Abstract
This paper describes the research done in order to valorise the biomass ash and evaluate its use as supplementary cementitious material (SCM) in the cement industry. The biomass ash samples used in this study were collected from three different power plants. The characterisation of the ashes as SCMs was performed after two different valorisation processes: (i) a vitrification process in order to obtain a new material with high hydraulicity and (ii) an easy de-alkalisation process in order to reduce the alkali content. The results of this work show that biomass ash derived from the combustion of woodchips and straw, properly treated, is characterised by pozzolanic activity and latent hydraulicity that could be exploited for the manufacture of low embodied energy concrete. The ultimate strength of mortars prepared using vitrified biomass ash becomes higher than that of the parent Portland cement after 28 days.
Introduction
Worldwide, biomass generated electrical capacity of ∼88 GW. This made biomass the most widely used renewable source of energy within the global energy system in 2013.Citation1 Since the production of energy from biomass combustion is continuously increasing, the processing and disposal of the resulting ash has become an environmental and economic issue. Biomass ash is the solid residue produced during the incineration of plant biomass for heat and electricity production and represents ∼2–20% of the input material.Citation2 The composition of the biomass ash differs considerably, even within the same facility, because the physical and chemical characteristics depend strongly on plant type, origin and parts used for combustion, the process parameters, and the storage conditions. Ash contains valuable macro- and micronutrients from the combusted biomass and thus requires a suitable recycling strategy. Because of its value, nowadays, biomass ash is usually disposed of in landfills, creating significant costs for biomass plant operators.
An extended overview of the potential utilisation of biomass ash and the related technological advantages and challenges was conducted by Vassilev et al.Citation3 Biomass ash has many potential areas of application: in forest ecosystems to return nutrients to harvested fields or to counteract soil acidification, for agricultural and horticultural purposes as fertilizer,Citation4–Citation6 and for geotechnical construction and industrial processes. Typical applications in this last field are the construction of roads, surface layer in landfills, and admixture of ash for concrete, brick or cement production.
The possibility to use fly and bottom ash as raw materials for geopolymers has been studied by several authors.Citation7–Citation9 The typical SiO2/Al2O3 ratio in the ash for producing good quality geopolymers has been determined to be between 3.3 and 4.5.Citation9 The potential use of biomass ash as filler in bituminous mixture has been recently investigated by Melotti et al.Citation10: biomass ash, if subjected to a sieving process, may be considered as a valid alternative to natural filler. Mortars produced by replacing natural sand with bottom bed ash (composed of sand, soil, small stones and unburnt biomass fraction) from forest biomass combustion showed similar properties of the standard ones.Citation11 High calcium wood ash has been used as partial cement replacement up to 15 wt-%,Citation12 thus obtaining mortars with higher compressive strengths as compared to the control mortar after a 90 day curing. Concrete formulations produced by replacing up to 10% of cement with biomass ash from bark combustion showed mechanical properties comparable with the reference ones after 28 days and a very good leaching resistance in both marine and freshwater medium.Citation13 The high content of chlorides in this ash is still an open issue, and the durability of these mortars has to be further investigated.Citation11
Besides, biomass ashes have complicated alkali silica reaction (ASR) performances because of their different chemical compositions and particle size.Citation14 However, Wang has recently demonstrated that biomass containing fly ash increases strength and reduces ASR expansion in mortar even with their high available alkalies.Citation14
With the increasing attention towards CO2 emission reduction and cost optimisation in cement production, the replacement of the clinker quota with supplementary cementitious materials (SCMs) in cement and concrete production is becoming increasingly widespread.Citation15 The utilisation of cement with increasing content of SCMs would allow the development of mortars and concrete with a reduced environmental impact and lower embodied energy. Moreover, most of the SCMs used in concrete have a beneficial effect on the durability properties thanks to the densely packed hydrated silicate phases that are forming and give high resistance to the concrete against chemical (chloride and sulphate) attack as well as very low porosity of the cement paste.Citation16 As an example, the granulated slags obtained as a secondary product from the steel industry, the fly ash resulting from the de-dusting of coal based energy production facilities and the natural pozzolans (mainly excavated in Italy, Greece and Indonesia) have been widely used for several years and approved in Europe by standardisation authorities (EN 196-1) and contribute to the development of the strength and durability in concrete. Several other SCMs are the object of intensive study such as metakaolin,Citation17 calcined clayCitation18 or municipal waste incinerator bottom ash.Citation19 The feasibility of the use of the previously mentioned SCMs in the replacement of clinker or cement is first regulated by standard and by the regional availability of the materials (pozzolans, fly ash, slag, kaolin and clay) and second by their costs and by the extra cost eventually required for their production (metakaolin and calcined clays). SCMs deriving from industrial waste have additionally restrictions due to the possible heavy metals and alkali content or to the risk of undesired effects such as expansion and workability loss typically observed in case of the use of municipal waste ashes.Citation19 The use of secondary processes such as vitrification or washing have been proposedCitation20,Citation21 in order to improve the quality of the SCMs or to remove most of the undesired effects in concrete.
