Abstract
Multiple myeloma (MM) remains incurable despite important recent advances in treatment due to its inherent resistance, characterized by highly complex and heterogeneous molecular abnormalities, as well as the support from myeloma bone marrow (BM) microenvironment. A novel therapeutic strategy that effectively targets specific molecules on myeloma cells and also potentially overcomes tumor microenvironment-mediated drug resistance and the downstream effects of genetic instability is thus urgently needed. Over the last 2 years, an anti-CD38 monoclonal antibody daratumumab (DARA) has emerged as a breakthrough targeted therapy for patients with MM. Early-stage clinical trials have found DARA to be safe and to have encouraging clinical activity as a single agent and in combination with lenalidomide in heavily pretreated, relapsed patients in whom other novel agents (such as bortezomib, thalidomide and lenalidomide) as well as stem cell transplant has already failed. DARA may, therefore, be the first mAb with significant anti-MM activity both as a monotherapy and in combination. It is currently being further evaluated both alone and in combination with conventional and novel anti-MM agents as part of prospective clinical trials. This review discusses the preclinical and clinical development of DARA, its pathophysiological basis, and its prospects for future use in MM.
1. CD38 is a cell-surface receptor and an ectoenzyme
The CD38 gene encodes a 46-kDa single-chain, transmembrane receptor glycoprotein with a short 20-aa N-terminal cytoplasmic tail and a long 256-aa extracellular domain Citation[1]. Under normal conditions, CD38 is expressed at relatively low levels on the cell surface of lymphoid and myeloid cells, as well as on some nonhematopoietic tissues (including epithelia, striated muscle and nerve cells) Citation[2]. Most mature resting lymphocytes express low levels of surface CD38, and pluripotent/primitive hematopoietic precursor cells (HPC) that are crucial for a long-term (sustained) marrow recovery do not express CD38 (CD34+CD38-).
CD38 not only serves as a cell-surface antigen but also serves as a receptor-mediated signaling cascade to regulate cell adhesion as well as an ectoenzyme to catalyze metabolism of cyclic adenosine diphosphate ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) Citation[2]. Functional CD38 appears as a dimer or a multimer Citation[3] on the cytoplasmic side of the membrane, where its catalytic site is defined Citation[4]. As a receptor, CD38 is preferentially localized in lipid rafts, microdomains of plasma membrane and is in close contact with the BCR complex and with other molecules (i.e., CXCR4) regulating signaling, homing, adhesion and migration Citation[5,6]. Lipid raft localization and association with signaling complexes are prerequisites for signals mediated through CD38 to phosphorylate ERK1/2 and activate NF-κB Citation[7,8]. Ligation of CD38 with agonistic mAbs, such as IB4, induces T-cell activation evidenced by Ca2+ mobilization, as well as further triggers proliferation of T lymphocytes Citation[9], and induces significant proliferation of peripheral blood mononuclear cells (PBMCs) as well as increased secretion of IL-6 and IFN-γ Citation[10].
CD31 is a CD38 nonsubstrate ligand that can trigger the signaling and recapitulate the biological events observed in vitro using surrogate agonistic mAbs Citation[11,12]. CD38/CD31 interaction has been analyzed extensively in a number of different settings including T, B, NK and myeloid cells in normal and pathological conditions. For example, binding of endothelial cells to CD38+ chronic lymphocytic leukemia (CLL) cells via CD31 provides specific survival signals via activation of NF-κB and the induction of downstream target genes Citation[7].
As an ectoenzyme, CD38 uses NAD+ as substrate for the formation of cyclic ADP-ribose (cADPR) and ADPR, as well as nicotinamide and NAADP Citation[13]. Importantly, cADPR and NAADP are structurally and functionally distinct calcium messengers that regulate endoplasmic reticulum and lysosomal calcium stores, respectively Citation[14,15]. CD38 is the major NAD glycohydrolase (NADase) in mammalian cells, regulating extracellular NAD+ levels and maintaining NAD+ homeostasis, with novel additional roles of extracellular NAD+ including regulation of immune and inflammatory responses, especially regulatory T-cells Citation[16,17]. CD38 signaling also occurs via cross-talk with antigen-receptor complexes on T- and B-cells or other types of receptor complexes, associating with different co-receptors depending on the cell type Citation[5], and cross-talk between CD38 and MHC molecules involved in switching and secretion of IgG1 as well as activation of NK cells Citation[18-20].
