Abstract
Metformin is the most commonly prescribed antidiabetic oral agent. It has also been used off-label for polycystic ovarian syndrome, steatohepatitis, and HIV-associated metabolic abnormalities. However, this oldie is a newbie for the oncologist. Population studies have suggested that metformin decreased the incidence and mortality rates of cancer in diabetic patients. With better understanding of its mechanisms of antitumor activity, metformin may become a new drug for cancer in combination with chemotherapy or targeted therapy.
1. Introduction
Metformin is one of the most prescribed drugs in the offices of internists and endocrinologists, with over half a century of use as an antidiabetic agent. Recently, this pharmaceutical old-timer has become a new kid on the block for oncologists. In this editorial, we discuss this old-but-new drug from the perspectives of an endocrinologist, an internist, and an oncologist.
2. Background
The chemical basis of metformin (1-(diaminomethylidene)-3,3-dimethylguanidine) is derived from Galega officinalis (goat's rue or French lilac) Citation[1]. Metformin is a member of the biguanide class of compounds. Like other biguanides, it was developed as a therapeutic for hyperglycemia and diabetes mellitus in the 1950s Citation[2-5]. Metformin absorption occurs mainly in the small intestine, it has an oral bioavailability of 50 – 60%, and it is excreted unmetabolized from the kidneys. Its half-life is about 5 h Citation[6,7].
3. Clinical use in diabetes mellitus
One-third of adults in the United States have pre-diabetes Citation[8-10]. Type 2 diabetes mellitus occurs in 3.7% of adults between 20 and 44 years in age, but the prevalence reaches 27% in adults over 65 years Citation[11]. Metformin use has been associated with about a 31% reduced risk of developing type 2 diabetes mellitus in a high-risk population Citation[12]. In the UK Prospective Diabetes Study, metformin was superior to sulfonylureas and insulin in reducing the incidence of macrovascular and microvascular complications in patients with type 2 diabetes with lower risk of hypoglycemia. Furthermore, metformin use in overweight patients was associated with 36% lower risk of all-cause mortality compared to patients treated with diet alone Citation[13]. In a separate prospective trial, adding metformin to insulin therapy ameliorated insulin-induced weight gain, improved diabetes control, as well as reduced macrovascular complications () Citation[14].
Metformin therapy improves insulin sensitivity and reduces the fasting plasma glucose and insulin concentrations. It decreases hepatic glucose output by inhibiting gluconeogenesis and enhances peripheral glucose uptake by muscles and adipose tissues. Additionally, it increases the intestinal use of glucose and decreases fatty acid oxidation. When compared with sulfonylurea, metformin was associated with lower risk of decline in kidney function. This effect was independent of weight, blood pressure, and glycated hemoglobin level Citation[15]. Rarely does metformin cause hypoglycemia since it neither stimulates insulin secretion nor modulates glucose-counter-regulatory mechanisms Citation[16].
The anti-hyperglycemic effects of metformin are complex and likely multifactorial. In the past decade, many details have emerged about metformin's molecular mechanisms of action. The key function of metformin is reducing hepatic gluconeogenesis but the exact molecular targets are elusive. The activation of AMP-activated protein kinase (AMPK) in hepatocytes was thought to be the key mechanism to explain metformin's antidiabetic activity Citation[17], but this intriguing theory was challenged by other alternative mechanisms that were uncovered to explain metformin's effects. Metformin was found to reduce the activity of glucose 6 phosphatase, and thus, reduced hepatic glucose production. Also, the inhibiting cell respiration by modulating mitochondrial respiratory chain complex-1 is gaining traction as the most likely explanation for metformin effects Citation[18,19].
Simultaneously, metformin indirectly enhances glucose-dependent insulin secretion by increasing the secretion of glucagon-like peptide-1 (GLP-1) from intestinal L cells and possibly by reducing GLP-1 degradation Citation[20,21]. Recently, metformin was also shown to antagonize the hyperglycemic effect of glucagon in the liver by increasing intracellular AMP leading to the inhibition of key intracellular enzymes (such as protein kinase A and adenylate cyclase) Citation[22].
4. Clinical use for non-diabetes indications
4.1 Polycystic ovarian syndrome
Metformin has also been used off-label for the treatment of polycystic ovarian syndrome (PCOS), a condition that may include hirsutism and reduced fertility, in the context of obesity and insulin resistance Citation[23]. Metformin improves hirsutism and acne in patients with PCOS Citation[24]. In patients with PCOS, metformin normalizes the quality and activation of molecules that regulate insulin-dependent glucose transporter (GLUT4). Metformin reverses GLUT4 endocytosis and increases GLUT4 mRNA expression, which, in turn, improves endometrial metabolic function and reproductive outcomes () Citation[25,26].