The recycling of biomass ash as construction materials meets the recommendations of the European Directive on waste 2008/98/CE and has significant environmental benefits related to CO2 emission reduction and to the reduction of waste carried to landfills.Citation10 Besides, in Italy, a simplified procedure (regulation DM 05/02/98) is sufficient for recycling waste from biomass combustion in concrete, cement, brick production, embankment construction and environmental reuse.
In this paper, the potential use of ash from biomass combustion as SCMs in mortars and concrete is discussed with the objective of finding innovative solutions through processes that could recover valuable raw materials from industrial waste, allowing the development of a ‘greener-concrete’ with a reduced environmental impact and lower embodied energy compared to the current one.
Experimental
Biomass ash and pretreatments
The ashes (mixture of bottom and fly ashes) used in this study were collected from three different power plants (moving grate technology): two are woodchips power plant located in Italy (Chieri, referred to as TO, and Sondrio, referred to as SO) and one is a straw power plant located in Sweden (Skurups Fjärrvärme, referred to as SW). The geometrical, physical and chemical characteristics of the ashes have already been reported in previous studies.Citation10,Citation22 Ashes derived from woodchip combustion have a similar composition with relatively high CaO, SiO2 and K2O content. SW ash, from a straw combustion plant, contains high quantities of K2O, SiO2, Cl − and SO3.Citation22 The sintering/melting behaviour of the ashes has been determined using a hot stage microscopy (Expert System Solutions, Italy) at a heating rate of 20°C min − 1.
The TO, SO and SW ashes were submitted to vitrification processes. The vitrification of the TO, SO and SW ashes was performed without the addition of any additives at 1500°C for 1 h, in air, in a chamber furnace (Bicasa Supertherm HT/17) using alumina crucibles. The resulting glasses (referred to as TO-glass, SO-glass and SW-glass respectively) appear as dark brown in colour (TO-glass in Citation23). The chemical and mineralogical compositions of the three glasses—TO-glass, SO-glass and SW-glass—were determined by X-ray diffraction (XRD) analysis (Philips PW1710) and X-ray fluorescence (XRF; Panalytical Axios). Microhardness tests were performed on the three glasses by Leica Leitz microindenter. The chemical composition of the ashes and vitrified ashes, already reported by Ferraris et al.,Citation22 is given in .
1 TO-glass obtained by vitrification of ash from combustion of woodchipsCitation23

Table 1 Chemical composition (wt-% oxides, XRF) of SW ash before (SW-ash) and after washing process (SW-ash48) and glasses obtained by vitrification of TO, SO and SW ashes (TO-glass, SO-glass and SW-glass) (LOI, loss on ignition at 950°C)
In order to investigate the influence of the vitrification temperature, TO and SO ashes were also heat treated at 1300°C for 1 h, in air, in a chamber furnace. The resulting glass–ceramics (referred to as TO-1300 and SO-1300 respectively) appear as dark brown in colour.
The SW ash, because of the high alkali and SiO2 content, was also tested after a de-alkalisation process with water. SW ash was washed with distilled water (100 g of ash for 2 L of water) for 24 h by continuous mixing. Then, the ash was dried in an oven at 120°C and rewashed with the same procedure. The washed SW ash is referred to as SW-ash48 in the text.
