA and LGA children. Furthermore, impaired CoQ10 metabolism and catalase activity have been documented in overweight LGA children in comparison with SGA children.Citation59
Reduced vitamin E levelsCitation69 and beta-carotene valuesCitation37 have also been detected in children with JIA, together with low vitamin C concentrations.Citation60
Low vitamin E levels have been found in children with inadequately controlled asthma,Citation53 with a deficit in bronchial function, especially in children with an insufficient antioxidant vitamin intake.Citation70 Among antioxidants, lack of vegetables and fruits has been demonstrated to be related to coughing, while low fish intake was the main predictor of reduced lung function. These data suggest that these foods, rich in antioxidant vitamins, could have a role in reducing the risk of developing asthma.Citation71
Conclusion
In summary, oxidative stress plays a critical role in developing and exacerbating the pathogenesis of several disorders, even in pre-pubertal children. Some useful surrogate biomarkers have been proposed to evaluate the oxidant injury and the antioxidant defense systems in most childhood diseases. In order to minimize the degree of oxidative damage, early identification of these young patients becomes imperative. Merely by prompt diagnosis and close follow-up, it would be possible to identify those children who could benefit from therapeutic approaches targeting oxidative stress. As every disease has a characteristic pathological pathway, further investigations are required to assess the utility of specific antioxidant treatments during childhood.
References
- Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, et al. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis 2005;179(1):43–9.
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 2005;39:359–407.
- Menke T, Niklowitz P, Wiesel T, Andler W. Antioxidant level and redox status of coenzyme Q10 in the plasma and blood cells of children with diabetes mellitus type 1. Pediatr Diabetes 2008;9(6):540–5.
- Mohn A, Catino M, Capanna R, Giannini C, Marcovecchio M, Chiarelli F. Increased oxidative stress in prepubertal severely obese children: effect of a dietary restriction-weight loss program. J Clin Endocrinol Metab 2005;90(5):2653–8.
- D'Adamo E, Giannini C, Chiavaroli V, de Giorgis T, Verrotti A, Chiarelli F, et al. What is the significance of soluble and endogenous secretory receptor for advanced glycation end products in liver steatosis in obese pre-pubertal children? Antioxid Redox Signal 2011;14(6):1164–72.
- Chiavaroli V, Giannini C, D'Adamo E, de Giorgis T, Chiarelli F, Mohn A. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics 2009;124(2):695–702.
- Mohn A, Marzio D, Giannini C, Capanna R, Marcovecchio M, Chiarelli F. Alterations in the oxidant-antioxidant status in prepubertal children with growth hormone deficiency: effect of growth hormone replacement therapy. Clin Endocrinol 2005;63(5):537–42.
- Dut R, Dizdar EA, Birben E, Sackesen C, Soyer OU, Besler T, et al. Oxidative stress and its determinants in the airways of children with asthma. Allergy 2008;63(12):1605–9.
- Brik R, Rosen I, Savulescu D, Borovoi I, Gavish M, Nagler R. Salivary antioxidants and metalloproteinases in juvenile idiopathic arthritis. Mol Med 2010;16(3–4):122–8.
- Wu G, Fang Y, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 2004;134:489–92.
- Sies H, Stahl W. Vitamins E and C, β-carotene, and other carotenoids as antioxidants. Am J Clin Nutr 1995;62:1315S–21S.
- Yokoyama M. Oxidant stress and atherosclerosis. Curr Opin Pharmacol 2004;4:110–5.
- Obrosova IG, Van Hysen C, Fathallah L, Cao X, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism and antioxidative defense. FASEB J 2002;16:123–5.
- Cominacini L, Rigoni A, Pasini AF, Garbin U, Davoli A, Campagnola M, et al. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells of through an increased production of superoxide. J Biol Chem 2001;276:13750–5.
- Smitko PE, Wang CH, Weisel RD, Jeffries GA, Anderson TJ, Verma S. Biomarkers of vascular disease linking inflammation to endothelial activation. Part 2 Circulation 2003;108:2041–8.
- Jakus V, Bauerova K, Michalkova D, Carsky J. Values of markers of early and advanced glycation and lipoxidation in serum proteins of children with diabetes mellitus. Bratisl Lek Listy 2000;101:484–9.
- Gleisner A, Martinez L, Pino R, Rojas IG, Martinez A, Asenjo S, et al. Oxidative stress markers in plasma and urine of prepubertal patients with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2006;19(8):995–1000.
- Belch JJF, Greene SA, Littleford R, Jennings PE, Khan F. Impaired skin blood flow response to heat in children with insulin-dependent diabetes. Int Angio 1996;l5:189–91.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–61.
