Abstract
Objective:
Current therapies for diabetic peripheral neuropathy with pain mask the painful symptoms while the underlying pathology continues to progress. This study assessed changes in symptoms and quality of life in patients taking a novel prescription medical food, L-methylfolate-methylcobalamin-pyridoxal-5-phosphate (LMF-MC-PP, Metanx), intended to address the underlying metabolic needs of patients with diabetic peripheral neuropathy.
Research design and methods:
Between November 2010 and April 2012, patients rated their experiences before and after using LMF-MC-PP through an automated telephone system that included symptomatic items from the Neuropathy Total Symptom Score-6 (NTSS-6) questionnaire and questions related to quality of life and medication satisfaction.
Results:
A total of 544 patients participated in the study. Patients reported a mean reduction of 35% in NTSS-6 scores from after 12 weeks on LMF-MC-PP. Mean (standard deviation) score was reduced by 1.5 (1.8) at 12 weeks from a baseline of 4.3 (1.5) (p < 0.05). Patients achieved significant reductions in self-reported disruptions in work/school activities, social life, and family life, respectively. Overall pain rating decreased by 32% (p < 0.05). Patients previously treated with medications reported a 52% improvement in medication satisfaction (p < 0.05).
Conclusions:
In a real-world clinical setting, patients with diabetic peripheral neuropathy treated with LMF-MC-PP achieved significant improvements in total symptom score (NTSS-6) and in quality of life and functioning, together with greater medication satisfaction. A limitation of this study was the use of a survey instrument to collect data on patient outcomes.
Introduction
The epidemic of both type 1 and type 2 diabetes mellitus affects 29.1 million children and adults in the United States – constituting 9.3% of the population. Neuropathy is the most common complication of diabetes – it has been estimated that about 60% to 70% of people with diabetes have mild to severe forms of nervous system damageCitation1. Diabetic peripheral neuropathy (DPN) is a debilitating complication of diabetes and causes sensory symptoms that impact health and functionalityCitation2. The prevalence of chronic DPN has been estimated at 13% to 26% of diabetic patientsCitation3,Citation4, the prevalence increases with both age and duration of diabetes, and the diagnosis of DPN is more common in those whose glycemic control has been suboptimal in previous yearsCitation5.
While there may not be complete agreement on the pathogenesis of DPN, evidence suggests that several proposed mechanisms are collectively responsible for the development and progression of DPN. De Vriese et al.Citation6, Feldman and VincentCitation7, and TesfayeCitation8 provided a summary of a unified DPN pathogenesis. Under these hypotheses, diabetes leads to chronic hyperglycemia, which appears to stimulate four pathways: advanced glycation end-product (AGE) formation, polyol pathway hyperactivity, free radical formation/glucose autoxidation, and protein kinase C (PKC) activationCitation9. Increased activity in these pathways leads to oxidative and nitrative stress, which in turn leads to endothelial dysfunction. Therefore, while glycemic control remains the cornerstone of DPN treatment, nutritional treatments that impact on the metabolic processes surrounding endothelial dysfunction provide sound alternatives to pharmacologic treatments.
Currently available pharmacologic treatments recommended for DPN include duloxetine, gabapentin, and pregabalin and are directed towards symptom management, primarily pain relief, rather than addressing the underlying pathophysiology of DPNCitation10,Citation11. In addition, these drugs associated with substantial significant side effects including dizziness, drowsiness, gastrointestinal distress, constipation, headaches, dry mouth, and sexual dysfunctionCitation10,Citation11. Nutritional therapies that promote endothelial function may not only improve symptoms of neuropathy but may also slow or even reverse the progression of DPNCitation12–14. Research data on the use of nutritional management to address the underlying metabolic abnormalities and endothelial dysfunction that are implicated as causes of DPN have shown promising resultsCitation15.