Biomass ash as SCM
The vitrified ashes (TO-glass, SO-glass, SW-glass, TO-1300 and SO-1300) and SW after a 48 h washing (SW-ash48) were tested for pozzolanic and hydraulic activity, after grinding and sieving (Φ < 90 μm). The evaluation of pozzolanic activity was performed according to the UNI-EN 196-5. The evaluation of the latent hydraulic activity was performed on standard mortars, prepared according to UNI-EN 196-1 by replacing 30 wt-% of CEM II A-LL 42.5 R with the glass obtained from vitrifying the ashes or with the SW ash after a 48 h washing. Mortar specimens (40x40x160 mm) were prepared by mixing 450 g of binder with 225 g of water and 1350 g of standard sand and using steel mould. Each testing sample was repeated twice. Mortar samples were cured for the first 24 h at 20°C and 95% R.H., then in water at 20°C up to 1 year and submitted to compressive mechanical tests after 1, 7, 28, 60, 90, 180 and 360 days. The obtained results were compared to the performance of four reference mortars based on CEM II A-LL 42.5 R (referred to as Ref. 1), on CEM II A-LL 42.5 R obtained by replacing 30 wt-% of cement with a limestone filler (referred to as Ref. 2), with fumed silica (referred to as Ref. 3) or natural pozzolan (referred to as Ref. 4). The water/cement ratio was kept constant at 0.5 for all mixes. The composition of mortars prepared according to UNI-EN 196-1 is shown in .
Table 2 Composition of mortars prepared according to UNI-EN 196-1
Scanning electron microscopy (SEM; Philips 525M) and energy dispersive X-ray spectroscopy (EDS; Philips SW9100 EDAX) analyses were carried out on the pastes after 28 days of curing in water at 20°C.
Results and discussion
Because of its high SiO2 content, the SW ash could potentially be used as SCM after a reduction of the alkali content, which would limit its use. Cement with high soluble alkali content (>1%) can have a negative impact on the durability properties of the concrete (risk of alkali aggregate attack), on the visual appearance of the concrete (efflorescence formation on the surface) and on the workability properties of the concrete.Citation24 Therefore, the SW ash was tested after a de-alkalisation process with water. In , the compositions of the SW ash before and after the 48 h washing with distilled water are reported. After the washing process, Cl − and SO3 content decreased to about one-third of the initial content and the K2O content decreased from ∼19 to ∼13 wt-%. Although this value remains high, the main target of the washing process is the removal of the soluble alkalies, which are the most dangerous for the durability of the concrete. The presence of insoluble alkalies has no effect on the durability properties of the concrete, at least in the normal operating temperature range ( − 10 to 35°C).
Additionally, the loss on ignition of SW-ash and SW-ash48 are 2.0% and 11.9% respectively (). The higher loss on ignition measured in SW-ash48 shows that, during the washing process, the SW ash undergoes a first hydration step, which leads to the formation of hydrated products.
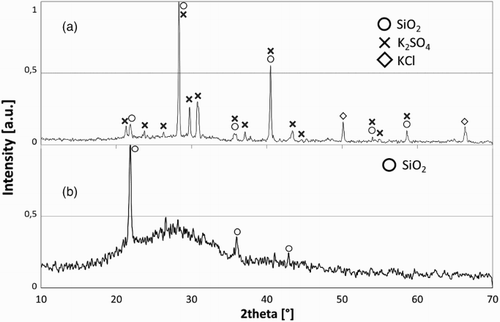
shows the XRD patterns of the SW ash before and after washing. The unwashed SW ash contains K2SO4, KCl and SiO2, while after 48 h of the washing process, the only detectable crystalline phase is cristobalite, thus confirming that the washing process was successful in removing the majority of the soluble alkalies. The amorphous phase in SW-ash48 () can reasonably be attributed to C–S–H gel hydration products formed during the washing process.
The hot stage microscopy has been used to evaluate the softening/melting temperature of the three ashes (TO, SO and SW-ash48). The TO and SO ashes melt at ∼1280–1300°C, while SW-ash48 shows an initial softening at ∼800°C and melts at ∼1100°C. These results are comparable with the data reported by James et al.Citation25 for similar ashes. Owing to the high viscosity of the melts, the vitrification of all ashes was performed at 1500°C, without the addition of any additives.
The chemical composition (wt-% oxides) of the vitrified biomass ashes is shown in . As expected, the glass obtained by direct vitrification of ashes from the woodchip combustion plants (TO-glass and SO-glass) showed almost the same composition; the vitreous systems are mainly composed of calcium, silicon, aluminium and potassium oxides, with minor quantities of iron and magnesium oxide. Obviously, the similarity of the composition for the glasses derived from different woodchips ashes is an important aspect in their hypothetical use as SCMs in mortars and concrete. Besides, SW-glass is mainly composed of SiO2 (>60 wt-%), CaO (12 wt-%) and K2O (11.5 wt-%) with smaller amounts of MgO and P2O5.
