Abstract
Tellurium based glasses have interesting thermoelectric characteristics. However, their high electrical resistivity is still an obstacle to considering them for thermoelectric applications. In this work, the (Te85Se15)60 − 0.6xAs40 − 0.4xCux glass system was studied. This revealed that Cu can act as glass former and increase both glass thermal stability and electrical conductivity. The best candidate, (Te85Se15)45As30Cu25, was chosen to prepare composites with Bi0.5Sb1.5Te3 using spark plasma sintering. These glass ceramic samples exhibited a much better thermoelectric performance. Glass ceramics with 50 mol. % of Bi0.5Sb1.5Te3 show a maximum ZT value equal to 0.37 at 413 K. Meanwhile, the advantages of glass including low sintering temperature and high formability are well maintained.
Introduction
The thermoelectric (TE) phenomena can provide the direct conversion of applied temperature gradient into electricity (Seebeck effect) or electricity into temperature difference (Peltier effect). This solid state technology can be applied in a variety of applications,Citation1 and the most well known is as a thermocouple for temperature measurement.Citation2 Nowadays, as the continuous fossil fuel supply decreases and world demand increases, TE materials have drawn renewed interest due to their potential to provide a sustainable supply of energy. Compared with other conventional electric generators, the reliability and simplicity of thermoelectricity enable niche applications even though many conventional processes are more efficient. One example is radioisotope TE generator,Citation3 which can provide electric power for spacecraft and satellites.
The ability of a given TE material to efficiently produce TE power is related to its dimensionless figure of merit given by ZT = S2T/ρκ, which depends on the Seebeck coefficient S, Kelvin temperature T, thermal conductivity κ and electrical resistivity ρ. Thus, a good TE material requires the maximisation of S2/ρ (power factor) and the minimisation of thermal conductivity. However, S, ρ and κ are mutually interdependent via the carrier concentration. In the early 1990s, the development of the ‘phonon glass electron crystal’ concept by SlackCitation4 led to a better understanding of the mechanism. In a good TE material, the phonons should be disrupted like in a glass, but the electrons should have high mobility like they do in crystalline semiconductors. A maximum S2/ρ is often obtained in heavily doped low gap semiconductors or semimetals. Other strategies are also employed to lower thermal conductivity including the presence of nanoscale inclusions and impurities, mass fluctuations and the presence of loosely bound atoms.
Tellurium based glasses, as the most conductive glasses, possess all the properties mentioned above and exhibit intrinsically low thermal conductivity thanks to their inherent disorder. The typical thermal conductivity of Te glasses is of the order of ∼0.1 W K − 1 m − 1.Citation5–Citation7 Thus, power factor is the parameter that needs to be optimised. Recent approaches have shown that the tellurium based glasses have a high intrinsic Seebeck coefficient (>500 μV K− 1).Citation8–Citation10 However, for TE application, their electrical resistivity is still too high to achieve a large ZT value. To overcome this obstacle, there is the possibility of varying the compositions to control the carrier concentration.Citation11 The Cu–Ge–Te, Cu–Si–Te, Cu–Ga–Te and Cu–As–Te glassy systems were developed by Gonçalves et al.Citation10,Citation12,Citation13 and Lucas et al.Citation14 The electrical resistivity is dramatically decreased by Cu addition. Up to now, the most conductive tellurium based glass is Cu30As15Te55 prepared by melt spinning with an electrical conductivity of ∼0.1 Ω cm.Citation10 However, this value is still not sufficient for commercial application.
To further reduce the electrical resistivity, another strategy relies on the preparation of glass ceramic composites. The glass ceramics prepared by partial crystallisation of Cu15As30Te55 using spark plasma sintering (SPS) exhibit a power factor increase from 1 to 257 μW m − 1 K− 2.Citation11 On this basis, glass ceramics prepared by introducing other TE phases as particles into glass matrix could also be interesting due to the controllability of crystalline phase fraction.
In the present work, investigations were carried out on Te–As–Se–Cu glasses. The glass with a relatively low electrical resistivity and proper thermal stability was chosen from the Te–As–Se–Cu glass system for the preparation of glass ceramics with Bi0.5Sb1.5Te3 (BST) using SPS to densify the composites. The synthesis process and the influence of BST percentage on TE properties were systematically studied.