L-methylfolate (LMF) or 5-methyltetrahydrofolate (5-MTHF) is the active form of folic acid. In a randomized, placebo-controlled clinical trial to evaluate the effects on endothelial function, LMF restored endothelial-dependent vasodilation among hypercholesterolemic patientsCitation16. Among patients with type 2 diabetes, 5-MTHF improved nitric-oxide-mediated vasodilation, which suggests a role for 5-MTHF in improving the status of nitric oxide (NO) – an important vasodilator – and endothelial functionCitation12. In an experimental model of DPN, folic acid protected against DPN, which was related to neuroprotective effects of folic acidCitation17. Research on methylcobalamin (MC), the active form of vitamin B12, found that MC improved the somatic and autonomic symptoms of diabetic neuropathy among DPN patients (without causing side effects)Citation18, enhanced nerve regeneration in rats with DPNCitation19, and delayed onset of DPN in ratsCitation20. An in vitro study showed that pyridoxine (P), the active form of vitamin B6, along with pyridoxamine (PM) were found to inhibit superoxide radical production, reduce lipid peroxidation and glycosylation, and increase the (Na+ + K+)-ATPase activity in high glucose-exposed red blood cells (RBCs) – suggesting that P or PM supplementation may delay or inhibit DPNCitation21.
Several studies evaluated the combined effect of these three substances, LMF, MC and pyridoxal-5-phosphate (PP). In a study of patients with type 2 diabetes and DPN, an oral combination of LMF-MC-PP was shown to improve and restore cutaneous sensationCitation22. A study of micronutrients including vitamins B6, B12, and folic acid in patients with type 2 diabetes, found an improvement in neuropathy symptomsCitation23. LMF-MC-PP also reduced pain among DPN patients who were partial responders to pregabalinCitation24. Most recently, a randomized, double-blind, placebo-controlled trial showed that LMF-MC-PP significantly improved neuropathy symptoms and health-related quality of lifeCitation25. Thus, a substantial body of preclinical and clinical literature supports the use of micronutrients for treating the underlying pathophysiology of DPN.
Metanx is a prescription medical food containing LMF-MC-PP. The goal of this study was to assess, within a real-world clinical setting, changes in total neuropathy sensory symptoms and quality of life among DPN patients who were taking LMF-MC-PP.
Methods
Data for this study were collected between November 2010 and April 2012 from surveys of patients who had been prescribed LMF-MC-PP to manage their DPN symptoms. To be eligible to participate in these surveys, patients were required to be at least 18 years old and naive to treatment with LMF-MC-PP. Patients were asked to complete a baseline survey prior to using LMF-MC-PP and a follow-up survey at 12 weeks post-treatment. Surveys were conducted using an interactive voice response system. A verbal consent to participate in the study was obtained from all participants prior to enrollment.
The baseline survey included questions on demographics (age and gender), and disease and treatment history such as duration of time since diabetes diagnosis, duration of DPN symptoms, prescription medications taken for their DPN symptoms, and medication satisfaction. Patients were asked to rate their overall pain from DPN and level of disruption to their daily activities due to DPN symptoms. Additional questions in the follow-up survey asked about what other medications were taken with LMF-MC-PP to manage DPN symptoms, compliance with LMF-MC-PP, and satisfaction with LMF-MC-PP.
For both the baseline and follow-up surveys, patients were surveyed for the presence of symptoms assessed by a modified 6-item Neuropathy Total Symptom Score-6 (NTSS-6) questionnaireCitation25. The NTSS-6 was developed to evaluate individual neuropathy sensory symptoms in patients with diabetes mellitus and DPN so that a response to therapy in clinical trials could be identifiedCitation26. The NTSS-6 assesses symptoms on six domains: numbness and/or insensitivity; prickling and/or tingling sensation; burning sensation; aching pain and/or tightness; sharp, shooting, lancinating pain; and allodynia and/or hyperalgesia. Only patients with a baseline NTSS-6 score ≥1 were included in the study.
Overall pain rating was measured on a 10 point visual analog scale, with 0 indicating no pain and 10 indicating the worst possible pain. Impact of DPN symptoms on quality of life was measured by rating the level of disruption to work and school activities, social life, and family life on a 0 to 10 scale, with 0 indicating no disruption and 10 indicating complete disruption. Medication satisfaction was measured on a 0 to 10 scale with 0 indicating ‘not at all satisfied’ and 10 indicating ‘very satisfied’.
The primary outcome measure was mean change in NTSS-6 from baseline to follow up at the end of LMF-MC-PP treatment. Secondary outcome measures were change in overall pain rating, impact of DPN symptoms on quality of life measures, and change in medication satisfaction, before and after LMF-MC-PP treatment. Patient characteristics and outcomes of interest were summarized using frequency distributions or descriptive statistics (means/medians and standard deviations) as appropriate. Statistical significance between baseline and follow-up responses was tested using the Wilcoxon signed rank test for continuous/ordinal variables and McNemar test for nominal variables. Statistical Package for Social Sciences (SPSS) v20 was used for all statistical analyses. A p-value of <0.05 was considered significant.
