Abstract
Cholesterol homeostasis maintenance is regulated by a cellular feedback system that senses cholesterol amount in cellular membranes. 3-hydroxy 3-methylglutaryl coenzyme A reductase (HMGR) plays a pivotal role in cholesterol metabolism as it is the key rate-limiting enzyme of its biosynthetic pathway; its inhibition provokes a feedback response capable of reducing plasma cholesterol content. HMGR inhibition is a keystone in the treatment and prevention of cardiovascular disease and, therefore, statins (HMGR inhibitors) are widely prescribed even though they may sometimes induce side effects. These drugs are prescribed indifferently to both man and women even if there are several well-known differences in cholesterol metabolism depending on the gender and the age. Thus, gender-related differences in cholesterol metabolism should be taken into account to identify new targets for customized pharmacological treatments for hypercholesterolemia.
1. Introduction
Cholesterol plays a crucial role in the synthesis of new membranes, in the turnover of lipids in existing membranes, and in the biosynthesis of molecules such as steroid hormones and bile acids. Plasma cholesterol, in humans, originates from two main sources: cholesterol biosynthesis (peripheral and hepatic) and diet; cholesterol alimentary intake ranges from 0.2 to 0.4 g/day, whereas de novo synthesis ranges from 1 to 1.2 g/day. Cholesterol homeostasis maintenance is regulated by a cellular feedback system that senses cholesterol amount in membranes. This system controls the transcription of genes encoding both proteins involved in cholesterol biosynthesis and proteins involved in cholesterol uptake from plasma lipoproteins. Therefore, cellular cholesterol homeostasis is regulated by both the de novo cholesterol synthesis via the rate-limiting enzyme 3-Hydroxy-3-MethylGlutaryl Coenzyme A Reductase (HMGR) and the receptor-mediated endocytosis of low density lipoproteins (LDL) via LDL receptors (LDLr) Citation[1]. Due to its pivotal role in cholesterol biosynthesis, the HMGR enzyme is an important target for pharmaceutical intervention aimed to modulate cholesterolemia, and several HMGR inhibitors have been synthesized.
2. Cholesterol level regulation: 3-hydroxy-3-methylglutaryl coenzyme A reductase and low density lipoprotein receptor
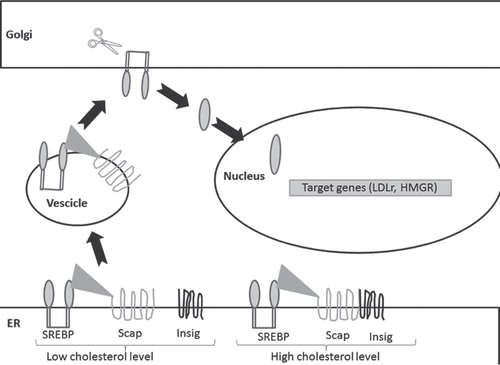
Cholesterol homeostasis maintenance is a balance between HMGR activity and LDLr lipoprotein uptake. The transcription of both HMGR and LDLr is assured by the nuclear transcriptionally active fragment of the Sterol Regulatory Element Binding Proteins (nSREBPs) whose levels are regulated by a molecule named SREBP Cleavage Activating Protein (Scap). In sterol-deprived cells, Scap binds SREBPs and escorts them from the endoplasmic reticulum (ER) to the Golgi apparatus where SREBPs are proteolytically processed to yield nSREBPs that enter the nucleus and induce the expression of their target genes. When cholesterol level rises in ER, the Scap/SREBP complex fails to migrate, the proteolytic processing of SREBPs is abolished, and the transcription of the target genes declines. ER retention of Scap/SREBP is mediated by sterol-dependent binding of Scap/SREBP to Insig (INSulin Induced Gene), an ER-resident protein () Citation[1]. Intracellular accumulation of sterols triggers the binding of HMGR to Insig, which, in turn, initiates the ubiquitination and the subsequent proteasomal degradation of the enzyme. LDLr degradation, on the other hand, depends on Proprotein Convertase Subtilisin Kexin 9 (PCSK9), a protease highly expressed in the liver and secreted into the blood. PCSK9 binds LDLr driving it to degradation in lysosomes and preventing it from recycling to the cell surface Citation[2]. PCSK9 inhibition is considered an interesting target for therapy against hypercholesterolemia. Several strategies have been developed to target PCSK9, the most successful one being monoclonal antibodies that bind PCSK9 targeting the circulating protein and as a consequence its extracellular function.