- Atabek ME, Keskin M, Yazici C, Kendirci M, Hatipoglu N, Koklu E, et al. Protein oxidation in obesity and insulin resistance. Eur J Pediatr 2006;165(11):753–6.
- Giannini C, de Giorgis T, Scarinci A, Ciampani M, Marcovecchio ML, Chiarelli F, et al. Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre-pubertal children. Atherosclerosis 2008;197:448–56.
- Giannini C, de Giorgis T, Scarinci A, Cataldo I, Marcovecchio ML, Chiarelli F, et al. Increased carotid intima-media thickness in pre-pubertal children with constitutional leanness and severe obesity: the speculative role of insulin sensitivity, oxidant status, and chronic inflammation. Eur J Endocrinol 2009;161(1):73–80.
- Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010;51:679–89.
- Day CP, James OF. Steatohepatitis: a tale of two ‘hits’? Gastronterology 1998;114:842–5.
- Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in non-alcoholic fatty liver disease patients. Clin Sci 2004;106:635–43.
- Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal 2005;7(7–8):1040–52.
- Angulo P. NAFLD, obesity, and bariatric surgery. Gastroenterology 2006;130:1848–52.
- Videla LA, Rodrigo R, Araya J, Poniachik J. Oxidative stress and depletion of hepatic polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med 2004;37(9):1499–507.
- Videla LA, Rodrigo R, Araya J, Poniachik J. Insulin resistence and oxidative stress interdependency in non-alcoholic fatty liver. TRENDS Mol Med 2006;12(2):555–8.
- Nobili V, Parola M, Alisi A, Marra F, Piemonte F, Mombello C, et al. Oxidative stress parameters in pediatric non-alcoholic fatty liver disease. Int J Mol Med 2010;26(4):471–6.
- Mohn A, Chiavaroli V, Cerruto M, Blasetti A, Giannini C, Bucciarelli T, et al. Increased oxidative stress in prepubertal children born small for gestational age. J Clin Endocrinol Metab 2007;92(4):1372–8.
- Thomas AM, Berglund L. Growth hormone and cardiovascular disease: an area in rapid growth. J Clin Endocrinol Metab 2001;86:1871–3.
- Vasanthi P, Nalini G, Rajasekhar G. Status of oxidative stress in rheumatoid arthritis. Int J Rheum Dis 2009;12(1):29–33.
- Martini A, Lovell DJ. Juvenile idiopathic arthritis: state of the art and future perspectives. Ann Rheum Dis 2010;69(7):1260–3.
- Simonini G, Matucci Cerinic M, Cimaz R, Anichini M, Cesaretti S, Zoppi M, et al. Evidence for immune activation against oxidized lipoproteins in inactive phases of juvenile chronic arthritis. J Rheumatol 2001;28(1):198–203.
- Sklodowska M, Gromadzińska J, Biernacka M, Wasowicz W, Wolkanin P, Marszalek A, et al. Vitamin E, thiobarbituric acid reactive substance concentrations and superoxide dismutase activity in the blood of children with juvenile rheumatoid arthritis. Clin Exp Rheumatol 1996;14(4):433–9.
- Araujo V, Arnal C, Boronat M, Ruiz E, Domínguez C. Oxidant-antioxidant imbalance in blood of children with juvenile rheumatoid arthritis. Biofactors 1998;8(1–2):155–9.
- Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol 2008;8:49–56.
- Caballero Balanzá S, Martorell Aragonés A, Cerdá Mir JC, Belda Ramírez J, Navarro Iváñez R, Navarro Soriano A, et al. Leukotriene B4 and 8-isoprostane in exhaled breath condensate of children with episodic and persistent asthma. J Investig Allergol Clin Immunol 2010;20(3):237–43.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86.
- Kawamura M, Heinecke JW, Chait A. Pathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxidependent pathway. J Clin Invest 1994;94(2):771–8.
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 1988;318:1315–21.
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 2003;17(1):24–38.
- Vazzana N, Santilli F, Cuccurullo C, Davì G. Soluble forms of RAGE in internal medicine. Intern Emerg Med 2009;4:389–401.
- Desco MC, Asensi M, Márquez R, Martínez-Valls J, Vento M, Pallardó FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 2002;51:1118–24.
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000;49:1939–45.
- Savino A, Pelliccia P, Schiavone C, Primavera A, Tumini S, Mohn A, et al. Serum and urinary nitrites and nitrates and doppler sonography in children with diabetes. Diabetes Care 2006;29(12):2676–81.
- Chiarelli F, Cipollone F, Romano F, Tumini S, Costantini F, di Ricco L, et al. Increased circulating nitric oxide in young patients with Type 1 diabetes and persistent microalbuminuria. Diabetes 2000;49:1258–63.