Results
Baseline patient characteristics
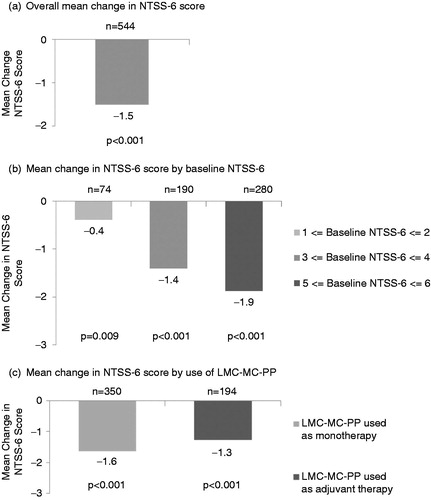
A total of 544 patients completed the two-survey study. Mean (SD) time between baseline survey completion and follow-up survey completion was 95 (21) days. A summary of baseline patient characteristics revealed more females than males, mean (SD) age of 65.5 (10.9) years, and a majority (84%) in the 55 years and over age group (). Approximately half (51%) of patients had been diagnosed with diabetes mellitus longer than 5 years, and 35% had had DPN symptoms for more than 3 years. About one in four patients (25.7%) reported using prescription medication to treat DPN symptoms prior to use of LMF-MC-PP. Mean (SD) baseline NTSS-6 was 4.3 (1.5) with slightly more than half (51.5%) of patients reporting an NTSS-6 score of 5 or 6.
Table 1. Baseline patient characteristics (N = 544).
Medication experience with LMF-MC-PP
During the study, 350 (64%) patients used LMF-MC-PP as monotherapy (). The remainder of the patients used a variety of medications approved for the treatment of DPN. Compliance with LMF-MC-PP was high with 96% of patients reporting taking most doses or every dose of LMF-MC-PP as prescribed by their physician.
Table 2. Medication behavior (N = 544).
Treatment response
At baseline, the mean (SD) score was 4.3 (1.5). Overall, patients reported a significant (p < 0.001) reduction from baseline for the NTSS-6 score of 1.5 (1.8) points after a mean of 95 (21) days of LMF-MC-PP treatment (). This change represents a 35% reduction from baseline. When stratified by baseline NTSS-6 score, significant (p < 0.05) reductions in NTSS-6 were observed across all groups (). Patient subgroups with the greatest severity (highest baseline NTSS-6 scores) reported the greatest reductions. Improvements in NTSS-6 score were reported regardless of whether LMF-MC-PP was used as adjuvant therapy or as monotherapy. Patients who used LMF-MC-PP as monotherapy and patients who used LMF-MC-PP with other DPN medications had significant (p < 0.001) reductions in NTSS-6 ().
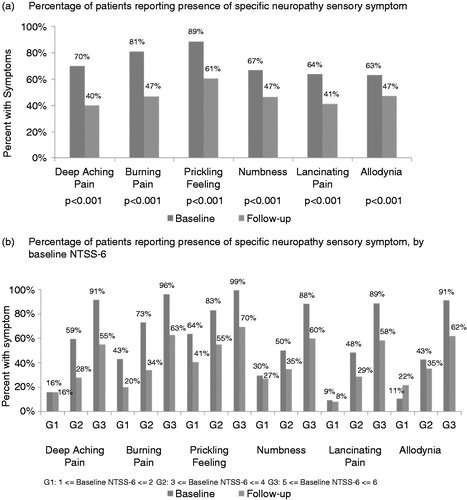
When individual neuropathy sensory symptoms that make up the NTSS-6 score were examined, statistically significant (p < 0.001) reductions from baseline were observed across all 6 items with LMF-MC-PP treatment (). Symptoms exhibiting the highest percentage reduction after treatment with LMF-MC-PP were ‘deep aching pain’ and ‘burning pain’. Mean change from baseline for each NTSS-6 item stratified by baseline severity showed that mean reductions were observed for most sensory symptom regardless of baseline severity ().