Experimental
(Te85Se15)60 − 0.6xAs40 − 0.4xCux (x = 0, 10, 16.7, 20, 25) glasses (named as TEA1, TEA2, TEA3, TEA4 and TEA5 respectively) were prepared using the melt quenching method. Appropriate amounts of high purity (5N) raw materials Te, Se, As were mixed with Cu and sealed in evacuated (∼10− 3 Pa) silica tubes and melted in a rocking furnace at 1023 K for 10 h. Then, the glasses were quenched in water and annealed at a temperature 5 K lower than their glass transition temperature Tg for 3 h to relieve internal stresses.
For composition optimisation, the differential scanning calorimetry (DSC) experiments were performed using a DSC Q20 (TA Instruments) at a heating rate of 10 K min− 1 in order to determine the temperature difference ΔT between the glass transition temperature Tg and the crystallisation temperature Tx, which are the criteria of glass stability. Electrical conductivity measurements were also carried out on glass discs of 7 mm diameter and 1 mm thickness at room temperature with an S-302 four-point probe system from Lucas Labs.
Based on previous characterisation, the best glass composition was chosen and milled for 3 h to micrometre sized powders using the planetary ball milling technique (Retsch PM100) under the protection of argon. Rotation cycles of 3 min at 300 rev min− 1 were scheduled with direction reversal and a pause of 3 min between each cycle. Bi0.5Sb1.5Te3 (BST) was also synthesised by ball milling raw elements (5N) at 350 rev min− 1 for 10 h. The grain size of both the glass and the BST powders was selected to be < 50 μm using a sieve. The mixtures of glass with different ratios of BST (0, 10, 30 and 50%) were prepared by magnetic stirring at 200 rev min− 1 in ethanol (3N) solution at 70°C for 30 min and dried at 353 K for 30 min in a drying oven. Ethanol was chosen based on its high removability. Spark plasma sintering of the glass and glass ceramics powders was realised with a FCT System GmbH SPS furnace at 463 K for 10 min under a pressure of 40 MPa. The samples obtained were named as BST0, BST10, BST30 and BST50 according to the percentage of Bi0.5Sb1.5Te3 powder.
The density of the glass and glass ceramic bulk samples was determined using Archimedes' method. X-ray diffraction measurements were also performed using a PANalytical X'Pert Pro to identify the sample phases in the 2θ range of 5 to 90°. For the ZT calculation, the Seebeck coefficient S and electrical resistivity ρ were measured simultaneously using a homemade system at ∼298, 333, 373, 413 and 438 K. Note that both the S and ρ values of BST0 exceed the measuring range of the equipment. Another Seebeck measurement system (up to 373 K) and an S-302 four-point probe system (at 298 K) designed for high resistivity semiconductors were used to measure the S and ρ values of BST0 respectively. Thermal conductivity κ was also obtained by multiplying the thermal diffusivity α, density ρdensity and specific heat capacity cp. The thermal diffusivity α was measured at different temperatures using the laser flash method (NETZSCH, LFA457, Germany). The specific heat capacity cp was measured from 298 K up to 413 K using a DSC 1 STARe System (Mettler Toledo).
Results and discussion
The glass transition temperatures Tg and ΔT of the (Te85Se15)60 − 0.6xAs40 − 0.4xCux samples, determined from DSC measurements, are shown in . It is worth noting that Tg increases monotonically with Cu content in the whole compositional range. The same phenomenon has also been observed in both Se60 − 0.6xAs40 − 0.4xCux and Te60 − 0.6xAs40 − 0.4xCux glass systems.Citation15,Citation16 This can be explained from a structure point of view. Both As2Te3 and As2Se3 glasses are structurally made of distorted layers bound together by van der Waals interactions.Citation13,Citation17 Thus, (Te85Se15)60As40 should have the same structure. Similar to previous work,Citation16 the tetrahedrally coordinated Cu atoms can occupy interlayer positions and form bonds with the elements of the parent glass. The uniform distribution of Cu atoms develops stronger binding between the layers and strengthens the network. The density evolution was proven in previous studies. Compared with pure As2Se3 (4.462 g/cm3) and As2Te3 (5.535 g/cm3) glasses,Citation18,Citation19 the density of TEA5 glass (6.030 g cm− 3) is much higher, indicating that the glass became more constrained after doping. This network rigidity increase results in an overall increase in Tg with Cu content. Indeed, Cu can substitute As randomly and connect with Se or Te.Citation16,Citation20 This can effectively decrease the Te–Te nucleating agent and prevent the glass from crystallisation. As a result, ΔT increased from TEA1 (65 K) to TEA3 (100 K). Basically, Cu could be viewed as a glass former, and the stability of the glasses increases with increasing amounts of Cu. However, too much Cu (30%), due to its metallic behaviour, can destroy the thermal stability of the glass and Cu2Se nucleation (PDF 46-1129) occurs.
