Abstract
Background:
This article summarizes the outcome from an international consensus meeting, which took place in Vienna on 4 November 2014.
Scope:
The aim of the meeting was to provide the state of the art on the pathophysiology and treatment of acute pain with special emphasis on nimesulide, a non-steroidal anti-inflammatory drug (NSAID) indicated for the treatment of acute pain and primary dysmenorrhea. Besides the data on the mechanisms of acute inflammatory pain and on the efficacy and safety of nimesulide in patients affected by different forms of acute pain, the clinical experience of attending experts was discussed based on selected case reports.
Results:
The members of this consensus group recognized that nimesulide is a NSAID highly effective in the treatment of several painful situations with an acute inflammatory component including primary dysmenorrhea. Although safety concerns regarding nimesulide have emerged in recent years, both robust new epidemiological data and clinical experience confirm a positive benefit/risk profile of nimesulide in the treatment of several forms of acute pain.
Conclusions:
The members of this international consensus group concluded that nimesulide, when used appropriately, remains a particularly valuable and safe option for the treatment of several conditions characterized by the presence of acute inflammatory pain because of the rapid onset of the analgesic action, and the positive evidence-based benefit/risk profile.
Keywords: :
Introduction
Pain is one of the leading causes for primary care consultations. While acute pain serves as a warning signal of a disease or a threat to the body by a noxious insult and is expected to resolve within the normal anticipated healing period, chronic pain persists despite the fact that the initial injury – if there was one at all – has healed. Unrelieved or undertreated acute pain, however, can lead to chronic pain.
Pharmacologic management is the cornerstone of both acute and chronic pain management. Particularly for acute pain treatment, nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most often used analgesic agents. NSAIDs are a chemically diverse group of drugs that share more or less the same therapeutic anti-inflammatory, analgesic, antipyretic and – with the exception of the COX-2-selective agents – platelet-inhibitory properties. Due to the opioid-receptor-independent COX inhibition as their common mode of action, they have several advantages, but also to a greater or lesser extent similar
side effects, which are mostly dose dependent. Even in therapeutic doses they can cause cerebrovascular and gastrointestinal disorders including ulcers and bleeding, and in high doses they may damage heart, kidneys and liverCitation1–3.
The non-selective NSAID nimesulide is a preferential COX-2 inhibitor with potent analgesic, anti-inflammatory and antipyretic activities that have been proven in numerous clinical trails and for a broad spectrum of pain conditions. Thirty years of clinical use of nimesulide have shown its rapid and sustained control of inflammation and pain as well as its favorable safety profile especially with regard to a reduced propensity to cause adverse gastrointestinal effects.
However, rare and unpredictable hepatic injury has been reported under nimesulide therapy, and a full assessment of the benefits and risks of nimesulide by the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) was undertaken in January 2010. In 2012, the CHMP concluded that the benefits of systemic nimesulide-containing medicines continue to outweigh their risks in the treatment of patients with acute pain and primary dysmenorrheaCitation4. Moreover, the duration of treatment was limited to 15 consecutive days only.
Liver damage is a rare adverse event that has already been known for the entire class of NSAIDs; recent pharmaco-epidemiological studies concluded that safety concerns with nimesulide are no higher than with other NSAIDs, and that the risk/benefit profile for hepatic side effects is comparable with other drugs of its classCitation5–7.
The purpose of this commentary is to provide current evidence on the efficacy and in particular safety of nimesulide in the treatment of a variety of clinical conditions characterized by the presence of acute inflammatory pain.
Acute pain
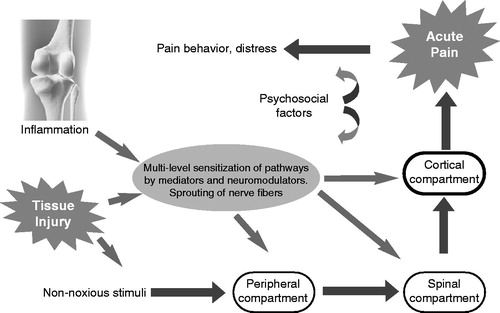
The 17th century French philosopher Rene Descartes was the first who described acute pain as a phenomenon of the brain. However, he was only partly right, since pain is not a ‘hard wired’ straightforward transmission of painful stimuli to the brain as postulated by him, but a complex phenomenon. The sensory experience of acute pain caused by a noxious stimulus is mediated by the specialized high-threshold nociceptive systemCitation8. This sensory system extends from the peripheral nerves through the dorsal root ganglion into the spinal cord, brain stem, and thalamus to the cerebral cortex, where the sensation – together with the simultaneously generated affective components – is finally perceived as pain. Acute pain is a normal response to tissue damage, and signal transmission and conduction always occur along the classic nociceptive pathways (). The final perception of pain intensity and character is the net result of the balance between afferent excitatory and descending inhibitory pathways involved at different levels of the central nervous system.
Thermal, mechanical, or chemical stimuli activate the peripheral endings of a highly specialized subset of primary sensory neurons, the nociceptors, which normally only respond to intense, high-threshold stimuliCitation8. Once a noxious stimulus is detected by the primary afferent nerve fiber the information is transduced into the language of the nervous system, namely electrical current or action potentials that convey nociceptive signals to synapses in the dorsal horn of the spinal cordCitation9.
Any tissue damage results in profound changes in the chemical environment of the peripheral nociceptorsCitation10. Damaged cells release intracellular factors such as adenosine triphosphate (ATP) and ions, activated inflammatory cells migrate into the injured area where they produce cytokines, chemokines, and growth factorsCitation9. Collectively, they produce the so-called ‘inflammatory’ or ‘sensitizing soup’, which consists of a wide array of signaling molecules and mediators, including neurotransmitters and peptides (substance P, CGRP, bradykinin), eicosanoid derivatives (prostaglandins, thromboxanes, leukotrienes, endocannabinoids) and related lipids at the site of tissue injury. This sensitizing soup changes the transduction sensitivity of the nociceptors in the periphery by decreasing the threshold of high-threshold and silent nociceptors. This process is called peripheral sensitization and also occurs in joints, where the damage to the joint tissues modifies the stimulus–response properties of nociceptor afferents in the same wayCitation11. These pro-inflammatory mediators not only enhance the damage, they also induce synovitis and intra-osseous inflammation, finally resulting in acute arthritisCitation12,Citation13.