Change in pain severity
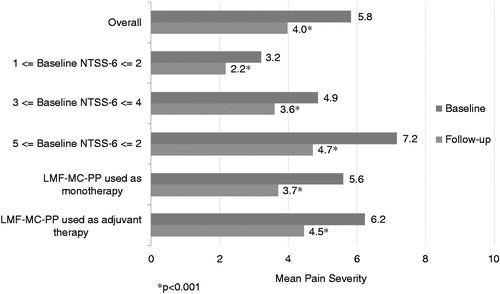
Overall pain severity was significantly (p < 0.05) reduced by 32% after LMF-MC-PP from a mean score of 5.8 at baseline to 4.0 at follow up (). Reductions in pain severity were consistent across the patient subgroups stratified by baseline NTSS-6 score, with each patient subgroup reporting statistically significant (p < 0.001) reductions from baseline to follow up. In addition, the patient subgroup using LMF-MC-PP as monotherapy and the patient subgroup using LMF-MC-PP as adjuvant therapy had significant (p < 0.001) reductions in pain from baseline to follow up.
Change in quality of life measures
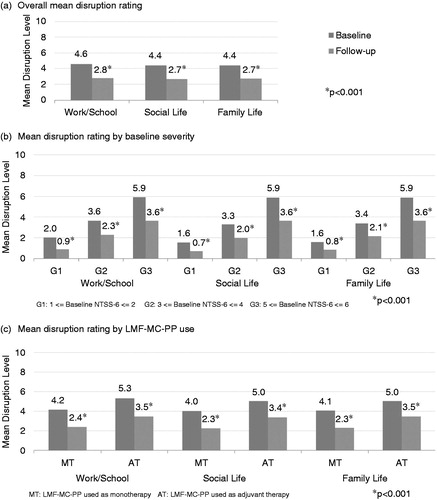
LMF-MC-PP was associated with a significant (p < 0.001) decrease in self-reported levels of disruption to patients’ normal activities due to DPN symptoms. Levels of disruption to work/school activities, social life, and family life declined significantly by 39%, 40%, and 38% respectively, from baseline to follow up (). Reductions in the impact of DPN symptoms on quality of life measures were significant (p < 0.001) across patient subgroups according to baseline severity (), and significant (p < 0.001) across patient subgroups using LMF-MC-PP as monotherapy or adjuvant therapy ().
Medication satisfaction
Among patients who had previously used medication for their DPN symptoms, patients reported a significantly (p < 0.05) higher satisfaction level with LMF-MC-PP (6.7 [2.8]) than with their prior DPN medication (4.4 [2.8]). Mean (SD) level of satisfaction was 7.1 (2.7) with LMF-MC-PP among patients who had no previous experience with prescription medication for DPN.
Change in patient outcomes by various patient subgroups
The mean change from baseline to endpoint in NTSS-6, pain severity, and disruptions to work/school activities, social life, and family life were examined when stratified by age, gender, time since diabetes diagnosis, and time since first DPN symptoms (). LMF-MC-PP produced a consistent improvement for each outcome measure across all patient subgroups.
Table 3. Change by baseline age, gender, time since diagnosis, and duration of DPN symptoms.
Discussion
In this real world study, results showed that patients using LMF-MC-PP as a nutritional therapy for DPN: (1) had significant reduction in their symptoms as reflected by lower NTSS-6 scores and lower pain rating, (2) achieved quality of life improvements as measured by the reduction in the degree of disruption to their normal activities, and (3) had higher satisfaction with their DPN therapy. Improvements in patient outcomes were consistent across patient subgroups with varying baseline severity. Furthermore, patients using LMF-MC-PP alone and patients using LMF-MC-PP as adjuvant therapy had similar improvements in NTSS-6 symptom scores, pain severity, and quality of life scores. Additional analyses showed that these improvements were achieved across different patient subpopulations. Similar patient outcomes were observed regardless of gender, age, time since diabetes diagnosis or time since onset of DPN symptoms.
Baseline NTSS-6 was positively correlated with duration of DPN symptoms in this study. However, we did not find any significant difference in the magnitude of change in NTSS-6 before and after LMF-MC-PP treatment among patient groups with differing durations of DPN symptoms. In a previous study, reduction in burning pain after LMF-MC-PP treatment was greater among patients who had DPN symptoms for less than 3 years. This survey found that the magnitude of improvement in quality of life measures was related to the baseline severity of DPN on the NTSS-6. Another survey of patients with DPN found a similar association between neuropathic symptoms measured with the NTSS-6 and quality of lifeCitation27. Others have reported results from surveys showing that the impact of DPN on health care resource use, quality of life, and costs that are related to pain and symptom severityCitation3,Citation28,Citation29.