Figure 1. Regulation of LDLr and HMGR transcription in dependence on cellular cholesterol content. In the presence of high cholesterol levels, Insig interaction with SERBP-Scap complex is restrained in ER; in the presence of low cholesterol content SREBP-Scap complex migrates from ER to Golgi apparatus where the SREBP nuclear active fragment is released. Once into the nucleus, this transcription factor activates its target genes such as HMGR and LDLr.
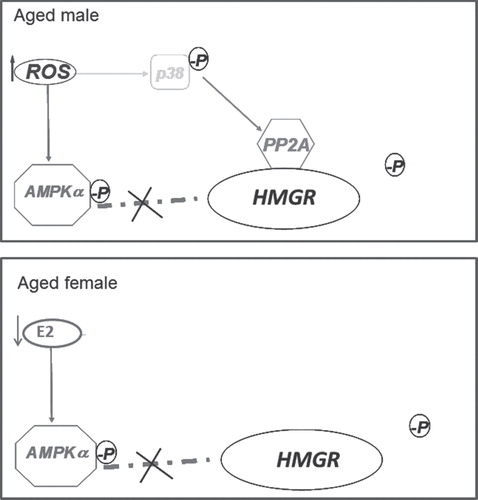
Moreover, HMGR is also subjected to a short-term regulation that is mainly assured by phosphorylation and dephosphorylation processes guaranteed respectively by AMP-activated kinase (AMPK) α, and protein phosphatase 2A (PP2A) Citation[3], respectively. The reduction of cellular cholesterol synthesis (i.e., by HMGR inhibition) leads to a homoeostatic response which induces the up-regulation of LDLr that binds lipoproteins which are taken up into cells and degraded. Rat HMGR is differently regulated according to gender, ageing, and the redox state of the cells Citation[4,5]. During ageing, the enzyme results to be completely dephosphorylated and activated. The mechanisms underlying this modification are also sexually dimorphic. In the aged male rat the total enzyme activation is due to the well-known age-related ROS increase without modulating AMPK activity Citation[4]; conversely, during the female rat aging process, the diminished AMPK activation state, (triggered by estrogen decline at the onset of estropause), induces the total HMGR activation Citation[5] (). Intriguingly, HMGR shows lower activity and expression in adult females rather than in males, in dependence on plasma 17-βestradiol levels. These gender differences could account for the different human cholesterol-related diseases, whose incidence is different between genders and between adult and elder subjects. Cholesterol metabolism is well-studied in the liver, where a major part of lipid metabolism takes place. However, this metabolism is present in all body districts, and recent studies demonstrate a pivotal role for cholesterol biosynthetic pathway also, for example, in skeletal muscle and brain Citation[6,7]. The loss of cholesterol homeostasis into the liver leads to hypercholesterolemia that is one of the primary risk factors for pathologies such as coronary artery, carotid artery, brain vessel diseases Citation[8]. The disruption of cholesterol homeostasis in other body districts, for example, in the brain, has been associated to neurodegenerative disorders, including Alzheimer’s, Huntington’s and Parkinson diseases Citation[7]. Thus, cholesterol homeostasis maintenance is very important to preserve health at multiple levels.
Figure 2. De-regulation of HMGR during ageing in dependence on sex. In aged male rat (top panel) the enzyme dephosphorylation is due to the age-related ROS increase without affecting AMPK activity as PP2A is associated to HMGR via p38 activation. In aged female rat (bottom panel), the diminished AMPK activation state, due to estropause-related estrogen decline, induces the total HMGR dephosphorylation and activation.