4.2 Steatohepatitis
In non-diabetic pediatric patients with non-alcoholic steatohepatitis (NASH), metformin enhances insulin sensitivity, reduces liver fat, improves liver function, and enhances quality of life Citation[27]. By reducing accumulation of free fatty acid in liver significantly, metformin suppresses oxidation of fatty acids which is responsible for hepatocyte inflammation and injury. However, it is noteworthy to observe that improvement in liver histology is linked to weight reduction contributed by metformin () Citation[28,29].
Metformin-activated AMPK is a major cellular regulator of lipid and glucose metabolism. This activation leads to inhibition of glucose production reduction, induction of oxidation of fatty acid, and suppression of expression of lipogenic enzymes. Additionally, metformin treatment leads to suppression of expression of SREBP-1, a key lipogenic transcription factor in rat primary hepatocytes Citation[17]. More recent studies, using genetic-loss-of function models, show that metformin also has AMPK-independent effects on the counter-regulatory hormone glucagon Citation[30]. Further study is needed in this area to clarify how to incorporate metformin into currently available treatments for NASH.
4.3 HIV-associated metabolic abnormalities
Among HIV-infected patients – particularly in HIV-infected patients on treatment with protease inhibitors and nucleoside analogs – abnormalities in lipid and glucose metabolism, as well as lipodystrophy and lipoatrophy, are increasingly recognized. Elevated glucose, triglyceride, total cholesterol, and low-density lipoprotein (LDL) levels are commonly observed. In HIV-associated lipodystrophy, metformin leads to a reduction in insulin area under the curve and insulin levels 120 min after oral glucose tolerance test, suggesting an overall improvement in insulin sensitivity. There is also a decrease in visceral abdominal fat, which is increased in patients with HIV and is considered a risk factor for coronary artery disease in non-HIV-infected patients Citation[31]. A potential benefit of use of metformin in combination with exercise training in this population has been suggested by a randomized trial; however, another randomized trial suggested that metformin use be reserved for patients with adequate peripheral fat and marked insulin resistance () Citation[32,33].
4.4 Cardiovascular protective effect
By positively affecting cardiac risk factors, metformin offers cardiovascular protection. Metformin lowers glucose level and hence reduces oxidative stress and lipid oxidation. It lowers total cholesterol, LDL, and triglyceride levels while maintaining or increasing high-density lipoprotein levels Citation[4,34]. In both diabetic and non-diabetic patients, metformin decreases the level of plasminogen activator inhibitor (PAI-1), thus improving fibrinolysis and reducing clot formation. Moreover, it reverses platelet aggregation Citation[35,36]. Metformin improves vascular function and has acute sympathetic inhibitory actions in spontaneously hypertensive rats () Citation[37,38].
5. Metformin and cancer
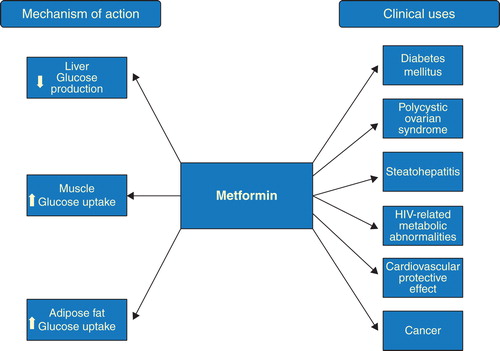
5.1 Mechanisms of action
Several mechanisms of action of metformin have been proposed for its activities as an antineoplastic agent (). Activation of AMPK by metformin plays an important role in mediating its effects. AMPK activation results in phosphorylation and activation of tuberous sclerosis complex 2 (TSC2), a tumor suppressor gene, which exerts an inhibitory effect on the mammalian target of rapamycin (mTOR), a serine-threonine kinase involved in initiating mRNA translation Citation[39]. Ultimately, AMPK activation results in inhibition of cellular protein synthesis and cell growth, making metformin an effective treatment for cancer Citation[2,40,41].
Metformin-induced activation of AMPK has also been shown to disrupt crosstalk between insulin/IGF-1 receptor and G-protein-coupled receptor signaling in pancreatic cancer cells and inhibits the growth of these cells in xenograft models Citation[42]. Another explanation of the antiproliferative effect of metformin is that it reverses hyperinsulinemia and thus decreases the stimulation that insulin exerts on tumor cells via the insulin receptor. Thereby, metformin decreases intracellular signaling through the phosphoinositide 3-kinase inhibitor/AKT/mTOR leading to the inhibition of cancer cell growth and proliferation. Metformin further decreases intracellular signaling through the activated Ras/Raf/MEK/ERK pathway, which is known to increase cell proliferation and inhibit apoptosis Citation[43,44].