Central sensitization is triggered by a release of transmitters from central nociceptive nerve terminals, which leads to alterations in synaptic receptor density, threshold, kinetics, and activation, thus dramatically increasing the synaptic transmission of nociceptive signals finally perceived as painCitation8. There is an activity-dependent increase in excitability of dorsal horn neurons, which means activation by inputs that are normally sub-threshold and an increase in response to supra-threshold inputs. Central sensitization manifests as either a reduced threshold for normally innocuous stimuli now eliciting pain – allodynia – or as an exaggerated or amplified response to noxious stimuli – hyperalgesia – or as the spread of hypersensitivity to non-injured innervation fields – secondary hyperalgesiaCitation14.
Consensus point
Central and peripheral sensitization play a fundamental role in the development of hyperalgesia associated with acute inflammatory pain.
NSAIDs and acute pain
Sensitization plays an important role in acute pain and also in the development of chronicity of painCitation15. Therefore, it is desirable to interrupt the chain of events as early as possible by drugs, which reduce not only peripheral sensitization at the nociceptors but also central sensitization processes at the spinal cord level. NSAIDs are able to functionally modify these nociceptive pathways at peripheral and central sites of the nervous system.
The most important property of NSAIDs is the reduction of the biosynthesis and accumulation of prostaglandins (PGs) at the site of injury or inflammation by inhibition of the cyclo-oxygenases (COX-1 and the inducible COX-2). NSAIDs usually normalize the decreased pain threshold associated with inflammation through their effects on cyclo-oxygenases, in particular on inducible COX-2Citation15. Some NSAIDs irreversibly inactivate COX (e.g. aspirin) or are reversible competitive inhibitors of COX (e.g. ibuprofen)Citation16.
Consensus point
Prostaglandins are involved in the development of neuronal sensitization and hyperalgesia. NSAIDs inhibit the formation of prostaglandins. For these reasons, they are highly effective in the treatment of acute inflammatory pain.
From a pharmacological point of view, NSAIDs are a heterogeneous group in terms of COX-2 selectivity, and also a chemically diverse group of substances whose overall anti-nociceptive action at peripheral and central sites depends on the site of drug delivery (e.g. systemic, local–peripheral, epidural, spinal, intracerebro-ventricular) and on the rate of GI absorption and uptake into inflamed cells, synovial tissues or cerebrospinal fluid, which is determined by the physical and chemical properties of the drug, specific transport mechanisms, local and systemic blood flow and tissue barriers to drug permeation such as the blood–brain barrierCitation16,Citation17.
NSAIDs not only differ with regard to their pharmacokinetic characteristics and pharmacologic properties, but also with respect to their clinical efficacy. Differences in clinical efficacy and in tolerability may be related to the different selectivities for COX-1 and COX-2 inhibitionCitation18. A major clinical difference lies in the time to onset and duration of pain relief. Compared to other oral NSAIDs nimesulide has a faster onset of analgesic action, i.e. 15 minutes after oral administrationCitation19,Citation20,Citation21, and it is noteworthy that this fast onset has been observed in patients with inflammatory hyperalgesia associated with a chronic painful condition.
Consensus point
Differences in COX-1 and COX-2 selectivity may explain the different benefit/risk profiles in the group of NSAIDs.
Pain and comorbidities
Multiple concomitant acute and chronic medical conditions in the same pain patient are increasingly common in the clinical setting, also due to the progressive aging of the populationCitation22. Multimorbidity affects between 55% and 98% of the elderly populationCitation23. In most cases, a patient with multiple disorders will also have one or more conditions that are painful. For example, myofascial pain syndromes significantly co-occur with headache, fibromyalgia, and various types of visceral painCitation24. Different visceral pain syndromes very often co-exist in the same patients, e.g., ischemic heart disease and biliary calculosis, and acute painful disorders of multiple pelvic visceraCitation25.
Headache disorders, affecting nearly half the world’s adult population significantly co-exist with fibromyalgia, dysmenorrhea, endometriosis, or irritable bowel syndrome, with epidemiological findings suggesting that comorbid pain conditions may enhance the transition of episodic to chronic headacheCitation26–28.
These painful conditions may significantly influence each other, with trigger points exacerbating headache and fibromyalgia, or two types of visceral pain enhancing each other when originating from organs sharing at least part of their central sensory projection (viscero-visceral hyperalgesia).
In comorbidities that are not necessarily painful, there may be important symptom interactions and implications for diagnosis and therapyCitation22. Cardiac conduction disturbances or arrhythmias can limit the use of first-line drug treatments for neuropathic pain, and a concurrent cardiovascular disease may affect the therapeutic approach to migraine therapy with vaso-active compounds. Obesity is an important risk factor for headache and other pain conditions such as osteoarthritis and low back pain. Comorbidity in pain patients thus may not only result in enhanced or reduced pain perception, but can also have a significant impact on the patient’s treatment and the choice of analgesic drugs. Finally, affective disorders – such as depression or anxiety – frequently accompany many forms of pain, requiring careful evaluation for diagnosis of their primary or secondary nature and for integrated treatmentCitation29.
Consensus point
Headache often co-exists with fibromyalgia and different forms of visceral pain including dysmenorrhea.
Nimesulide for the treatment of acute pain
Pharmacological features
Nimesulide is a preferential COX-2 inhibitor with a wide range of additional biological actions that help to explain its specific anti-inflammatory and analgesic activity ().
Table 1. Main inhibitory mechanisms involved in the multi-factorial action of nimesulide against inflammatory pain.
Consensus point
The ability of nimesulide to affect different mediators and intracellular pathways involved in inflammatory pain provides this NSAID with a unique multi-factorial mechanism of action.
With regard to pharmacokinetics, nimesulide is rapidly absorbed from the gastrointestinal tract after oral administration and is mainly distributed in the extracellular fluids with a small volume of distribution in the range of 0.18–0.39 l/kg. The peak plasma concentration (Cmax) is reached 2–3 hours after administration. Plasma concentrations decline mono-exponentially, with an elimination half-life of approximately 4 hoursCitation44. Studies in patients with osteoarthritis (OA) have shown that relatively high concentrations of nimesulide are rapidly reached in the synovial fluid as well, thus indicating that nimesulide can also modulate inflammatory mediators at the joint levelCitation45. Nimesulide is extensively metabolized, and metabolites are excreted in the urine (≈70%) and in the feces (≈30%)Citation46. As for other drugs, hepatic insufficiency may significantly impair the elimination of this NSAID.