Table 1 Tg, ΔT and electrical resistivity of (Te85Se15)60 − 0.6xAs40 − 0.4xCux glasses
From , Cu played a significant role in reducing the resistance of (Te85Se15)60 − 0.6xAs40 − 0.4xCux glasses. The minimum resistivity ρ of ∼3.5 × 105 μΩ m was achieved for the TEA5 glass. Compared with TEA1 glass, Cu addition up to 25 mol. % results in a decrease in ρ by four orders of magnitude. However, the minimum ρ value obtained is still too high for effective TE devices application.
Thus, composites based on both (Te85Se15)45As35Cu25 (TEA1) glass and Bi0.5Sb1.5Te3 (BST) crystals were prepared. To ensure that the Seebeck coefficient is large, there should only be a single type of charge carrier. Mixed n type and p type conduction in one material will lead to both charge carriers moving to the cold end, cancelling out the induced Seebeck voltages. Te–Se–As–Cu glasses are known to be p type TE materials according to previous work.Citation5 As a result, BST, as a typical p type commercially available TE material, was chosen due to its high power factor and low thermal conductivity.Citation21 The BST used in this work was prepared by mechanical alloying with its room temperature power factor, thermal conductivity and ZT value to be 3500 μW m− 1 K− 2, 1.2 W m− 1 K− 1 and 0.9 respectively.Citation22
The glass ceramic samples prepared using SPS are shown in . After polishing, all of the glass and glass ceramic samples show a flat shiny surface. This demonstrated the good consolidation of the composites. During synthesis, due to the semiconducting properties of both the glass and BST phase, Joule heating occurs in both the carbon die and the powder, generating densification through the viscous sintering of the glass. Thus, the sintering temperature of glass ceramics (463 K) is much lower compared with pure BST (>700 K).
1 (Te85Se15)45As35Cu25/Bi0.5Sb1.5Te3 glass ceramic samples prepared by SPS technique. After polishing, all of glass and glass ceramic samples show flat shiny surface, indicating good consolidation of composites
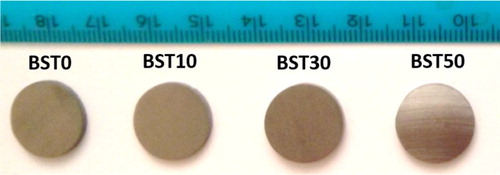
The density of the obtained pellets was compared with the theoretical density, which was calculated from pure TEA5 glass (6.03 g cm− 3) and pure BSTCitation23 (6.878 g cm− 3). The relative density lies between 97.7 and 99% (). This value confirmed good densification during sintering.
Table 2 Density and relative density of sintered (Te85Se15)45As35Cu25/Bi0.5Sb1.5Te3 glass ceramics
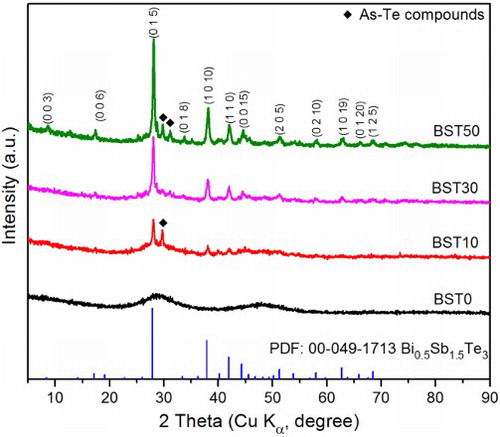
The X-ray diffraction pattern of the sintered BST0 pellet () demonstrates that fully densified amorphous samples can be successfully obtained by SPS processing. For the glass ceramics, the diffraction peaks of the BST crystals can be clearly observed, and the intensity increases obviously with increasing BST content. Nevertheless, extra peaks attributed to arsenic telluride can also be observed. Indeed, ball milling of both glass and ceramics powder can introduce extra surface energy owing to the broken bonds. In addition, BST crystals can act as nucleation agents during sintering. The thermal stability of the glass ceramic composites is thus greatly deteriorated, generating new diffraction peaks. However, the peak intensities are quite low, indicating that the proportion of the TEA5 glass remaining amorphous after sintering is large.