2. Rationale for targeting CD38 for cancer immunotherapy
Overexpression of CD38 is seen in the majority of lymphoid tumors, notably malignant plasma cells in all stages of MM Citation[21]. Along with CD138 antigen, CD38 is most commonly used as a marker for myeloma cell immunophenotyping. Furthermore, long-lived and/or MM-initiating cells from which MM plasma cells are derived are CD38++(high)CD19-(negative) plasma cells that are either CD138+ or CD138− Citation[22,23], suggesting that these may in turn identify MM stem cells that are relatively resistant to therapy. Interestingly, CD38 expression in CLL is associated with a more aggressive clinical behaviour, as CD38+ patients have a shorter progression-free and overall survival Citation[6]. In vitro studies have shown that interaction between CD38 and CD31 promotes the proliferation and survival of CLL cells both directly and through up-regulation of CD100 Citation[24], which interacts with plexin-B1 on stromal cells and some subsets of T cells Citation[25]. Consistent with this observation, CD38-positive (CD38+) CLL cells express higher levels of other activation markers than CD38-negative cells Citation[24].
3. Targeting CD38-based therapy for MM
While there are no approved mAb treatments for MM patients to date, several show promise, especially in combination with other agents. Monoclonal antibodies specifically targeting CD38 may inhibit uncontrolled growth circuits, therefore, increasing susceptibility selectively of MM cells to conventional chemotherapy. In addition, human T cells with the anti-CD38 chimeric antigen receptor (CAR) were shown to be highly cytotoxic against MM cells strongly expressing CD38 Citation[26]. Specifically, T cells expressing anti-CD38-CAR induce > 91% lysis of MM cell lines and patient MM cells with CD38 expression, whereas no detectable cytotoxicity was seen against U266 cells lacking CD38 expression at both mRNA and protein levels. Autologous T-cell immunotherapy with anti-CD38-CAR might, therefore, be a novel treatment strategy for myeloma Citation[27].
Several antibodies or antibody variants to human CD38 have been generated that induce killing of CD38-positive neoplastic B malignancies, including MM Citation[28-32]. However, these studies using anti-CD38 mAb with or without an immunotoxin (ricin) have not led to useful clinical applications, in part due to concerns about toxicity associated with an antibody targeting this epitope. Most recently, Daratumumab (DARA) targeting CD38 has showed single-agent activity in a Phase I clinical trial in MM as discussed later Citation[33,34]. This promising data has led to the clinical development of two other CD38 mAbs: a humanized mAb (SAR650984) and a HuCAL-derived fully human mAb (MOR03087).
4. Basic characteristics of daratumumab
DARA was one of 42 antibodies generated by immunization of human immunoglobulin transgenic HuMab-mice® with recombinant CD38 protein (HA-CD38), alone or alternating with CD38-transfected cells. It was chosen after extensive screening for its ability to induce cytotoxicity in CD38+ cells and binding specificity for a unique epitope on CD38 Citation[35]. It displays low nanomolar affinity to CD38 and recognizes the protein on all MM cell lines with CD38 mRNA expression as well as all CD138-purified patient MM cells tested in FACS analysis.
5. DARA-induced potent anti-MM activities via multiple mechanisms of action in preclinical studies
Preclinical studies indicate that DARA is effective in killing primary CD38+CD138+ patient MM cells and a range of MM/lymphoid cell lines by both antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) Citation[35,36]. In addition, CDC and ADCC are induced in the bone marrow (BM) microenvironment since bone marrow stromal cells (BMSC) do not significantly impair its activity. In a SCID mouse animal model using sensitive bioluminescence imaging, DARA monotherapy significantly inhibits CD38+ MM cell growth in both preventive and therapeutic settings Citation[35]. It is also effective in vivo against primary MM cells freshly isolated from therapy-naive or refractory patients in a humanized microenvironment in an immune-deficient mouse Citation[37-39].