In patients with moderate renal impairment the pharmacokinetic profiles of nimesulide are not altered.
Consensus point
The pharmacokinetic features of nimesulide are consistent with the rapid onset of its analgesic effect.
Clinical evidence in patients with different types of acute inflammatory pain
More than 200 clinical trials evaluated the efficacy of nimesulide in a wide spectrum of inflammatory and painful conditions. In these studies nimesulide has consistently shown relief of inflammatory pain significantly superior to placebo and at least equivalent, or in some cases even superior, to that of other NSAIDsCitation17,Citation47.
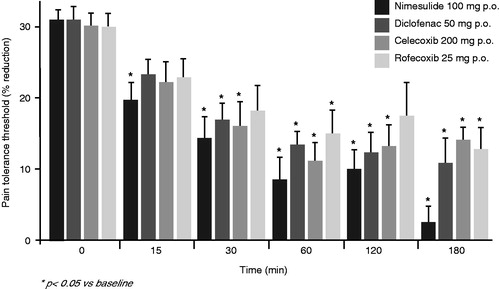
Nimesulide is particularly indicated for the treatment of acute pain, where inflammation is the predominant component. Hyperalgesia is a characteristic feature of inflammatory pain. The anti-hyperalgesic activity of nimesulide, diclofenac, celecoxib and rofecoxib was investigated in patients with rheumatoid arthritis who suffered from acute inflammatory hyperalgesia associated with their chronic painful rheumatic conditionCitation21. After a single oral dose, all these NSAIDs reduced inflammatory hyperalgesia to mechanical stimulation. However, with nimesulide the onset of action was more rapid than with the other drugs tested, and only in nimesulide-treated patients was the effect already evident 15 minutes after taking (). Such a fast onset of analgesia could only be approached with special rapid release formulations of other NSAIDsCitation48,Citation49.
Figure 2. Anti-hyperalgesic effects of nimesulide compared to diclofenac, celecoxib and rofecoxib in patients with joint inflammation: percentage reduction of pain tolerance threshold. With kind permission from Bianchi and Broggini, 2002Citation21.
The efficacy of nimesulide (100 mg b.i.d.) in the relief of postoperative pain after orthopedic surgery was compared with that of naproxen (500 mg b.i.d.) in a multicenter, double-blind, double-dummy, randomized, parallel group studyCitation50. Ninety-four patients with at least moderate postoperative pain after arthroscopy and meniscectomy were randomized to receive nimesulide, naproxen, or placebo for a maximum of 3 days. Nimesulide was significantly more effective than placebo for the treatment of postoperative pain, as measured by the primary efficacy variable of summed pain intensity difference within 6 hours after first treatment. Furthermore, nimesulide also provided significantly better pain relief than naproxen on this end point. At 1 hour after treatment, more than 70% of nimesulide-treated patients experienced a 50% reduction in pain intensity, compared with less than 50% of patients treated with naproxen and 40% of patients receiving placebo. Overall, nimesulide demonstrated superior analgesic activity compared with naproxen and placebo for the majority of secondary efficacy variables. Although the sodium salt of naproxen would be expected to have a faster onset of action compared to the formulation used in this study, these findings demonstrated that nimesulide is an effective, fast-acting and well tolerated oral anti-inflammatory drug with a distinct analgesic activity in patients undergoing orthopedic surgery.
Joint pain is the main complaint in patients affected by osteoarthritis (OA). The analgesic efficacy of nimesulide (100 mg) was compared with equivalent doses of celecoxib (200 mg) and rofecoxib (25 mg) in a prospective, randomized, double-blind, within-patient study performed in 30 patients with OA of the kneeCitation51. The results of this short-term trial demonstrated that a single dose of nimesulide provided greater efficacy and a more rapid onset of the analgesic action than a comparable single dose of celecoxib and rofecoxib.
A prospective, randomized, double-blind, between-patient study compared the analgesic effects of nimesulide and celecoxib at indicated doses for the symptomatic treatment of knee OACitation41. The primary efficacy endpoint was the intensity of joint pain during walking measured with a 100 mm VAS. In patients with joint effusion the effects of nimesulide were more marked than for celecoxib, with evidence of a faster onset of analgesic action. Both after single or repeated (14 days) administration, nimesulide significantly reduced the synovial fluid concentrations of substance P (SP) and the proinflammatory cytokine IL-6, whereas celecoxib did not change the concentrations of SP and significantly reduced the levels of IL-6 only on day 14. This effect of nimesulide on inflammatory pain mediators is consistent with its fast analgesic action.
Nimesulide has also proven to be very effective in the treatment of acute low back pain. In a prospective, randomized, double-blind, comparative trial, a total of 104 patients with acute lumbosacral pain were randomly allocated either to a treatment with oral nimesulide (100 mg twice daily for 10 days) or oral ibuprofen (600 mg three times daily for 10 days)Citation52. From the third day of treatment onward, a clear improvement in all measured parameters for pain and functioning was observed with the two study drugs. The patients’ capacity for daily tasks showed improvement in both groups (p < 0.001), but after 10 days a statistically significant difference was found between the two groups in favor of nimesulide (p < 0.05). Nimesulide and ibuprofen provided similar improvement in the modified Schober tests, but overall more gastrointestinal adverse events were reported with ibuprofen.
A double-blind, multicenter study demonstrated that nimesulide was at least as effective as diclofenac in the short-term treatment of acute shoulder pain (acute subdeltoid bursitis and bicipital tendinitis)Citation53. Over 82% of the patients rated nimesulide as good/very good compared to 78% of patients rating diclofenac as good/very good. Global tolerability was judged by the investigators to be good/very good in 96.8% of patients in the nimesulide group compared with 72.9% of patients treated with diclofenac, and judgments by the patients were 96.8 and 78.0%, respectively. Both differences were statistically significant.
In several smaller studies, nimesulide was investigated as a treatment option for an acute gout attackCitation54–57. In all studies, nimesulide induced a rapid reduction of inflammatory pain. In one study, the efficacy of nimesulide exceeded that of diclofenacCitation57.
Consensus point
Nimesulide has proven to be very effective for the treatment of acute joint pain.