2 X-ray diffraction patterns of (Te85Se15)45As35Cu25/Bi0.5Sb1.5Te3 glass ceramics. Besides Bi0.5Sb1.5Te3 diffraction peaks, extra peaks attributed to arsenic telluride can also be observed. Nevertheless, high proportion of (Te85Se15)45As35Cu25 glass remained amorphous after sintering
To determine the TE potential of these materials, the Seebeck coefficient S and electrical resistivity ρ were measured separately for each composition at different temperatures (). Many previous researchesCitation24,Citation25 showed that both S and ρ are not independent because they have a close relationship with carrier concentration. For all of the samples, S was always positive, indicating a dominant p type conduction. BST0, due to its limited charge carrier concentration, exhibits a high S value (693 μV K− 1 at 298 K). By adding BST, S was initially decreased from BST0 to BST30. To explain this, it is important to note that a material's Seebeck coefficient is inversely related to its carrier density. Therefore, insulators tend to have higher Seebeck coefficients, while metals have lower values due to their high carrier concentrations. Hence, from SPS-BST0 to SPS-BST30, the sudden increase in charge carrier concentration induced by BST significantly decreased the Seebeck coefficient of the glass. Then, from BST30 to BST50, the Seebeck coefficient rises again. This could be explained by the increasing content of BST, which starts to play a dominant role and compensate for the S decrease in the glass. The dependence of the Seebeck coefficient on the temperature also corroborates the previous analysis. The S of BST0 decreases as the temperature increases, which is typical of the behaviour of tellurium based glasses.11,13 However, S of BST50 show a monotonous increase with temperature. This tendency is consistent with the properties of BST crystals in both this work and the reference.Citation26
Table 3 Seebeck coefficient and electrical resistivity of sintered (Te85Se15)45As35Cu25/Bi0.5Sb1.5Te3 glass ceramics
BST crystal, as a dopant, also determines the electrical resistivity of the glass ceramics. A large drop of four orders of magnitude from BST0 to BST50 is observed. This is also attributed to the introduction of extra charge carriers from BST. In addition, the ρ of BST10 is decreasing with increasing temperature. This is quite usual with semiconductorsCitation27 because the electrical conduction is a thermally activated process usually visualised as jumping over energy barriers. On the contrary, ρ of BST30 and BST50 slightly increased with temperature, which is the characteristic of semimetals and metals.
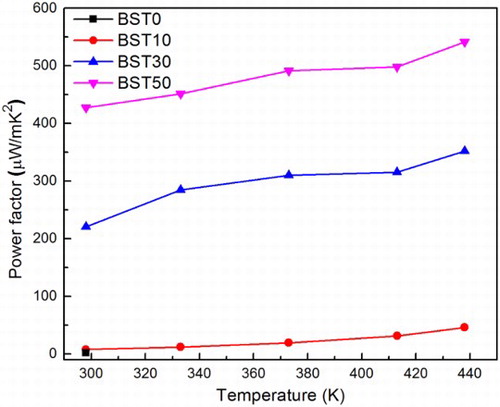
On the basis of the large decrease in ρ and reasonable values of S, the power factor was obtained using equation S2/ρ (). The power factor at room temperature shows a dramatic enhancement from BST0 (1.6 μW m− 1 K− 2) to BST50 (427 μW m− 1 K− 2). The maximum value is 541 μW m− 1 K− 2 obtained at 438 K in the BST50 exhibiting the highest BST crystalline fraction.