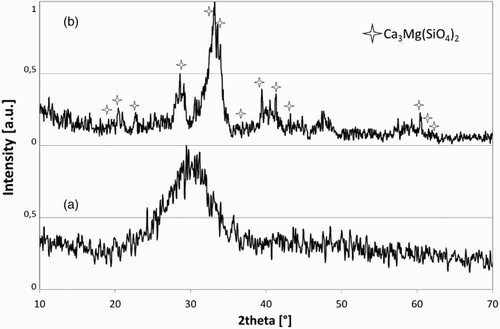
The XRD analysis of the TO, SO and SW glasses, obtained by the biomass ash vitrification at 1500°C, indicates that they are completely amorphous (TO-glass in ). The Vickers hardness () of the glass samples is between 750 and 780 HV50. This value corresponds to 6 in the Mohs’ scale,Citation26 the hardness of a medium hard aggregate.
Table 3 Vickers hardness of vitrified TO, SO and SW ashes
In order to investigate the influence of the vitrification temperature, TO and SO ashes were also heat treated at a lower temperature (1300°C). The XRD analysis of TO-1300 and SO-1300, obtained by a heat treatment at 1300°C for 1 h, shows the presence of crystalline phases in addition to the amorphous phase (XRD pattern of TO-1300 in ). The main identified crystalline phase is a calcium magnesium silicate with formula Ca3Mg(SiO4)2.
The heat treated ashes (TO-glass, SO-glass, SW-glass, TO-1300 and SO-1300) and SW after a 48 h washing (SW-ash48) were tested for pozzolanic activity according to UNI-EN 196-5. The pozzolanicity tests () showed that only SW-ash48, SW-glass and SO-glass are characterised by pozzolanic activity at both 8 and 15 days. SW-ash48 and SW-glass show the higher pozzolanic activity (0.95 after 8 days and 0.74–0.78 after 15 days). SO-glass and SO-1300 have a very low pozzolanic activity after 15 days (0.91 and 0.98 respectively), and TO-glass does not have any significant pozzolanic activity (1.05), probably because of the lower content of SiO2, 26–28 wt-%, compared to 57 and 54 wt-% for SW-glass and SW-ash48 respectively.
Table 4 Pozzolanic activity of vitrified ashes (TO-glass, SO-glass, SW-glass, TO-1300 and SO-1300) and SW after 48 h washing (SW-ash48) according to UNI-EN 196-5
The evaluation of the latent hydraulic activity of the heat treated ashes (TO-glass, SO-glass, SW-glass, TO-1300 and SO-1300) and SW ash after a 48 h washing (SW-ash48) was performed on standard mortars, prepared by replacing 30 wt-% of CEM II A-LL 42.5 R with TO-glass, SO-glass, SW-glass, TO-1300, SO-1300 or SW-ash48. The obtained results were compared to the performance of four reference mortars based on CEM II A-LL 42.5 R and on CEM II A-LL 42.5 R obtained by replacing 30 wt-% of cement with a limestone filler, fumed silica or natural pozzolan ().
The latent hydraulicity tests () demonstrate that all the materials contribute to strength development when tested in combination with a CEM II A-LL 42.5 R. TO-1300, SO-1300 and SW-ash48 are less active than the other materials but perform better than the reference mortar n. 2 (30 wt-% of limestone). The SW-ash48 mortars still show resistances that are higher than 80% of the reference cement (ref. 1); similar results have been obtained by Rajamma et al.Citation27 with different biomass ashes. The SW-ash48, obtained by a simple washing process and not involving any heat treatment, could potentially be used as an inexpensive SCM for medium strength cementitious components (>30 MPa after 28 days), which is high enough to serve for many common applications.