Oral surgical pain models, such as the extraction of third molars, are considered particularly useful for the evaluation of the analgesic activities of NSAIDs. There is accompanying production of prostaglandin E2 (PGE2), bradykinin and other pro-inflammatory mediators in the extraction site. Interestingly, the production of PGE2 in the oral surgical extraction site is inhibited by NSAIDs in parallel with the reduction in painCitation58. A considerable number of studies have been reported showing the efficacy of nimesulide in controlling postoperative pain following dental surgery. Among the early investigations, a study by CornaroCitation59 compared the effects of nimesulide and placebo in 49 patients who had undergone oral surgery for various conditions. Overall, pain relief judged as ‘excellent’ or ‘good’ was found in 64% of patients treated with nimesulide compared with 25% in those given placebo. Nimesulide 100 mg b.i.d. proved to be significantly superior to placebo in analgesic and anti-inflammatory activity. Salvato and co-workersCitation60 compared the effects of 6 days of treatment with either nimesulide 200 mg/day, serratiopeptidase 15 mg/day or no therapy in 100 patients who had undergone tooth extraction or surgery for osteolysis. Reduction in pain and inflammation was rated to be ‘excellent’ or ‘good’ in 95% of patients that received nimesulide, 65% of those given the peptidase preparation and in 25% of the non-treatment group. Nimesulide had faster onset of analgesia than the other treatments. Finally, using the third molar surgery model, Ragot and co-workersCitation61 showed in a randomized double-blind placebo-controlled trial in 134 patients that pain intensity difference (PID) and pain relief (PAR) scores after the intake of 100 or 200 mg nimesulide or 250 mg niflumic acid were significantly greater than placebo over the 6 hour period of the study. This study was of particular importance for nimesulide, since it showed no advantage in taking the higher dose of 200 mg compared to the 100 mg dose of the drug.
Another large randomized, multicenter, double-blind, placebo-controlled study in 469 patients undergoing molar tooth extraction compared the effects of single doses of 100 or 200 mg nimesulide with 500 mg mefenamic acidCitation62. Rescue medication (paracetamol) was allowed and the quantities consumed by the different groups were recorded. The percentage of responders in the 100 and 200 mg nimesulide groups was 77.7 and 74.5%, respectively, whereas the mefenamic acid group had 43.4% and placebo group had 16.5% of responders. The PID values over the period of 0.5–6 hours of the study and cumulative or SPID values showed the greatest pain relief with the 100 and 200 mg doses of nimesulide, without any difference between the two doses. Excellent or good pain relief was achieved in 87/110 (79.1%) of patients that received 100 mg nimesulide, in 91/112 (81.3%) of patients that had 200 mg nimesulide, in 51/98 (52%) of patients who had mefenamic acid, and in 33/103 (32%) of patients who received placebo. The percentage of patients who required additional paracetamol use was 27% in the nimesulide groups, 57% in the mefenamic acid group and 70% in the placebo group. These comparative studies demonstrated that nimesulide was as effective as niflumic acid and better than mefenamic acid in the treatment of postoperative dental pain, with a rapid onset of analgesic action (already evident in the first 30 minutes). Levrini and colleaguesCitation63 performed an observational, multicenter, prospective survey to evaluate the pattern of administration of NSAIDs in 616 patients undergoing surgery for impacted third molar extraction. This study also aimed to collect information on the efficacy, onset and duration of the analgesic effect of routinely prescribed NSAIDs. Nimesulide was the most used drug (68%) followed by diclofenac, ketoprofen and ibuprofen. Especially when given before patients started experiencing pain after surgery, nimesulide was more effective than other NSAIDs in reducing the intensity of pain, in delaying the time to maximum intensity of pain, and in providing complete pain relief on the day of surgery. On day 1, 72.6% of patients who had taken nimesulide experienced complete pain relief versus 54.7% of patients treated with other NSAIDs. Furthermore, nimesulide provided a fast onset of the analgesic action, evident after 15 minutes in about 70% of patients. The results of this observational study confirm that nimesulide is a particularly effective NSAID for the treatment of acute postoperative pain induced by dental surgery.
Prostaglandins play an important role in the pathogenesis of primary dysmenorrhea. These inflammatory mediators stimulate myometrial contraction, produce ischemia and induce the peripheral sensitization of nerve endings. Pain is produced through these three mechanisms. Moreover, the penetration of prostaglandins into general circulation accounts for a number of possible systemic symptoms of dysmenorrhea (nausea, fatigue, fever, headache). Most symptoms of primary dysmenorrhea, therefore, can be explained by the action of uterine prostaglandins, namely PGF2α. This explains the usefulness of NSAIDs in the treatment of this painful condition. Numerous clinical studies in patients with dysmenorrhea showed nimesulide to be superior to placebo and similar to or more effective than other NSAIDs such as diclofenac, naproxen and mefenamic acidCitation17. In particular, data showed the activity of nimesulide on intrauterine pressure and the reduction of PGF2α, which both play a fundamental role in this type of perimenstrual painCitation64. As with joint pain, nimesulide again showed a faster onset of pain relief compared to the widely used NSAID diclofenacCitation65.
Consensus point
Nimesulide is particularly suitable for the treatment of primary dysmenorrhea.
Approximately 60% of all headaches in women are related to the menstrual cycle. To be more precise, 25% of migraines occur exclusively during the perimenstrual period (menstrual migraine), and 75% occur during this period as well as at other times of the cycle (menstrually related migraine)Citation66. NSAIDs are frequently used as first line acute treatment for migraine in both out-patient and in-patient settings, as well as for preventive treatment in menstrual migraineCitation67. Nimesulide has a well documented efficacy for relieving symptoms associated with migraine and non-migraine headachesCitation17.
In a controlled, double-blind clinical trial, 30 women with menstrual migraine were randomly assigned to receive either granular nimesulide or placeboCitation68. Starting from the onset of migraine symptoms, each treatment was continued for 10 days and was repeated for the two following menstrual cycles. The overall efficacy was assessed with hourly self-evaluation of pain on each study day. In patients treated with nimesulide, pain intensity and duration were significantly reduced compared with placebo (p = 0.0001).