Dowling et al. demonstrated that metformin treatment led to a 30% decrease in global protein synthesis in MCF-7 breast cancer cells, which express liver kinase B1 (LKB1) Citation[2]. However, metformin had no effect on protein synthesis in MDA-MB-231 breast cancer cells, which do not express LKB1 Citation[2]. In MCF-7 cells, metformin treatment led to a dose-dependent increase in the phosphorylation of AMPK and a strong decrease in the phosphorylation of S6K and 4E-BP1, the two major downstream effectors of mTOR. Interestingly, metformin treatment had no effect on the phosphorylation of AMPK, S6K, and 4E-BP1 in MDA-MB-231 cells. This provides strong evidence that LKB1 is required for AMPK activation and mTOR inhibition by metformin, as well as for metformin's inhibition of mRNA translation Citation[2].
Additionally, Dowling et al. also demonstrated that TSC2 is necessary for metformin's inhibitory effects Citation[2]. Metformin inhibited the growth of TSC2+/+ mouse embryonic fibroblasts (MEFs) by 53% but did not affect TSC2-/- MEFs. Treatment of cells with an AMPK inhibitor prevented metformin's inhibitory effect on translation. These observations, taken together, provide proof that metformin's effect on translation is mediated by the activation of AMPK, which ultimately results in mTOR pathway inhibition and in inhibited translation and cell growth Citation[2].
Additionally, nuclear factor-κB, a regulatory factor for MDR1 gene transcription, is suppressed by metformin, leading to decreased expression of P-glycoprotein and reduced drug resistance Citation[45-47].
In vitro, metformin inhibits proliferation in multiple cancer cell lines, an effect likely mediated through activation of AMPK. Tumor types that have been studied in vitro include breast, colon, and prostate Citation[40,44,48,49]. Metformin, in combination with a histone deacetylase inhibitor, exerted an antitumor effect against an osteosarcoma cell line in vivo and in vitro Citation[50]. Moreover, phenformin, another biguanide, induced apoptosis in LKB1-mutant non-small cell lung cancer cells Citation[51].
5.2 Cancer prevention
Population studies have suggested that metformin may decrease the incidence and mortality rate of cancer in diabetic patients Citation[52-54]. In a retrospective study of early-stage breast cancer, the rate of pathologic complete response was higher in diabetic patients taking metformin (24%) than in the non-metformin diabetic group (8%) or in patients without diabetes (16%). Metformin was independently predictive of a pathologic complete response Citation[55].
5.3 Metformin in cancer treatment
It would be naïve to assume that metformin alone may be enough to treat various types of cancer. However, metformin can be combined with chemotherapy or targeted therapy. These combinations should be driven by preclinical findings. Suggested cancers include, but not limited to, endometrial, breast, prostate, non-small cell lung cancer, and hepatocellular cancers. For example, metformin is being considered as a novel drug for treating endometrial cancer Citation[56]. Metformin reversed progestin resistance and sensitized endometrial cancer cells to chemotherapy by repressing expression of glyoxalase 1 enzyme Citation[56,57]. Metformin has been shown to potentiate the effect of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation of the mTOR pathway Citation[58,59]. It has been shown that metformin promotes progestin receptor expression via inhibition of mTOR in endometrial cell lines; therefore, the combination of metformin with an mTOR inhibitor should be investigated as a synergistic therapeutic option for endometrial carcinoma Citation[60]. It would be of interest to observe whether there is a clinical response to metformin in patients with TSC2 mutation or LKB1 mutation.
6. Side effects, precautions, and contraindications
The most common side effects of metformin are diarrhea, nausea, vomiting, metallic taste, bloating, flatulence, and anorexia Citation[61]. Chronic treatment with metformin impairs absorption vitamin B12 and increases its accumulation in the liver, leading to a decrease in its accumulation in kidney and circulation. However, clinical vitamin B12 deficiency is not common Citation[62].
The most serious toxicity of metformin is thought to be lactic acidosis Citation[63]; however, there is still controversy as to whether use of metformin truly increases the risk of this condition. In a systematic review of over 200 trials, the incidence of lactic acidosis was 5.1 cases per 100,000 individuals treated with metformin, compared with 5.8 cases per 100,000 non-metformin-treated individuals Citation[64]. Even if the risk exists, it can be mitigated when metformin use is avoided in patients with hepatic, cardiac, or renal failure and in patients aged 80 years or older.