4 Compressive strength of mortars prepared according to UNI-EN 196/1: a reference mortars n.2 and 4 and mortars prepared by replacing 30 wt-% of CEM II A-LL 42.5 R with TO-1300 and SO-1300 glass–ceramics and SW-ash48; b reference mortars n.1 and 3 and mortars prepared by replacing 30 wt-% of CEM II A-LL 42.5 R with vitrified biomass ashes (TO-glass, SO-glass and SW-glass); compositions of mortars in
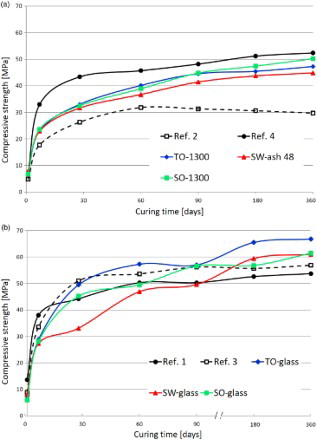
SW-glass based mortars reach almost the same compressive strengths of the reference cement (ref. 1) after 60 days, while TO-glass and SO-glass based mortars overtake all the reference formulations even after 28 days of hydration. All the mortars prepared with the three glasses show a compressive resistance higher than the fumed silica based mortars’ one after 180 days (TO-glass after only 28 days), thus confirming the high long term latent hydraulic reactivity of the glasses and that no detrimental reactions between the matrix and the vitrified ashes have occurred. In a previous study, alkali aggregate tests (according to UNI 8520-22 and ASTM C 298) have been performed on mortars when standard sand have been replaced by vitrified municipal solid waste incinerator bottom ash.Citation20 Results indicated that the vitrified ash does not generate deleterious alkali–silica reactivity or other detrimental reactions.
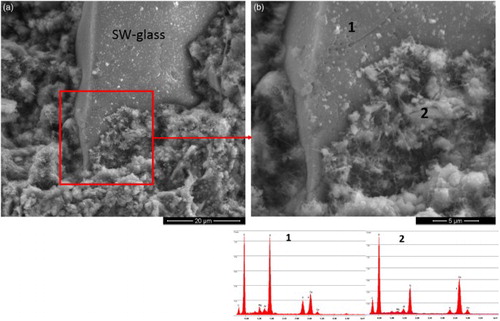
shows the morphology of the polished surface of a SW-glass based paste (after 28 days of curing) observed with SEM; the crack deflects from the original path and deviates at the SW-glass particle/cement interface. This deviation results in an increase in energy absorption that could lead to an increase of the toughness of the conglomerate.Citation28 shows a magnification of the SW-glass/cement interface (fracture surface), where a good adherence of the growing hydrate phases formed in the hydration of cement was observed. The matrix around the glass is very dense, and the nucleation of the hydrate phase is represented by the white round agglomerates on the glass surface. The EDS analysis performed in the white region (labelled with ‘2’ in ) shows a Ca/Si atomic ration of ∼1.9 and the presence of small amount of aluminium (Al/Si atomic ratio ∼0.15) and potassium. Aluminium can enter C–S–H (calcium silicate hydrate) mainly at the bridging sites in the silicate chains, and similar Al/Si ratios were reported in the literature for other aluminium containing SCMs.Citation14 For comparison purpose, the EDS analysis of the SW-glass is also reported in (‘1’ region).
6 a, b magnification of SW-glass/cement interface (fracture surface) and EDS analysis in regions ‘1’ (SW-glass particle) and ‘2’ (interface zone); SW-glass based paste after 28 days of curing
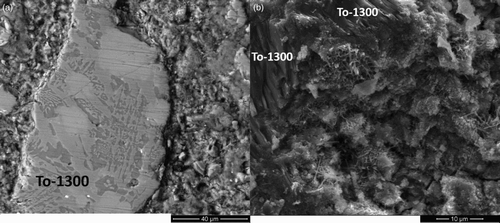
In , the polished and fracture surfaces of a TO-1300 based paste (after a 28 day curing) are shown. The glass–ceramic microstructure of the TO-1300 particles is clearly observable in (left top side). Besides, in , flaky and needle-like C–S–Hs are clearly visible in a largely cracked cement matrix, with a porosity higher than the one observed in the SW-glass based mortar (), thus explaining the lower mechanical strength of the mortars prepared with the two glass–ceramic SCMs in comparison with the three glass based ones.
7 SEM analysis of a polished and b fracture surfaces of TO-1300 based paste (after 28 days of curing)
The improvement of the mortar microstructure obtained using the vitreous SCMs (TO-glass, SO-glass and SW-glass) is to be closely correlated with the long term high compressive strengths (). In blended cementitious conglomerates, the SCM incorporation affects the development of strength through different mechanisms: the filler effect (i.e. small SCM grains fill in the interstitial space between the cement particles, resulting in a much denser binder matrix), the hydration acceleration effect, and the pozzolanic or hydraulic reaction.Citation15 The interface zone in ordinary Portland cement is a region of low C–S–H, high ettringite and Ca(OH)2 concentrations with decreased microhardness. In blended cements, additional C–S–H () can replace Ca(OH)2 and fill porosity. We cannot correlate the observed strength gain to a ‘filler effect’ that would have affected only the early age strengths and not the late age strengths. On the contrary, TO-glass and SW-glass samples show a remarkable strength gain after 90 days of hydration. At later curing ages, the mortars produced with the vitrified biomass ash show a higher rate of strength development due to the supplementary formation of products of the pozzolanic or hydraulic reactions, and the ultimate strength becomes higher than that of the parent Portland cement as illustrated in . The vitrified ashes show a slow reactivity and present positive strength contributions after 28 days (TO-glass and SO-glass) and 60 days (SW-glass) of curing.