In a recent retrospective evaluation of medical records of 741 migraine patients (137 men and 604 women, aged 18–76 years), who had used at least one type of NSAID for their migraine attacks in the 3 months preceding the first visit at an Italian Center for the Study of Headache, nimesulide was the most frequently used NSAID (55%), followed by ketoprofen (18%), ibuprofen (17%) and paracetamol (11%)Citation69. With nimesulide 72% of the patients achieved complete pain relief, in 28% partial relief was observed. These preliminary results of a wider study suggest a good efficacy of nimesulide in the treatment of migraine attacks, which is in line with the outcome of previous national surveysCitation70,Citation71, in which nimesulide was the most used compound for the treatment of migraine attacks.
Consensus point
Nimesulide is effective in the treatment of different forms of acute headache, including migraine.
Safety profile of nimesulide
In clinical practice the spectrum of adverse effects often represents the main discriminator for choosing an individual NSAIDCitation17. In general, the use of any NSAID, including nimesulide, is recommended for the shortest time needed to solve the symptoms of inflammation, and caution is required to ensure the safety of patients who are more vulnerable, such as the elderly and those with concomitant organ impairment.
Consensus point
As for other NSAIDs the use of nimesulide is recommended at its lowest effective dose for the shortest period of time necessary to obtain the control of symptoms.
Gastrointestinal safety
The frequent occurrence of gastrointestinal (GI) adverse events is a major concern in the use of NSAIDs. To quantify the risk of upper GI complications (UGIC: peptic ulcer, perforations, obstruction and bleeding) Castellsague and co-workers conducted a systematic review and meta-analysis of published observational studiesCitation6. The highest relative risk (RR) was found with piroxicam, ketorolac and azapropazone ().
Figure 3. Pooled relative risks of upper gastrointestinal complications (UGIC) associated with the use of individual NSAIDs. Data from Castellsague et al., 2012Citation6.
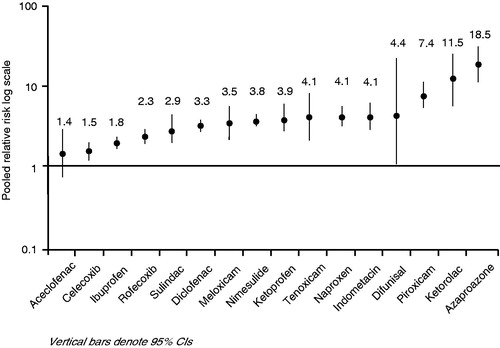
The use of high daily doses was associated with a significant increase of the relative risk for UGIC compared to low/medium doses of individual NSAIDs (e.g. naproxen 3.2 vs 6.4, diclofenac 2.5 vs 4.2, piroxicam 6.9 vs 20.3).
In a recent retrospective cohort study, the risk for UGIC in users of nimesulide was estimated and compared with that associated with the use of other NSAIDsCitation5. The analysis comprised 2001–2008 data from regional health databases in Friuli-Venezia-Giulia (FVG, Italy) and included 588,827 NSAIDs users and 3031 UGIC cases. Current use of different NSAIDs was compared to non-use by logistic regression analyses.
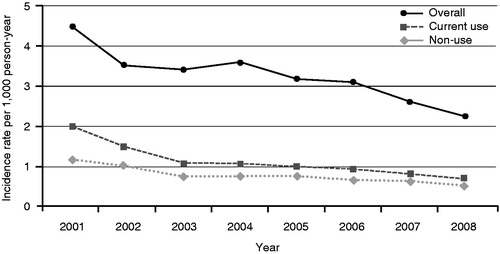
Among current users the incidence rate (IR) per 1000 person-years decreased from 4.45 cases in 2001 to 2.21 cases in 2008. This observation is consistent with the increased use of proton pump inhibitors in the same period ().
Figure 4. Time trends of incidence rates for upper gastrointestinal complications (UGIC), current single use vs non-use. Data with kind permission from Castellsague et al., 2013Citation5.
As reported in , the relative risk (RR) associated with a low/medium dose range of nimesulide resulted in an overall RR of 1.53%, compared with a RR of 2.83% in users of a single NSAID.
Table 2. Relative risk for upper gastrointestinal complications (UGIC) associated with the use of different NSAIDs: data from Castellsague et al., 2013Citation5.
As expected, this study also showed that patients older than 65 years of age have a significantly higher risk for UGIC with the use of NSAIDs than younger patients.
Consensus point
The risk of upper gastrointestinal complications with nimesulide is lower than with many other NSAIDs widely used in patients with acute pain.
Hepatic safety
NSAIDs have been involved in liver injury, and adverse hepatic reactions have been reported for most drugs of this familyCitation72–76. Some NSAIDs (bromfenac, ibufenac, benoxaprofen and, more recently, lumiracoxib) have been withdrawn from clinical use because of their hepatotoxicity. With regard to the relatively weak analgesic drug paracetamol, patients may tend to overdose the drug in case of insufficient pain relief, which is still the leading cause of accidental hepatotoxicity and liver failure worldwide.
The multinational SALT studyCitation7 evaluated the population event rates for NSAID-associated acute liver failure leading to liver transplantation. This non-interventional, retrospective study was agreed with the European Medicines Agency (EMA) to provide estimates of the rates of acute liver failure leading to registration for liver transplantation in patients who had been exposed to NSAIDs or paracetamol within 30 days before first clinical symptoms of liver injury. In this case–population study all adult patients that were registered for liver transplantation in France, Greece, Ireland, Italy, the Netherlands, Portugal and the United Kingdom were analyzed in the 3 year period 2005–2007. Cases were validated by a hepatologist in each country, and population exposures to NSAIDs and paracetamol were estimated from national sales. The analysis showed that acute liver failure leading to transplantation after exposure to NSAIDs is a rare event, with no major differences between most of the clinically used NSAIDs (). Event rates per million treatment-years were 1.59 for all NSAIDs pooled, and 1.88 for nimesulide. Notably, paracetamol in therapeutic doses led to liver failure twice as frequently as NSAIDs.
Figure 5. Event rates for exposure to a NSAID or paracetamol within 30 days before the date of the first clinical symptoms. Data with kind permission from Gulmez et al., 2013Citation7.
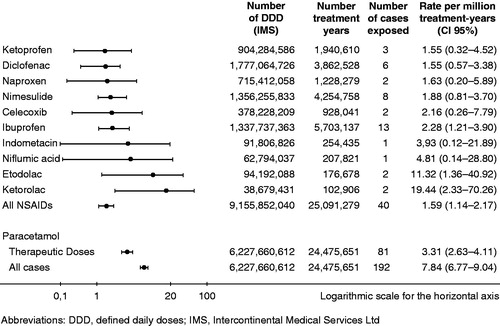
These results confirm other epidemiological studies demonstrating a small risk of severe liver injury in patients taking nimesulide and other non-steroidal anti-inflammatory drugsCitation77.