3. HMGR as the therapeutic target for cardiovascular disease
A positive association among high blood levels of cholesterol (LDL-Cholesterol in particular), atherosclerosis, and coronary heart disease (CHD) has been assessed, CHD is the main cause of mortality in Western industrialized countries, and as a consequence, dyslipidemia is one of the main risk factors leading to cardiovascular disease.
Statins, HMGR inhibitors, are often prescribed to treat hypercholesterolemia. Statin treatment strongly reduces HMGR activity and, as a consequence, cellular cholesterol content inducing an increase of LDLr and in turn a decreased cholesterolemia. The statins currently used are Simvastatin, Pravastatin, Atorvastatin, Rosuvastatin, Fluvastatin, and Pitavastatin. Although different pharmaceutical companies patented several HMGR inhibitors (well-reviewed in Citation[9]), statin therapy remains the current standard of care to treat hypercholesterolemia. Even though these drugs are usually well tolerated, statins can lead to several side effects, and the most recurrent are related to skeletal muscle. Statin-associated myopathy is characterized by a wide spectrum of symptoms, ranging from myalgia up to life-threatening rhabdomyolysis Citation[10]. These adverse effects could be due to the decrease of some HMGR end-products such as prenyls or ubiquinone Citation[10]. In addition, it could be taken into account that some patients with low cholesterol synthesis show high cholesterol intestinal absorption and are non-responders to statin therapy Citation[11]. These findings suggest that the diagnosis and, as a consequence, the treatment of hypercholesterolemia should be carefully considered.
4. Conclusion and expert opinion
In the very last years, few new interesting HMGR inhibitors have been patented. However, since they are devoid of particular pharmacological interest, statin therapy remains the most prescribed current treatment for all those pathologies that need HMGR inhibition. Statins are very effective and it is very difficult to find new compounds able to exert similar pharmacological effects. Therefore, despite several side effects complained by patients, statins prescription continues to be the preferential pharmacological intervention.
In 2013, the American College of Cardiology and the American Heart Association released new guidelines regarding the management of plasma cholesterol content in order to reduce cardiovascular disease risk. These new guidelines highlight statin treatment among other lipid lowering drugs. Thus, HMGR inhibition remains a cornerstone of the treatment and prevention of cardiovascular disease, although the ratio between cholesterol absorption and synthesis should be evaluated during diagnosis, to personalize and optimize the lipid-lowering therapy Citation[12].
In addition, increasing evidence has been provided that cholesterol metabolism could become an interesting pharmacological target to treat or ameliorate pathologies that are far from CVD Citation[7], such as Alzheimer disease Citation[13], autistic like pathologies Citation[14], Huntington disease Citation[15]. As a consequence, statin treatment may become increasingly necessary and more widespread. Unfortunately, these drugs present side effects, such as myopathy Citation[16,10], and therefore the synthesis of liver targeted statins would be strongly recommended, although to date there are no patents yet. Noteworthy, statin users are mainly aged people and it is now known that the mechanisms underlying the onset of hypercholesterolemia are different between male and female subjects, at least in rats in this specific age of life Citation[5]. Thus, gender-related differences in the cholesterol metabolism should be taken into account to identify new targets for customized pharmacological treatment at least for hypercholesterolemia. Unfortunately, this aspect is completely disregarded, and no gender-specific drugs have ever been patented nor do the new cholesterol guidelines take into consideration the increasing evidence of the different physiopathology between male and female subjects Citation[17]. In this regard, several papers debate both the gender and the aging effects on statin users showing different results. There is clinical evidence that women are consistently less likely to be at target not only for statin use but also for other drugs Citation[18]. Another research assessed that statins (atorvastatin and simvastatin) impair monocytes chemotaxis and that this impairment is gender-related, suggesting that statins may act differently in males and females with regard to their no-lipid lowering effect Citation[19]. Moreover, Gutierrez and collaborators showed that “statin therapy is an effective intervention in the secondary prevention of cardiovascular events in both sexes, but there is no benefit on stroke and all-cause mortality in women” Citation[20]. Thus, further research needs to be done regarding putative gender differences in response to statin medications, as unfortunately in epidemiological studies the sample size for men is definitively larger than that for women.