3 Power factor of (Te85Se15)45As35Cu25/Bi0.5Sb1.5Te3 glass ceramics. Power factor shows dramatic enhancement from pure glass to glass with 50% of Bi0.5Sb1.5Te3
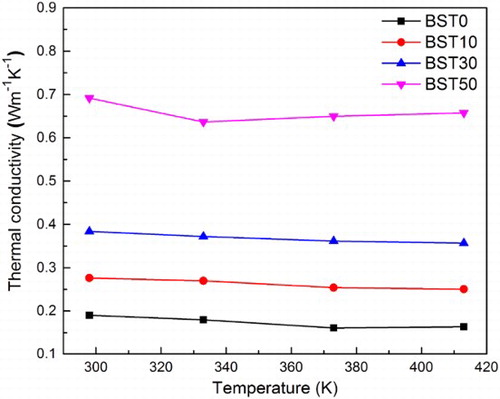
The thermal conductivities κ were calculated on the basis of the thermal diffusivity, density and specific heat capacity measurement. The results from 298 up to 413 K are shown in . The thermal conductivity of BST0 is < 0.2 W m− 1 K− 1 in all of the measuring range. Actually, the thermal conductivity of non-crystalline solids is several orders of magnitude smaller than that of crystalline materials. It decreases monotonically with decreasing temperature and is almost independent of the chemical composition.Citation28,Citation29 By adding BST with a thermal conductivity of ∼1.2 W m− 1 K− 1, the glass ceramic samples became more and more conductive. The thermal conductivity of BST50 is ∼0.65 W m− 1 K− 1. Compared with BST10 and BST30, this value is slightly higher than expected. This could be attributed to the formation of arsenic telluride crystals during sintering. Thus, to optimise the TE performance, the sintering parameters should be more precisely controlled to avoid undesirable crystallisation.
4 Thermal conductivity of (Te85Se15)45As35Cu25/Bi0.5Sb1.5Te3 glass ceramics. Glass ceramic samples became more thermal conductive by adding Bi0.5Sb1.5Te3. This can be explained by higher thermal conductivity of Bi0.5Sb1.5Te3 (1.2 W m− 1 K− 1) compared to glass
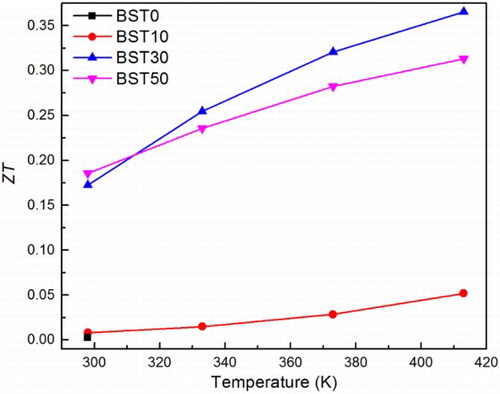
Based on the above measurements, we calculated the figure of merit ZT, which is shown in . Compared with the amorphous material BST0, the TE performance of BST50 exhibits a significant improvement. A tremendous increase in ZT value at room temperature can be observed from 0.002 up to 0.19. The best result obtained is 0.37 at 413 K for BST30. BST50, due to the thermal conductivity increase caused by the crystallisation of arsenic telluride, shows a lower TE performance. Nevertheless, its ZT value at 413 K is 0.31. These results prove that the substitution of (Te85Se15)45As35Cu25 glass by Bi0.5Sb1.5Te3 crystal is an effective method to improve TE performance. Meanwhile, the advantages of glass, including low sintering temperature and high formability, are well maintained. In the future, of the properties of glass ceramics composites with higher percentage of Bi0.5Sb1.5Te3 crystal should be investigated. The SPS processing parameters could be optimised to prevent crystallisation of second phases.
Conclusions
The thermal stability and electrical resistivity of the (Te85Se15)60 − 0.6xAs40 − 0.4xCux glasses were investigated by means of DSC and four-point probe respectively. Cu, which could be viewed as a glass former, can randomly substitute for As and connect with chalcogenide elements (Se, Te). This behaviour can strengthen the network and increase both the glass transition temperature and thermal stability. Cu also plays a significant role in reducing the electrical resistance of the glass. Cu (25 mol. %) can generate a decrease in ρ by four orders of magnitude. On this basis, (Te85Se15)45As35Cu25 was chosen for the preparation of composites with Bi0.5Sb1.5Te3 (0, 10, 30 and 50 mol. %) prepared by SPS. A systematic study of the Seebeck coefficient, electrical resistivity and thermal conductivity revealed that substitution of glass by Bi0.5Sb1.5Te3 crystal is an effective means of reducing electrical resistivity. The power factor at room temperature shows a dramatic enhancement from 1.6 up to 427 μW m− 1 K− 2. The maximum ZT value (0.365 at 413 K) was obtained for BST50. Compared with other amorphous TE material, the glass ceramics explored in this work exhibit a much better TE performance and at the same time maintain the advantages of glass.