Consensus point
The overall risk of severe hepatic reactions associated with the use of NSAIDs is low. The incidence of liver injury associated with the use of nimesulide is fully within the range of other NSAIDs.
Cardiovascular safety
All NSAIDs carry some cardiovascular risk, and in patients with hypertension, hypercholesterolemia, and cardiovascular diseases all NSAIDs should be used with cautionCitation78, particularly in the elderly. Risks and benefits must be individually weighed, and the lowest effective dose should be used for the shortest possible time. Many data suggest that nimesulide shows a low overall risk of cardiovascular events such as myocardial infarction or congestive heart failureCitation79,Citation80.
Summary on safety
When used appropriately, nimesulide has a relatively low tendency to induce severe GI complications compared to other NSAIDs. With regard to its hepatotoxicity, the EMA as well as several recently published reports have not found any higher risk of severe hepatotoxicity than other NSAIDs. Severe renal, cardiovascular and skin reactions are very rare in patients treated with nimesulide.
Based on a thorough review of all available safety data, the Committee for Medicinal Products for Human Use (CHMP) of the EMA stated that the benefits of systemic nimesulide outweigh its risks. From this point of view, it is important to note that the marketing authorizations for systemic formulations of nimesulide in the EU Member States were renewed via the Mutual Recognition Procedure with unlimited validity in April 2014. Its use, however, should be restricted to the treatment of acute pain and primary dysmenorrhea, for a maximum duration of 15 days.
Thanks to the appropriate use of nimesulide in clinical practice, no critical safety issues were identified in recent years based on spontaneous reports, reviews, or published studies. Consistent with this, Periodic Safety Update Reports should be submitted to the EMA according to a 3 year periodicity, instead of every 6 months, as in the pastCitation81.
Overall conclusions
Patients seek effective, rapid, and safe relief of acute pain. Nimesulide is a preferential COX-2 inhibitor with a unique multi-factorial mode of action. Its anti-inflammatory and analgesic effects have been established in numerous controlled trials, and in 30 years of clinical practiceCitation82. Notably, nimesulide is characterized by a rapid onset of the analgesic effect, with meaningful pain relief observed within 15–30 minutes from drug intake.
Labeled use of nimesulide is associated with good tolerability, and the overall safety profile of nimesulide is similar to other NSAIDs, with a lower risk of gastrointestinal toxicity than with many other drugs of this group. The incidence of liver reactions with nimesulide is about 0.1 per 100,000 treated patients, which is in the range of other NSAIDs extensively used in clinical practice. Nimesulide may therefore be considered a valuable alternative to coxibs because of its comparable efficacy and lower cardiovascular risks.
Based on the updated available evidence, the experts of the Consensus Meeting Group on the Role of Nimesulide in Acute Pain concluded that this NSAID is particularly suitable for the treatment of different forms of acute inflammatory pain, such as musculoskeletal and low back pain, postoperative or post-traumatic pain, headache and migraine attacks including menstrual migraine, primary dysmenorrhea, and acute gout attack.
summarizes the exemplary clinical cases – indications, therapeutic regimens, and outcome – describing the use of nimesulide for the treatment of different types of acute inflammatory pain that were presented and discussed by the expert panel during the consensus meeting.
Table 3. Summary of exemplary case reports discussed by the expert panel during the consensus meeting.
Transparency
Declaration of funding
The present article is based on a Consensus Meeting that was made possible through an unrestricted grant from CSC Angelini Pharma Austria and Helsinn Healthcare SA Switzerland.
The authors meet ICMJE criteria for authorship, take full responsibility for the content of this publication and confirm that it reflects their viewpoints and medical expertise.
Declaration of financial/other relationships
H.G.K. has disclosed that he is a consultant to CSC Angelini Pharma Austria and Helsinn Healthcare SA, Grunenthal GmbH, Mundipharma Int., Teva, Philips, Astellas Eisai, bene Chemie Munchen; and on the Speakers’ Bureau of CSC Angelini Pharma Austria and Helsinn Healthcare SA, Grunenthal GmbH, Mundipharma Int., and Linde group. A.B. has disclosed that he is a consultant to APP Pharmaceuticals. A.Bas., F.B., E.C. and J.W. have disclosed that they are consultants to Angelini Pharma Austria and Helsinn Healthcare SA. J.C. has disclosed that she/he has received sponsorship for participating in a nimesulide symposium from Helsinn Healthcare SA. C.C. has disclosed that she/he has received grants from, is a consultant to and is on the Speakers’ Bureau of the following companies: Amgen, AbbVie, MSD, Pfizer, Roche, Sanofi and UCB; is a consultant to Egis and Zentiva; and is also on the Speakers’ Bureau of Angelini, BMS, Richter, Servier, Teva and Zentiva. M.A.G. has disclosed that she/he has received grants from and is a consultant to Helsinn Healthcare SA. M.V.V. has disclosed that she/he is on the Speakers’ Bureau of Abilis. M.H., L.H., M.K., J.L., L.N., R.S., A.S., and T.T. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work. Peer reviewer 1 has disclosed that he is on the Speakers’ Bureau of Pfizer. Peer reviewer 2 has no relevant financial or other relationships to disclose.
Acknowledgments
The Authors thank Monika Peretz from Update Europe, Vienna, for her editorial support, which was paid for by the sponsors.