The hydraulic behaviour of the vitrified ashes can be considered similar in terms of kinetic and mechanism of hydration (latent hydraulic activity) to the ground granulated slags that are currently used for the production of CEM III cement. This consideration enables to propose the vitrified ashes as beneficial for protecting the concrete in terms of attack by aggressive agents (i.e. chloride attack). This work will be part of a future research.
The vitrification of industrial waste (biomass ash included) is a well consolidated technological process,Citation29 offers the advantage of complete inertisation,Citation20 and is expected to be an attractive alternative for a sustainable long term use of biomass ash. In order to encourage the use of this technology, more work should be done to reduce the cost of the vitrification process (i.e. using inexpensive fluxes and increasing the vitrification time at lower temperature).
Conclusions
The production of biomass ash as a residue of biomass combustion is increasing worldwide. The chemical and mineralogical composition of biomass ash is extremely broad, and a general consideration upon its use cannot be really made. The present paper deals with two series of ashes with different chemical and mineralogical compositions. The results demonstrate that the biomass ash derived from the combustion of woodchips and straw could be successfully employed as SCMs in the building industry after valorisation processes based on vitrification or washing. The vitrification process transforms the ash into a non-crystalline material with high latent hydraulic reactivity, at a late age higher to the reference cement. The non-vitrified ash with a high SiO2 content is characterised by a pozzolanic activity that could be eventually exploited after a washing process that reduces the very high alkali content.
Acknowledgements
The research leading to these results received funding from the European Union's Seventh Framework Programme managed by REA-Research Executive Agency (http://ec.europa.eu/research/rea) through a Marie Curie Action (GlaCERCo GA 264526), from MIPAAF “Riutilizzo di ceneri provenienti da combustione di biomassa RICCO” DM 28384/7815/09 and from Regione Piemonte Ma2Re Project (POR FESR 2007/2013).
References
- xxx: ‘Biomass rises and falls with available feedstock supply’, Renewable Energy Focus, 2014, 15, (4), 32–35.
- B. M. Jenkins, L. L. Baxter and T. R. Miles: ‘Combustion properties of biomass’, Fuel Process. Technol., 1998, 54, 17–46.
- S. V. Vassilev, D. Baxter, L. K. Andersen and C. G. Vassileva: ‘An overview of the composition and application of biomass ash. Part 2. Potential utilisation, technological and ecological advantages and challenges’, Fuel, 2013, 105, 19–39.
- K. A. Aronsson and N. G. A. Ekelund: ‘Biological effects of wood ash application to forest and aquatic ecosystems’, J. Environ. Qual., 2004, 3, 1595–1605.
- I. Brunner, S. Zimmermann, A. Zingg and P. Blaser: ‘Wood-ash recycling affects forest soil and tree fine-root chemistry and reverses soil acidification’, Plant Soil, 2004, 267, 61–71.
- T. Kuba, A. Tschöll, C. Partl, K. Meyer and H. Insam: ‘Wood ash admixture to organic wastes improves compost and its performance’, Agr. Ecosyst. Environ., 2008, 127, 43–49.
- M. Komljenovic, Z. Bascarevic and V. Bradic: ‘Mechanical and microstructural properties of alkali-activated fly ash geopolymers’, J. Hazard. Mater., 2010, 181, (1–3), 35–42.
- S. Songpiriyakij, T. Kubprasit, C. Jaturapitakkul and P. Chindaprasirt: ‘Compressive strength and degree of reaction of biomass- and fly ash-based geopolymer’, Constr. Build. Mater., 2010, 24, (3), 236–240.