Metformin is contraindicated in lactating women and in patients with diabetic ketoacidosis, renal failure or dysfunction, hepatic insufficiency or alcohol abuse, heart failure, and conditions of tissue hypoxia, such as myocardial infarction or sepsis. Metformin is also contraindicated with the use of iodinated contrast dye (1 day before and 2 days after the imaging procedure) Citation[65,66].
7. Conclusions
Metformin is widely used in management of diabetes. It is frequently used as an off-label treatment for polycystic ovarian disease. Further clinical trials are needed to establish how to incorporate metformin into current treatment of NASH and when to use metformin for HIV-associated metabolic abnormalities.
In oncology, mechanisms of action of metformin are increasingly better understood. Retrospective and meta-analyses of trials showed that metformin had preventive effects against cancer. Neoadjuvant trials demonstrated anticancer activities in prostate and endometrial cancers. In the clinical setting, there is a trend toward increased use of metformin in non-diabetic cancer patients owing to emerging data on metformin's effects against cancer. Early-phase clinical trials combining metformin with a chemotherapy regimen or targeted therapy are needed to evaluate toxicity and efficacy and determine a recommended Phase II trial dose. Ultimately, these early trials should lead to randomized Phase II trials in mutation- or organ-specific cancers, which could result in transforming the newly discovered translational results for this old drug into practical use as a new cancer agent.
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care 1989;12:553-64
- Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 2007;67:10804-12
- Witters LA. The blooming of the French lilac. J Clin Invest 2001;108:1105-7
- Bailey CJ, Turner RC. Metformin. N Engl J Med 1996;334:574-9
- Bailey CJ, Day C. Metformin: its botanical background. Pract Diabetes Int 2004;21:115-17
- Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 2011;50:81-98
- Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 1996;30:359-71
- Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the US population in 1988–1994 and 2005–2006. Diabetes Care 2009;32:287-94
- Williamson DF, Cowie CC. Diabetes risk reduction behaviors among US adults with prediabetes. Am J Prev Med 2010;38:403-9
- Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: national Health And Nutrition Examination Survey 1999-2002. Diabetes Care 2006;29:1263-8
- Center for Disease Control, Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. US Department of Health and Human Services; 2011
- Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-65
- Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616-25
- Hung AM, Roumie CL, Greevy RA, et al. Kidney function decline in metformin versus sulfonylurea initiators: assessment of time‐dependent contribution of weight, blood pressure, and glycemic control. Pharmacoepidemiol Drug Saf 2013; [Epub ahead of print]
- Wright AD, Cull CA, Macleod KM, et al. Hypoglycemia in Type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis: UKPDS73. J Diabetes Complications 2006;20:395-401
- Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167-74
- Foretz M, Hebrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355-69
- Batandier C, Guigas B, Detaille D, et al. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr 2006;38:33-42
- Mannucci E, Ognibene A, Cremasco F, et al. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care 2001;24:489-94
- Mulherin AJ, Oh AH, Kim H, et al. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology 2011;152:4610-19
- Miller RA, Chu Q, Xie J, et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013;494:256-60
- Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ 2003;327:951-3
- Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol 2013; [Epub ahead of print]
- Pryor PR, Liu S, Clark AE, et al. Chronic insulin effects on insulin signalling and GLUT4 endocytosis are reversed by metformin. Biochem J 2000;348:83
- Carvajal R, Rosas C, Kohan K, et al. Metformin augments the levels of molecules that regulate the expression of the insulin-dependent glucose transporter GLUT4 in the endometria of hyperinsulinemic PCOS patients. Hum Reprod 2013; [Epub ahead of print]
- Schwimmer J, Middleton M, Deutsch R, et al. A phase 2 clinical trial of metformin as a treatment for non‐diabetic paediatric non alcoholic steatohepatitis. Aliment Pharmacol Ther 2005;21:871-9
- Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2009;29:172-82
- Uygun A, Kadayifci A, Isik AT, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2004;19:537-44
- Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia 2013;56(9):1898-906