References
- Sawdy R, Slater D, Fisk N, et al. Use of a cyclo-oxygenase type-2-selective non-steroidal anti-inflammatory agent to prevent preterm delivery. Lancet 1997;350:265-6
- Vane JR, Botting RM. Anti-inflammatory drugs and their mechanism of action. Inflamm Res 1998;47(Suppl 2):S78-87
- Bessone F. Non-steroidal anti-inflammatory drugs: what is the actual risk of liver damage? World J Gastroenterol 2010;16:5651-61
- European Medicines Agency. Assessment report for nimesulide containing medicinal products for systemic use. Procedure number: EMEA/H/A-31/1261. Referral under Article 31 of Directive 2001/83/EC, as amended
- Castellsague J, Pisa F, Rosolen V, et al. Risk of upper gastrointestinal complications in a cohort of users of nimesulide and other nonsteroidal anti-inflammatory drugs in Friuli Venezia Giulia, Italy. Pharmacoepidemiol Drug Saf 2013;22:365-75
- Castellsague J, Riera-Guardia N, Calingaert B, et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf 2012;35:1127-46
- Gulmez SE, Larrey D, Pageaux GP, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case–population SALT study. Drug Saf 2013;36:135-44
- Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 2004;140:441-51
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139:267-84
- Dawes JM, McMahon SB. Chemokines as peripheral pain mediators. Neurosci Lett 2013;557:1-8
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10:1361-8
- Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet 2005;365:965-73
- Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573-81
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl):S2-15
- Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth 2010;105(Suppl 1):i69-85
- Burian M, Geisslinger G. COX-dependent mechanisms involved in the antinociceptive action of NSAIDs at central and peripheral sites. Pharmacol Ther 2005;107:139-54
- Rainsford KD. Current status of the therapeutic uses and actions of the preferential cyclo-oxygenase-2 NSAID, nimesulide. Inflammopharmacology 2006;14:120-37
- Conaghan PG. A turbulent decade for NSAIDs: update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol Int 2012;32:1491-502
- Bennett A, Tavares IA. COX-2 inhibitors compared and contrasted. Expert Opin Pharmacother 2001;2:1859-76
- Sandrini G, Proietti Cecchini A, Alfonsi E, et al. Effectiveness of nimesulide in pain. A neurophysiological study in humans. Drugs Today 2001;37:21-9
- Bianchi M, Broggini M. Anti-hyperalgesic effects of nimesulide: studies in rats and humans. Int J Clin Pract Suppl 2002;128:11-19
- Kress HG, Ahlbeck K, Aldington D, et al. Managing chronic pain in elderly patients requires a CHANGE of approach. Curr Med Res Opin 2014;30:1153-64
- Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011;10:430-9
- Giamberardino MA, Affaitati G, Fabrizio A, Costantini R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol 2011;25:185-98
- Giamberardino MA, Costantini R, Affaitati G, et al. Viscero-visceral hyperalgesia: characterization in different clinical models. Pain 2010;151:307-22
- Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27:193-210
- Jamieson DJ, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol 1996;87:55-8
- Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. Lancet Neurol 2008;7:354-61
- Chai NC, Rosenberg JD, Peterlin BL. The epidemiology and comorbidities of migraine and tension-type headache. Tech Reg Anesth Pain Manag 2112;16:4-13
- Ottonello L, Dapino P, Pastorino G, et al. Nimesulide as a downregulator of the activity of the neutrophil myeloperoxidase pathway. Focus on the histoprotective potential of the drug during inflammatory processes. Drugs 1993;46(Suppl 1):29-33
- Rossoni G, Berti F, Buschi A, et al. New data concerning the antianaphylactic and antihistaminic activity of nimesulide. Drugs 1993;46(Suppl 1):22-8
- Pelletier JP, Martel-Pelletier J. Effects of nimesulide and naproxen on the degradation and metalloprotease synthesis of human osteoarthritic cartilage. Drugs 1993;46(Suppl 1):34-9
- Dapino P, Ottonello L, Dallegri F. The anti-inflammatory drug nimesulide inhibits neutrophil adherence to and migration across monolayers of cytokine-activated endothelial cells. Respiration 1994;61:336-41
- Bevilacqua M, Vago T, Baldi G, et al. Nimesulide decreases superoxide production by inhibiting phosphodiesterase type IV. Eur J Pharmacol 1994;268:415-23
- Pelletier JP, Mineau F, Fernandes J, et al. Two NSAIDs, nimesulide and naproxen, can reduce the synthesis of urokinase and IL-6 while increasing PAI-1, in human OA synovial fibroblasts. Clin Exp Rheumatol 1997;15:393-8
- Pelletier JP, Di Battista JA, Zhang M, et al. Effect of nimesulide on glucocorticoid receptor activity in human synovial fibroblasts. Rheumatology 1999;38(Suppl 1):11-13
- Henrotin YE, Labasse AH, Simonis PE, et al. Effects of nimesulide and sodium diclofenac on interleukin-6, interleukin-8, proteoglycans and prostaglandin E2 production by human articular chondrocytes in vitro. Clin Exp Rheumatol 1999;17:151-60
- Zheng SX, Mouithys-Mickalad A, Deby-Dupont GP, et al. In vitro study of the antioxidant properties of nimesulide and 4-OH nimesulide: effects on HRP- and luminol-dependent chemiluminescence produced by human chondrocytes. Osteoarthritis Cartilage 2000;8:419-25
- Fahmi H, He Y, Zhang M, et al. Nimesulide reduces interleukin-1beta-induced cyclooxygenase-2 gene expression in human synovial fibroblasts. Osteoarthritis Cartilage 2001;9:332-40
- Kullich W, Fagerer N, Schwann H. Effect of the NSAID nimesulide on the radical scavenger glutathione S-transferase in patients with osteoarthritis of the knee. Curr Med Res Opin 2007;23:1981-6
- Bianchi M, Broggini M, Balzarini P, et al. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int J Clin Pract 2007;61:1270-7
- Vellani V, Franchi S, Prandini M, et al. Nimesulide inhibits protein kinase C epsilon and substance P in sensory neurons - comparison with paracetamol. J Pain Res 2011;4:177-87
- Vellani V, Franchi S, Prandini M, et al. Effects of NSAIDs and paracetamol (acetaminophen) on protein kinase C epsilon translocation and on substance P synthesis and release in cultured sensory neurons. J Pain Res 2013;6:111-20