Although survey instruments have been widely used to evaluate the burden of DPN or treatment responseCitation3,Citation27–31, use of survey instruments is associated with some limitations. This survey program was designed to collect responses directly from patients about their experiences with LMF-MC-PP using automated media. Therefore, patients had no opportunity to provide additional insight about their experiences nor did patients undergo a clinical evaluation by a health care professional. However, research has shown that a self-administered NTSS-6 is consistently reliable compared to the clinical-administered version and correlates closely with health-related utility and quality of lifeCitation26,Citation32.
Limitations are that patients in this study voluntarily answered the survey questions. In addition, assessments were performed with an interactive voice response system that could have biased the results. Consequently, selection bias may be present because patients completing the survey could be different from those who chose not to respond to the surveys. No data were available to determine the extent to which any differences may have existed among survey participants vs. non-participants. Therefore, extrapolation of these results to a general population of diabetic patients with DPN may not be appropriate. Results from DPN patients choosing to participate and complete surveys may not necessarily be indicative of the experiences of all DPN patients using LMF-MC-PP as part of their treatment for DPN. Further, this study did not include a control group, which limits claims of efficacy.
Despite these limitations, patient feedback regarding experiences with a treatment regimen provides valuable information to health care practitioners. Disadvantages of randomized clinical trials include use of restrictive selection criteria, conducted under controlled, clinical study conditions, and inclusion of smaller sample sizes. While randomized controlled clinical trials clearly are the ‘gold standard’ for assessing clinical efficacy and safety of pharmacological therapy, these controlled trials are not designed to capture the everyday experiences of patients using a treatment in their real world environment. In contrast, survey programs, such the results reported here, have the advantage of including a larger number of patients. Further, they provide physicians with actionable information about the treatment from patients in their own practice during a time when the physician may not otherwise see the progress for a condition such as DPN, which is a slow progressive disease. Physicians and patients can then have more productive, informative discussions about patients’ care during subsequent office visits, leading to an enhanced understanding of DPN patient needs and treatment options.
These results support clinical findings that demonstrate to the efficacy and safety of orally administered LMF-MC-PP in patients with DPN. While this was not a randomized controlled clinical trial, data from this patient experience program provide valuable information about the use of LMF-MC-PP in a real-world setting and add to the growing body of research data on alternative treatments for DPN.
Transparency
Declaration of funding
Funding was provided by Nestlé Health Science – Pamlab Inc., Covington, LA, USA.
Author contributions: All authors were involved in the preparation and review of this manuscript, and all approved submission of the final manuscript to the journal.
Declaration of financial/other relationships
B.S.T. has disclosed that he is a speaker for Nestlé Health Science – Pamlab Inc., Covington, LA, USA. L.W.B. and M.V.C. have disclosed that they are employees of Nestlé Health Science – Pamlab Inc. E.T. has disclosed that she is an employee of Infomedics, a company that was funded by Nestlé Health Science – Pamlab Inc. to conduct this study.
CMRO peer reviewers on this study have no relevant financial relationships to disclose.
Acknowledgment
Editorial assistance in the preparation of this manuscript was provided by Richard S. Perry PharmD, which was supported by Nestlé Health Science – Pamlab Inc.