Although scientific research on women has been ignored in the last century, and the results obtained in men have been directly translated to women, recently more and more researches are carried out from a gender perspective. It would be highly desirable to increase the efforts in finding new stain-like drugs that should be targeted to specific districts of the human body and that would take into account the age- and the gender-depending differences.
HMGR inhibition remains a cornerstone of the treatment and prevention of cardiovascular disease.
Statins treatment remains the principal pharmacological intervention over other lipid lowering drugs.
Very few new patents are present in data bases regarding HMGR inhibitors.
Cholesterol metabolism is differently regulated and de-regulated in dependence on sex and ageing.
It is necessary to identify new targets for customized pharmacological treatment at least to treat hypercholesterolemia.
Acknowledgment
The English language revision performed by Dr Monica Vittoria Rega and is gratefully acknowledge.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
This box summarizes key points contained in the article.
Bibliography
- Martini C, Pallottini V. Cholesterol: from feeding to gene regulation. Genes Nutr 2007;2(2):181-93
- Peterson AS, Fong LG, Young SG. PCSK9 function and physiology. J Lipid Res 2008;49(7):1595-9
- Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annu Rev Genet 2007;41:401-27
- Pallottini V, Martini C, Cavallini G, et al. Age-related HMG-CoA reductase deregulation depends on ROS-induced p38 activation. Mech Ageing Dev 2007;128(11-12):688-95
- Trapani L, Violo F, Pallottini V. Hypercholesterolemia and 3-hydroxy-3-methylglutaryl coenzyme A reductase regulation in aged female rats. Exp Gerontol 2010;45(2):119-28
- Segatto M, Manduca A, Lecis C, et al. Simvastatin treatment highlights a new role for the isoprenoid/cholesterol biosynthetic pathway in the modulation of emotional reactivity and cognitive performance in rats. Neuropsychopharmacology 2014;39(4):841-54
- Segatto M, Leboffe L, Trapani L, Pallottini V. Cholesterol homeostasis failure in the brain: implications for synaptic dysfunction and cognitive decline. Curr Med Chem 2014;21(24):2788-802
- Li W, Prakash R, Chawla D, et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol 2013;304(11):R1001-8
- Pfefferkorn JA. Novel 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors: a patent review. Expert Opin Ther Pat 2011;21(2):187-203
- Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs 2008;8(6):373-418
- Weingartner O, Lutjohann D, Bohm M, Laufs U. Relationship between cholesterol synthesis and intestinal absorption is associated with cardiovascular risk. Atherosclerosis 2010;210(2):362-5
- Weingartner O, Lutjohann D, Elsasser A, Laufs U. Personalized lipid lowering therapy to further reduce residual cardiovascular risk. Atherosclerosis, Thrombosis and Vascular Biology 2015;35:990-5
- Williams MA, Silvestri V, Craig D, et al. The prevalence of age-related macular degeneration in alzheimer’s disease. J Alzheimers Dis 2014;42(3):909-14
- Wang H. Lipid rafts: a signaling platform linking cholesterol metabolism to synaptic deficits in autism spectrum disorders. Front Behav Neurosci 2014;8:104
- Patassini S, Giampa C, Martorana A, et al. Effects of simvastatin on neuroprotection and modulation of Bcl-2 and BAX in the rat quinolinic acid model of Huntington’s disease. Neurosci Lett 2008;448(1):166-9
- Trapani L, Melli L, Segatto M, et al. Effects of myosin heavy chain (MHC) plasticity induced by HMGCoA-reductase inhibition on skeletal muscle functions. FASEB J 2011;25(11):4037-47
- Marino M, Masella R, Bulzomi P, et al. Nutrition and human health from a sex-gender perspective. Mol Aspects Med 2011;32(1):1-70
- Bethel MA, Green JB, Milton J, et al. Regional, age and sex differences in baseline characteristics of patients enrolled in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Obes Metab 2015;17(4):395-402
- Ruggieri A, Gambardella L, Maselli A, et al. Statin-induced impairment of monocyte migration is gender-related. J Cell Physiol 2014;229(12):1990-8
- Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: a sex-based meta-analysis. Arch Intern Med 2012;172(12):909-19