- Hadigan C, Corcoran C, Basgoz N, et al. Metformin in the treatment of HIV lipodystrophy syndrome. JAMA 2000;284:472-7
- Driscoll SD, Meininger GE, Lareau MT, et al. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV-infected patients. AIDS 2004;18:465-73
- Kohli R, Shevitz A, Gorbach S, et al. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med 2007;8:420-6
- Hollenbeck CB, Johnston P, Varasteh BB, et al. Effects of metformin on glucose, insulin and lipid metabolism in patients with mild hypertriglyceridaemia and non-insulin dependent diabetes by glucose tolerance test criteria. Diabete Metab 1991;17:483-9
- Juhan-Vague I, Valadier J, Alessi M, et al. Deficient t-PA release and elevated PA inhibitor levels in patients with spontaneous or recurrent deep venous thrombosis. Thromb Haemost 1987;57:67
- Tremoli E, Ghiselli G, Maderna P, et al. Metformin reduces platelet hypersensitivity in hypercholesterolemic rabbits. Atherosclerosis 1982;41:53-60
- Verma S, Yao L, Dumont AS, et al. Metformin treatment corrects vascular insulin resistance in hypertension. J Hypertens 2000;18:1445-50
- Petersen JS, DiBona GF. Acute sympathoinhibitory actions of metformin in spontaneously hypertensive rats. Hypertension 1996;27:619-25
- Dancey JE. Agents targeting ras signaling pathway. Curr Pharm Des 2002;8:2259-67
- Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66:10269-73
- Vázquez-Martín A, Oliveras-Ferraros C, del Barco S, et al. mTOR inhibitors and the anti-diabetic biguanide metformin: new insights into the molecular management of breast cancer resistance to the HER2 tyrosine kinase inhibitor lapatinib (Tykerb). Clin Transl Oncol 2009;11:455-9
- Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res 2010;16:2505-11
- Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: time for action. J Clin Oncol 2009;27:3271-3
- Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009;8:909-15
- Deng L, Lin-Lee Y-C, Claret F-X, et al. 2-Acetylaminofluorene Up-regulates Rat mdr1bExpression through generating reactive oxygen species that activate NF-κB pathway. J Biol Chem 2001;276:413-20
- Kim HG, Hien TT, Han EH, et al. Metformin inhibits P‐glycoprotein expression via the NFκB pathway and CRE transcriptional activity through AMPK activation. Br J Pharmacol 2011;162:1096-108
- Anastasiou D. Metformin: a case of divide and conquer. Breast Cancer Res 2013;15:1-3
- Zakikhani M, Dowling RJ, Sonenberg N, et al. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila) 2008;1:369-75
- Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007;67:6745-52
- Duo J, Ma Y, Wang G, et al. Metformin synergistically enhances antitumor activity of histone deacetylase inhibitor trichostatin a against osteosarcoma cell line. DNA and Cell Biology 2013;32(4):156-64
- Shackelford DB, Abt E, Gerken L, et al. LKB1 Inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 2013;23(2):143-58
- Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620-5
- Dowling RJ, Niraula S, Stambolic V, et al. Metformin in cancer: translational challenges. J Mol Endocrinol 2012;48:R31-43
- Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304-5
- Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009;27:3297-302
- Zhang X, Shu L, Hosoi H, et al. Predominant nuclear localization of mammalian target of rapamycin in normal and malignant cells in culture. J Biol Chem 2002;277:28127-34
- Dong L, Zhou Q, Zhang Z, et al. Metformin sensitizes endometrial cancer cells to chemotherapy by repressing glyoxalase I expression. J Obstet Gynaecol Res 2012;38:1077-85
- Hanna RK, Zhou C, Malloy KM, et al. Metformin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and modulation of the mTOR pathway. Gynecol Oncol 2012;125:458-69
- Cantrell LA, Zhou C, Mendivil A, et al. Metformin is a potent inhibitor of endometrial cancer cell proliferation–implications for a novel treatment strategy. Gynecol Oncol 2010;116:92-8
- Xie Y, Wang YL, Yu L, et al. Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. J Steroid Biochem Mol Biol 2011;126:113-20
- Phillips BB. Managing therapy and adverse effects with antihyperglycemic agents: a focus on metformin and acarbose. Pharm Pract Manag Q 1997;17:21-31
- Greibe E, Miller JW, Foutouhi SH, et al. Metformin increases liver accumulation of vitamin B12 - An experimental study in rats. Biochimie 2013;95:1062-5
- Misbin RI, Green L, Stadel BV, et al. Lactic acidosis in patients with diabetes treated with metformin. N Engl J Med 1998;338:265-6
- Salpeter S, Greyber E, Pasternak G, et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2006;CD002967
- Sulkin TV, Bosman D, Krentz AJ. Contraindications to metformin therapy in patients with NIDDM. Diabetes Care 1997;20:925-8
- Jones GC, Macklin JP, Alexander WD. Contraindications to the use of metformin. BMJ 2003;326:4-5