Notes
*Metanx is a registered trade name of Nestlé Health Science – Pamlab Inc., Covington, LA, USA
*Metanx is a registered trade name of Nestlé Health Science – Pamlab Inc., Covington, LA, USA
References
- Center for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2014
- Diabetic Neuropathies: The Nerve Damage of Diabetes. Bethesda, MD: US Department of Health and Human Services; 2009 Feb. 12p. Report No.: NIH Publication No. 09–3185. Available at http://diabetes.niddk.nih.gov/dm/pubs/neuropathies/neuropathies.pdf. [Last accessed 12 May 2015]
- Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518-22
- Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes Care 2009;32(Suppl 2):S414-19
- Boulton AJM. Management of diabetic peripheral neuropathy. Clinical Diabetes 2005;23:9-15
- De Vriese AS, Verbeuren TJ, de Voorde JV, et al. Endothelial dysfunction in diabetes. Br J Pharmacol 2000;130:963-74
- Feldman EL, Vincent A. The prevalence, impact, and multifactorial pathogenesis of diabetic peripheral neuropathy. Adv Stud Med 2004;4:S642-9
- Tesfaye S. Epidemiology and etiology of diabetic peripheral neuropathies. Adv Stud Med 2004;4:S1014-21
- Sandireddy R, Yerra VG, Areti A, et al. Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int J Endocrinol 2014;2014:674987
- Duby JJ, Campbell RK, Setter SM, et al. Diabetic neuropathy: an intensive review. Am J Health Syst Pharm 2004;61:160-73
- Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev 2005;11:294-329
- van Etten RW, de Koning EJ, Verhaar MC, et al. Impaired NO-dependent vasodilation in patients with type II (non-insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia 2002;457:1004-10
- Jacobs AM, Cheng D. Management of diabetic small-fiber neuropathy with combination L-methylfolate, methylcobalamin, and pyridoxal 5’-phosphate. Rev Neurol Dis 2011;8:39-47
- Shevalye H, Watcho P, Stavniichuk R, et al. Metanx alleviates multiple manifestations of peripheral neuropathy and increases intraepidermal nerve fiber density in Zucker diabetic fatty rats. Diabetes 2012;61: 2126-33
- Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, et al. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol 2011;6: 260-73
- Verhaar MC, Wever RMF, Kastelein JJP, et al. Effects of oral folic acid supplementation on endothelial function in familial hypercholesterolemia: a randomized placebo-controlled trial. Circulation 1999;100:335-8
- Yilmaz M, Aktug H, Oltulu F, Erbas O. Neuroprotective effects of folic acid on experimental diabetic peripheral neuropathy. Toxicol Ind Health 2013 Dec 5. [Epub ahead of print], published online, doi: 10.1177/0748233713511513
- Yaqub BA, Siddique A, Sulimani R. Effects of methylcobalamin on diabetic neuropathy. Clin Neurol Neurosurg 1992;94:105-11
- Watanabe T, Kaji R, Oka N, et al. Ultra-high dose methylcobalamin promotes nerve regeneration in experimental acrylamide neuropathy. J Neurol Sci 1994;122:140-3
- Jian-bo L, Cheng-ya W, Jia-wei C, et al. The preventive efficacy of methylcobalamin on rat peripheral neuropathy influenced by diabetes via neural IGF-1 levels. Nutr Neurosci 2010;13:79-86
- Jain SK, Lim G. Pyridoxine and pyridoxamine inhibits superoxide radicals and prevents lipid peroxidation, protein glycosylation, and (Na+ + K+)-ATPase activity reduction in high glucose-treated human erythrocytes. Free Radical Biol Med 2001;30:232-7
- Walker MJ, Morris LM, Cheng D. Improvement of cutaneous sensitivity in diabetic peripheral neuropathy with combination L-methylfolate, methylcobalamin, and pyridoxal 5-phosphate. Rev Neurol Dis 2010;7:132-9
- Farvid MS, Homayouni F, Amiri Z, Adelmanesh F. Improving neuropathy scores in type 2 diabetic patients using micronutrients supplementation. Diabetes Res Clin Pract 2011;93:86-94
- Jacobs, AM, Cheng D. Addition of Metanx in pregabalin partial responders for painful diabetic neuropathy. J Diab Mellitus 2013;3:134-8
- Fonseca VA, Lavery LA, Thethi TK, et al. Metanx in type 2 diabetes with peripheral neuropathy: a randomized trial. Am J Med 2013;126:141-9
- Bastyr EJ III, Price KL, Bril V; for the MBBQ Study Group. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther 2005;27:1278-94
- Currie C, Covington M, McEwan P, et al. Diabetic peripheral neuropathy: evaluation of the association between neuropathic symptoms (NTSS-6-SA) and health related utility (EQ5D). Value Health 2005;8:A172-3
- Sadosky A, Schaefer C, Mann R, et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospective chart review and cross-sectional survey. Diabetes Metab Syndr Obes 2013;6:79-92
- Gore M, Brandenburg NA, Hoffman DL, et al. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain 2006;7:892-900
- Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Complications 2006;20:26-33
- Sadosky A, Hopper J, Parsons B. Painful diabetic peripheral neuropathy: results of a survey characterizing the perspectives and misperceptions of patients and healthcare practitioners. Patient 2014;7:107-14
- Perrin NA, Nichols GA, Oglesby A, Bastyr EJ. The reliability and validity of a self-administered version of the neuropathy total symptom score-6-SA (NTSS-6-SA). Diabetologia 2004;47(Suppl 1):A35