A third mechanism of DARA-induced cell death was shown by further cross-linking of DARA with anti-human immunoglobulin (hIgG) Ab () Citation[35] or FcγR-expressing IIA1.6 cells (IIA1.6-FcγR) Citation[40]. DARA triggered homotypic aggregation and adhesion of primary patient MM cells, followed by increased percentage of Annexin V+ and Annexin V+/PI+ cells (). In addition, the combination of DARA and dexamethasone (dex) or bortezomib (BTZ) induced synergistic activities against MM cells Citation[41,42]. Such improved anti-MM effect is most likely due to enhanced DARA-induced direct cytotoxicities when both drugs are combined. DARA-induced inhibition of CD38 ADP-ribosyl cyclase activity in target cells may also contribute to the effectiveness of DARA in killing both primary MM and plasma cell leukemia cells.
Figure 1. Daratumumab directly inhibits survival of CD138+ patient multiple myeloma cells. MM1S cells were treated with DARA with goat anti-human immunoglobulin for 2 days, followed by CellTiter-Glo luminescent cell viability assay (A) or Caspase 3/7 assay (B). (C) Freshly isolated CD138+ patient myeloma cells were treated with DARA (1, 10 g/ml) or isotype control (iso, 10 g/ml) in the presence of cross-linking Abs for 2 days. Percentages of Annexin V+/PI+ were determined by flow cytometry. Annexin V+ and combined Annexin V+/PI+ cells increased from 7.7 to 20.8% and 10.9 to 15.4%, indicating that DARA directly induces apoptosis of patient MM cells. (D) Light microscopy showed homotypic adhesion and aggregation of MM patient cells following DARA treatment.
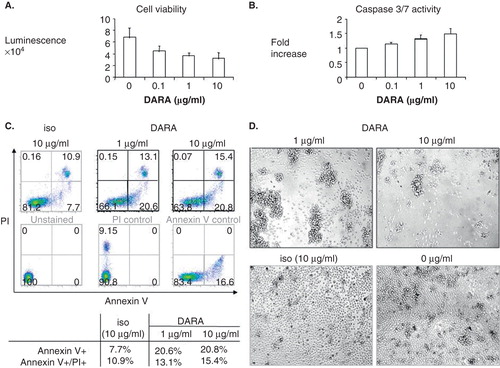
Excitingly, DARA is also able to induce tumor cell killing via antibody-dependent cellular phagocytosis (ADCP) by macrophages via an additional Fc-dependent effector mechanism () Citation[43]. As macrophages infiltrate the BM and may contribute to MM cell survival and resistance to chemotherapeutic treatment Citation[44], the addition of DARA may redirect tumor-associated macrophages and therefore overcome its protective effect.
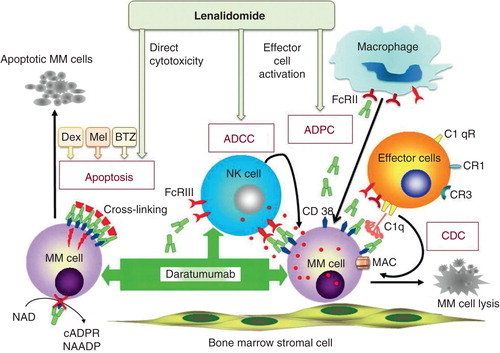
Figure 2. Mechanisms of action of daratumumab against multiple myeloma cells in the bone marrow microenvironment. DARA induces anti-MM effects via multiple mechanisms. It directly induces MM cell apoptosis when cross-linked with anti-human immunoglobulin or FcγRs expressed on various effector cells. DARA-induced inhibition of CD38 ADPR-ribosyl cyclase activity may also contribute to its direct killing of MM cells. Furthermore, antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADPC) and complement-mediated cytotoxicity (CDC) also contribute to DARA-induced MM cell death. ADCC and ADPC is achieved via binding of FcγRs on NK cells and macrophages (myeloid effector cells) by tumor cell-bound DARA. CDC is dependent on the interaction of the antibody Fc with the classic complement-activating protein C1q, resulting in the accumulation of C3b. Antibody opsonization and activation of complement leads to phagocytosis of tumor cells. C3b also binds to C3 convertase to form C5 convertase, inducing the membrane attack complex (MAC) that forms transmembrane channels. These channels in turn disrupt the phospholipid bilayer of MM cells, leading to cell lysis and death. DARA-induced anti-MM activities can be further enhanced by lenalidomide via both direct and indirect killing of MM cells. Dexamethasone (Dex)/prednisolone, bortezomib (BTZ) or melphalan (mel) also augment direct toxicity induced by DARA.