- Lotito AP, Muscará MN, Kiss MH, Teixeira SA, Novaes GS, Laurindo IM, et al. Nitric oxide-derived species in synovial fluid from patients with juvenile idiopathic arthritis. J Rheumatol 2004;31(5):992–7.
- Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med 2001;164:1376–81.
- Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Götz M, et al. European Pediatric Asthma Group. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy 2008;63:5–34.
- Bastain TM, Islam T, Berhane KT, McConnell RS, Rappaport EB, Salam MT, et al. Exhaled nitric oxide, susceptibility and new-onset asthma in the children's health study. Eur Respir J 2011;37(3):523–31.
- Bakkeheim E, Mowinckel P, Carlsen KH, Burney P, Lødrup Carlsen KC. Altered oxidative state in schoolchildren with asthma and allergic rhinitis. Pediatr Allergy Immunol 2011;22(2):178–85.
- Martín-Gallán P, Carrascosa A, Gussinyé M, Domínguez C. Biomarkers of diabetes-associated oxidative stress and antioxidant status in young diabetic patients with or without subclinical complications. Free Radic Biol Med 2003;34(12):1563–74.
- Stadtman ER. Protein oxidation and aging. Science 1992;257:1220–24.
- Renke J, Popadiuk S, Korzon M, Bugajczyk B, Wozniak M. Protein carbonyl groups' content as a useful clinical marker of antioxidant barrier impairment in plasma of children with juvenile chronic arthritis. Free Radic Biol Med 2000;29(2):101–4.
- Renke J, Szlagatys A, Hansdorfer-Korzon R, Szumera M, Kamińska B, Knap N, et al. Persistence of protein oxidation products and plasma antioxidants in juvenile idiopathic arthritis. A one-year follow-up study. Clin Exp Rheumatol 2007;25(1):112–4.
- Mandato C, Lucariello S, Licenziati MR, Franzese A, Spagnuolo MI, Ficarella R, et al. Metabolic, hormonal, oxidative, and inflammatory factors in pediatric obesity-related liver disease. J Pediatr 2005;147(1):62–6.
- Park E. Birth weight was negatively correlated with plasma ghrelin, insulin resistance, and coenzyme Q10 levels in overweight children. Nutr Res Pract 2010;4(4):311–6.
- Ashour M, Salem S, Hassaneen H, el-Gadban H, Elwan N, Awad A, et al. Antioxidant status in children with juvenile rheumatoid arthritis (JRA) living in Cairo, Egypt. Int J Food Sci Nutr 2000;51(2):85–90.
- Brik R, Livnat G, Pollack S, Catz R, Nagler R. Salivary gland involvement and oxidative stress in juvenile idiopathic arthritis: novel observation in oligoarticular-type patients. J Rheumatol 2006;33(12):2532–7.
- Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LA. Severe Asthma Research Program. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol 2009;123(1):146–52.
- Dozor AJ. The role of oxidative stress in the pathogenesis and treatment of asthma. Ann N Y Acad Sci 2010;1203:133–7.
- Babusikova E, Jesenak M, Kirschnerova R, Banovcin P, Dobrota D. Association of oxidative stress and GST-T1 gene with childhood bronchial asthma. J Physiol Pharmacol 2009;60 (Suppl 5):27–30.
- Murdzoska J, Devadason SG, Khoo SK, Landau LI, Young S, Goldblatt J, et al. In utero smoke exposure and role of maternal and infant glutathione s-transferase genes on airway responsiveness and lung function in infancy. Am J Respir Crit Care Med 2010;181(1):64–71.
- Strauss RS. Comparison of serum concentration of α-tocopherol and β-carotene in a cross-sectional sample of obese and non obese children (NHANES III). J Pediatr 1999;134:160–5.
- Barshop NJ, Sirlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2008;28(1):13–24.
- Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, et al. Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. J Pediatr Gastroenterol Nutr 2004;38(1):48–55.
- Honkanen VE, Pelkonen P, Konttinen YT, Mussalo-Rauhamaa H, Lehto J, Westermarck T. Serum cholesterol and vitamins A and E in juvenile chronic arthritis. Clin Exp Rheumatol 1990;8(2):187–91.
- Gilliland FD, Berhane KT, Li YF, Gauderman WJ, McConnell R, Peters J, et al. Children's lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am J Epidemiol 2003;158:576–84.
- Tabak C, Wijga AH, de Meer G, Janssen NA, Brunekreef B, Smit HA. Diet and asthma in Dutch school children (ISAAC-2). Thorax 2005;61:1048–53.