- Bernareggi A. Clinical pharmacokinetics of nimesulide. Clin Pharmacokinet 1998;35:247-74
- Bianchi M, Ferrario P, Balzarini P, et al. Plasma and synovial fluid concentrations of nimesulide and its main metabolite after a single or repeated oral administration in patients with knee osteoarthritis. J Int Med Res 2006;34:348-54
- Davis R, Brogden RN. Nimesulide. An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1994;48:431-54
- Bianchi M, Ehrlich GE, Facchinetti F, et al. Clinical applications of nimesulide in pain, arthritic conditions and fever. In: Rainsford KD (editor). Nimesulide. Actions and Uses. Basel: Birkhäuser, 2005:245-313
- Hersh EV, Levin LM, Cooper SA, et al. Ibuprofen liquigel in oral surgery pain. Clin Ther 2000;22:1306-18
- Zuniga JR, Noveck RJ, Schmidt WK, et al. Onset of action of diclofenac potassium liquid-filled capsules in dental surgery patients. Curr Med Res Opin 2011;27:1733-9
- Binning A. Nimesulide in the treatment of postoperative pain: a double-blind, comparative study in patients undergoing arthroscopic knee surgery. Clin J Pain 2007;23:565-70
- Bianchi M, Broggini M. A randomised, double-blind, clinical trial comparing the efficacy of nimesulide, celecoxib and rofecoxib in osteoarthritis of the knee. Drugs 2003;63(Suppl 1):37-46
- Pohjolainen T, Jekunen A, Autio L, Vuorela H. Treatment of acute low back pain with the COX-2-selective anti-inflammatory drug nimesulide: results of a randomized, double-blind comparative trial versus ibuprofen. Spine 2000;25:1579-85
- Wober W, Rahlfs VW, Büchl N, et al. Comparative efficacy and safety of the non-steroidal anti-inflammatory drugs nimesulide and diclofenac in patients with acute subdeltoid bursitis and bicipital tendinitis. Int J Clin Pract 1998;52:169-75
- Klumb EM, Pinheiro GRC, Ferrari A, Albuquerque EMN. The treatment of acute gout arthritis. Double-blind randomized comparative study between nimesulide and indomethacin. Revista Brasileira de Medicina 1996;53:540-6
- Barskova VG, Iakunina IA, Nasonova VA. Nimesil treatment of gouty arthritis. Ter Arkh 2003;75:60-4
- Barskova VG, Nasonova VA, Tsapina TN, et al. The effectiveness and safety of nimesulide (nimesile) in patients with gouty arthritis. Klin Med (Mosk) 2004;82:49-54
- Kudaeva FM, Eliseev MS, Barskova VG, Nasonova VA. Comparison of the time to analgetic and anti-inflammatory effect in the treatment of gouty arthritis with nimesulide and sodium diclofenac. Ter Arkh 2007;79:35-40
- Valanne J, Korttila K, Ylikorkala O. Intravenous diclofenac sodium decreases prostaglandin synthesis and postoperative symptoms after general anaesthesia in outpatients undergoing dental surgery. Acta Anaesthesiol Scand 1987;31:722-7
- Cornaro G. A new non steroidal anti-inflammatory drug in the treatment of inflammation due to parodental surgery. Curr Ther Res 1983;33:982-9
- Salvato A, Zambruno E, Ventrini E, Savio G. Sperimentazione clinica di un nuovo antiedemigeno orale: nimesulide. Giorn Stomat Ortognat 1984;3:184-91
- Ragot JP, Monti T, Macciocchi A. Controlled clinical investigation of acute analgesic activity of nimesulide in pain after oral surgery. Drugs 1993;46(Suppl 1):162-7
- Ragot JP, Giorgi M, Marinoni M, et al. Acute activity of nimesulide in the treatment of pain after oral surgery – double blind, placebo and mefenamic acid controlled study. Eur J Clin Res 1994;5:39-50
- Levrini L, Carraro M, Rizzo S, et al. Prescriptions of NSAIDs to patients undergoing third molar surgery – an observational, prospective, multicentre survey. Clin Drug Invest 2008;28:657-68
- Pulkkinen MO. Is there a rationale for the use of nimesulide in the treatment of dysmenorrhea? Drugs Today 2001;37:31-8
- Facchinetti F, Piccinini F, Sgarbi L, et al. Nimesulide in the treatment of primary dysmenorrhoea: a double-blind study versus diclofenac. Drugs Today 2001;37:39-45
- Brandes JL, Freitag FG, Zacur HA. Understanding and diagnosing menstrually related migraine. Adv Stud Med 2005;5:S774-82
- Silberstein SD, Stirpe JC. COX inhibitors for the treatment of migraine. Expert Opin Pharmacother 2014;15:1863-74
- Giacovazzo M, Gallo MF, Guidi V, et al. Nimesulide in the treatment of menstrual migraine. Drugs 1993;46(Suppl 1):140-1
- Affaitati G, Fabrizio A, Lopopolo M, et al. Use of NSAIDs for migraine attacks: results from a retrospective analysis in an Italian Headache Center. 15th World Congress of Pain 2014, Abs PT293
- Motola D, Vaccheri A, Silvani MC, et al. Pattern of NSAID use in the Italian general population: a questionnaire-based survey. Eur J Clin Pharmacol 2004;60:731-8
- Ferrari A, Pasciullo G, Savino G, et al. Headache treatment before and after the consultation of a specialized centre: a pharmacoepidemiology study. Cephalalgia 2004;24:356-62
- Davidson DG, Eastham WN. Acute liver necrosis following overdose of paracetamol. Br Med J 1966;2:497-9
- Mitchell JR, Jollow DJ, Potter WZ, et al. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther 1973;187:185-94
- Schapira D, Bassan L, Nahir AM, Scharf Y. Diclofenac-induced hepatotoxicity. Postgrad Med J 1986;62:63-5
- Banks AT, Zimmerman HJ, Ishak KG, Harter JG. Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the Food and Drug Administration as adverse reactions. Hepatology 1995;22:820-7
- Walker AM. Quantitative studies of the risk of serious hepatic injury in persons using nonsteroidal anti-inflammatory drugs. Arthritis Rheum 1997;40:201-8
- Traversa G, Bianchi C, Da Cas R, et al. Cohort study of hepatotoxicity associated with nimesulide and other non-steroidal anti-inflammatory drugs. BMJ 2003;327:18-22
- Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol 2009;103:1227-37
- Suleyman H, Cadirci E, Albayrak A, Halici Z. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr Med Chem 2008;15:278-83
- Fanelli A, Romualdi P, Vigano R, et al. Non-selective non-steroidal anti-inflammatory drugs (NSAIDs) and cardiovascular risk. Acta Biomed 2013;84:1-7
- Franchi S, Heiman F, Visentin E, Sacerdote P. Survey on appropriateness of use of nimesulide in nine European countries. Drug Healthc Patient Saf 2015;7:51-5
- Mattia C, Ciarcia S, Muhindo A, Coluzzi F. Nimesulide 25 anni dopo. Minerva Med 2010;101:285-93