- S. K. Tyni, J. A. Karppinen, M. S. Tiaine and R. S. Laitinen: ‘Fly and bottom ash from FB combustion of peat as precursors to geopolymers’, in the Proceedings Venice 2012, Fourth International Symposium on Energy from Biomass and Waste, Cini Foundation, Venice, Italy, November 2012, Paper 212, 12–15.
- R. Melotti, E. Santagata, M. Bassani, M. Salvo and S. Rizzo: ‘A preliminary investigation into the physical and chemical properties of biomass ashes used as aggregate fillers for bituminous mixtures’, Waste Manage., 2013, 33, 1906–1917.
- R. C. E. Modolo, V. M. Ferreira, L. A. Tarelho, J. A. Labrincha, L. Senff and L. Silva: ‘Mortar formulations with bottom ash from biomass combustion’, Constr. Build. Mater., 2013, 45, 275–281.
- C. B. Cheah and M. Ramli: ‘Mechanical strength, durability and drying shrinkage of structural mortar containing HCWA as partial replacement of cement, Constr’, Build. Mater., 2012, 30, 320–329.
- R. Barbosa, N. Lapa, D. Dias and B. Mendes: ‘Concretes containing biomass ashes: mechanical, chemical, and ecotoxic performances, Constr’, Build. Mater., 2013, 48, 457–463.
- S. Wang: ‘Cofired biomass fly ashes in mortar: reduction of alkali silica reaction (ASR) expansion, pore solution chemistry and the effects on compressive strength’, Constr. Build. Mater., 2015, 82, 123–132.
- B. Lothenbach, K. Scrivener and R. D. Hooton: ‘Supplementary cementitious materials’, Review Article, Cem. Concr. Res., 2011, 41, (12), 1244–1256.
- R. Snellings, G. Mertens and J. Elsen: ‘Supplementary cementitious materials’, Rev. Mineral. Geochem., 2012, 74, 211–278.
- J. A. Kostuch, G. V. Walters and T. R. Jones: ‘High performance concrete containing metakaolin—a review’, in ‘Concrete 2000’, (ed. R. K. Dhir and M. R. Jones., 2, 1799–1811; 1993, London, E&FN Spon.
- R. Fernandez, F. Martirena and K. L. Scrivener: ‘The origin of the pozzolanic activity of calcined clay minerals: a comparison between kaolinite, illite and montmorillonite, Cem’, Concr. Res., 2011, 41, (1), 113–122.
- U. Müller and k. Rübner ‘The microstructure of concrete made with municipal waste incinerator bottom ash as an aggregate component’, Cem. Concr. Res., 2006, 36, 1434–1443.
- M. Ferraris, M. Salvo, A. Ventrella, L. Buzzi and M. Veglia: ‘Use of vitrified MSWI bottom ashes for concrete production’, Waste Manage., 2009, 29, 1041–1047.
- C. H. K. Lam, A. W. M. Ip, J. P. Barford and G. McKay: ‘Use of incineration MSW ash: a review’, Sustainability, 2010, 2, 1943–1968.
- M. Ferraris, M. Salvo, S. Rizzo, M. Caldirola, F. Canonico and M. Bianchi: ‘Recycling biomass ash into supplementary cementitious materials (SCMs) through innovative technologies’, in The Proceedings Venice 2012, Fourth International Symposium on Energy from Biomass and Waste, Cini Foundation, Venice, Italy, November 2012, Paper 401, 12–15.
- www.composites.polito.it/index.html?p=resources_waste_management&m=ricco.
- S. Diamond: ‘A review of alkali–silica reaction and expansion mechanisms 1. Alkalies in cements and in concrete pore solutions’, Cem. Concr. Res., 1975, 5, (4), 329–345.
- A. K. James, R. W. Thring, S. Helle and H. S. Ghuman: ‘Ash management review – applications of biomass bottom ash’, Energies, 2012, 5, 3856–3873.
- E. Wilfred Taylor: ‘Correlation of the Mohs's scale of hardness with the Vickers's hardness numbers’, Mineral. Mag., 1949, 28, 718–721.
- R. Rajamma, L. Senff, M. J. Ribeiro, J. A. Labrincha, R. J. Ball, G. C. Allen and V. M. Ferreira: ‘Biomass fly ash effect on fresh and hardened state properties of cement based materials’, Composites B, 2015, 77, 1–9.
- V. Li and M. Maalej: ‘Toughening in cement based composites. Part I. Cement, mortar, and concrete’, Cem. Concr. Compos., 1996, 18, (4), 223–237.
- www.enersoltech.com/index.htm.