Acknowledgements
Financial supports from European Community's Seventh Framework Programme through Marie-Curie Action: ‘Initial Training Networks’ (GlaCERCo GA 264526), and Brittany region through CATHER Project are gratefully acknowledged. The authors are also very grateful to S. Grasso and J. Khaliq for their help on sample preparation and characterisation.
References
- S. B. Riffat and X. Ma: ‘Thermoelectrics: a review of present and potential applications’, Appl. Therm. Eng., 2003, 23, (8), 913–935.
- P. A. Kinzie: ‘Thermocouple temperature measurement’, 1973, New York, Wiley.
- H. Xi, L. Luo and G. Fraisse: ‘Development and applications of solar-based thermoelectric technologies’, Renewable Sustainable Energy Rev., 2007, 11, (5), 923–936.
- G. A. Slack: ‘New materials and performance limits for thermoelectric cooling’, CRC Handb. Thermoelectrics, 1995, 34, 407–439.
- P. Lucas, C. Conseil, Z. Yang, Q. Hao, S. Cui, C. Boussard-Pledel, B. Bureau, F. Gascoin, C. Caillaud and O. Gulbiten: ‘Thermoelectric bulk glasses based on the Cu-As-Te-Se system’, J. Mater. Chem. A, 2013, 1A, (31), 8917–8925.
- S.-N. Zhang, J. He, T.-J. Zhu, X.-B. Zhao and T. M. Tritt: ‘Thermal conductivity and specific heat of bulk amorphous chalcogenides Ge20Te80 − xSex (x = 0, 1, 2, 8)’, J. Non-Cryst. Solids, 2009, 355, (2), 79–83.
- J. Philip, R. Rajesh and C. P. Menon: ‘Carrier-type reversal in Pb-Ge-Se glasses: Photopyroelectric measurements of thermal conductivity and heat capacity’, Appl. Phys. Lett., 2001, 78, (6), 745–747.
- A. P. Gonçalves, E. B. Lopes, O. Rouleau and C. Godart: ‘Conducting glasses as new potential thermoelectric materials: the Cu-Ge-Te case’, J. Mater. Chem., 2010, 20, (8), 1516–1521.
- C. Seager, D. Emin and R. K. Quinn: ‘Electrical transport and structural properties of bulk As-Te-I, As-Te-Ge, and As-Te chalcogenide glasses’, Phys. Rev. B, 1973, 8B, (10), 4746.
- A. P. Gonçalves, E. B. Lopes, G. Delaizir, J. B. Vaney, B. Lenoir, A. Piarristeguy, A. Pradel, J. Monnier, P. Ochin and C. Godart: ‘Semiconducting glasses: A new class of thermoelectric materials?’, J. Solid State Chem., 2012, 193, 26–30.
- J. Vaney, G. Delaizir, E. Alleno, O. Rouleau, A. Piarristeguy, J. Monnier, C. Godart, M. Ribes, R. Escalier and A. Pradel: ‘A comprehensive study of the crystallization of Cu-As-Te glasses: microstructure and thermoelectric properties’, J. Mater. Chem. A, 2013, 1A, (28), 8190–8200.
- A. P. Gonçalves, G. Delaizir, E. B. Lopes, L. M. Ferreira, O. Rouleau and C. Godart: ‘Chalcogenide glasses as prospective thermoelectric materials’, J. Electron. Mater., 2011, 40, (5), 1015–1017.
- J. B. Vaney, A. Piarristeguy, A. Pradel, E. Alleno, B. Lenoir, C. Candolfi, A. Dauscher, A. P. Gonçalves, E. B. Lopes, G. Delaizir, J. Monnier, M. Ribes and C. Godart: ‘Thermal stability and thermoelectric properties of CuxAs40 − xTe60 − ySey semiconducting glasses’, J. Solid State Chem., 2013, 203, 212–217.
- P. Lucas, C. Conseil, Z. Yang, Q. Hao, S. Cui, C. Boussard-Pledel, B. Bureau, F. Gascoin, C. Caillaud, O. Gulbiten, T. Guizouarn, P. Baruah, Q. Li and J. Lucas: ‘Thermoelectric bulk glasses based on the Cu-As-Te-Se system’, J. Mater. Chem. A, 2013, 1A, (31), 8917–8925.
- K. Liang, A. Bienenstock and C. Bates: ‘Structural studies of glassy CuAsSe2 and Cu-As2Se3 alloys’, Phys. Rev. B, 1974, 10B, (4), 1528.
- A. Giridhar and S. Mahadevan: ‘Mean atomic volume, Tg and electrical conductivity of Cux(As0.4Te0.6)100 − x glasses’, J. Non-Cryst. Solids, 1998, 238, (3), 225–233.
- K. Tanaka and K. Shimakawa: ‘Amorphous chalcogenide semiconductors and related materials’, 2011, New York, Springer.
- M. Kotkata, M. El-Fouly, S. Fayek and S. El-Hakim: ‘The effect of Tl addition on the electrical and thermal transport properties of amorphous As2Se3’, Semicond. Sci. Technol., 1986, 1, (5), 313.
- S. Mahadevan and A. Giridhar: ‘Ga as an additive in the As2Te3 glass’, J. Mater. Sci., 2001, 36, (22), 5325–5332.
- N. Zotov, F. Bellido, M. Dominguez, A. Hannon and R. Sonntag: ‘Continuous random network models of Cu-As-Te glasses’, Physica B, 2000, 276B, 463–464.
- B. Poudel, Q. Hao, Y. Ma, Y. Lan, A. Minnich, B. Yu, X. Yan, D. Wang, A. Muto and D. Vashaee: ‘High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys’, Science, 2008, 320, (5876), 634–638.
- H. R. Williams, R. M. Ambrosi, K. Chen, U. Friedman, H. Ning, M. J. Reece, M. C. Robbins, K. Simpson and K. Stephenson: ‘Spark plasma sintered bismuth telluride-based thermoelectric materials incorporating dispersed boron carbide’, J. Alloys Compd, 2015, 626, 368–374.
- C.-J. Liu, G.-J. Liu, Y.-L. Liu, L.-R. Chen and A. B. Kaiser: ‘Enhanced thermoelectric performance of compacted Bi0.5Sb1.5Te3 nanoplatelets with low thermal conductivity’, J. Mater. Res., 2011, 26, (15), 1755–1761.
- P. Zhu, Y. Imai, Y. Isoda, Y. Shinohara, X. Jia, G. Ren and G. Zou: ‘Electrical transport and thermoelectric properties of PbTe prepared by HPHT’, Mater. Trans., 2004, 45, (11), 3102–3105.
- N. Mateeva, H. Niculescu, J. Schlenoff and L. Testardi: ‘Correlation of Seebeck coefficient and electric conductivity in polyaniline and polypyrrole’, Jpn. J. Appl. Phys., 1998, 83, (6), 3111–3117.
- Y. Ma, Q. Hao, B. Poudel, Y. Lan, B. Yu, D. Wang, G. Chen and Z. Ren: ‘Enhanced thermoelectric figure-of-merit in p-type nanostructured bismuth antimony tellurium alloys made from elemental chunks’, Nano Lett., 2008, 8, (8), 2580–2584.
- S. Stehlik, J. Kolar, M. Bartos, M. Vlcek, M. Frumar, V. Zima and T. Wagner: ‘Conductivity in Ag-As-S (Se, Te) chalcogenide glasses’, Solid State Ionics, 2010, 181, (37), 1625–1630.
- R. Zeller and R. Pohl: ‘Thermal conductivity and specific heat of noncrystalline solids’, Phys. Rev. B, 1971, 4, (6), 2029.
- C. Kittel: ‘Interpretation of the thermal conductivity of glasses’, Phys. Rev., 1949, 75, (6), 972–974.