Immunomodulatory drugs (IMiDs) such as lenalidomide (Len) are known to upregulate effector cell function, which is crucial for DARA-induced anti-MM activities. As expected, pretreatment of effector cells with Len results in improved DARA-induced ADCC in MM cell lines and primary MM cells by activating effector NK cells Citation[41,42,45]. The addition of DARA with bortezomib (BTZ), or Len, or with melphalan plus prednisolone enhances MM cell lysis Citation[42].
6. Clinical applications
Based on this promising preclinical data, DARA was brought to the clinic in a two-part Phase I study Citation[46-48] involving patients with relapsed/refractory (RR) MM who had received at least two prior lines of therapy, with the intent of characterizing the safety and pharmacokinetic profile of the agent as well as assessing efficacy based on response criteria of the International Myeloma Workshop Consensus Panel incorporating both Uniform and modified EBMT criteria Citation[49]. Utilizing a standard 3 + 3 dose escalation design, 10 cohorts were treated at doses ranging from 0.005 to 24 mg/kg administered intravenously once weekly for eight weeks. A predose at 10% of the full dose was given 1 day before the first dose of DARA.
Among 32 individuals treated in part 1 of the study, the median age was 59 and median number of prior lines of therapy 5.5. Of the study participants, 75% were refractory to both lenalidomide and bortezomib, 83% had previously undergone autologous stem cell transplantation and 33% had undergone allogeneic transplant. Infusion reactions occurred in 9% of patients during predose infusion, in 26% during the first full infusion and in an incrementally lower percentage of patients during subsequent infusions. Infusion reactions included bronchospasm, headache, dyspnea and fever. Toxicities were assessed according to NCI CTC version 4.0 criteria and two dose-limiting toxicities were reported – grade 3 anemia and grade 4 thrombocytopenia in one patient at 0.1 mg/kg dose and grade 3 AST elevation in one patient at the 1 mg/kg. Three additional patients experienced serious adverse events, including one with grade 2 cytokine release syndrome and two with grade 2/3 bronchospasm. With the introduction of additional premedication and prolonged infusion times these side effects have not proven to be a recurrent issue.
Pharmacokinetic analysis demonstrated rapid clearance at doses of 2 mg/kg or less, consistent with target-mediated clearance. At doses of 4 mg/kg and higher, on the other hand, trough levels were sustained at levels > 10 mg/ml, suggesting that at higher doses the impact of target-mediated clearance diminished and thus, higher doses might therefore improve activity. For the 16 mg/kg dose that is presumed to be target-saturating, the half-life of DARA ranges from 14 to 21 days. Parallel receptor saturation studies have not been done as the reliability of any validated assay has limited the scope of correlative pharmacodynamic studies, at least so far.
The overall rate of minimal response (MR) or better was 30%. Of note, however, and commensurate with the observations above, the rate of MR or better among patients treated at doses of 4 mg/kg and above was 67%, with a partial response (PR) rate or better of 42%.
Results from part 1 of this ongoing Phase I study thus provide initial evidence of a favorable toxicity profile and significant anti-MM activity associated with DARA in advanced and heavily pretreated patients. The implications of the bench-to-bedside development of a mAb with single-agent activity in RR MM are substantial. Although thalidomide, lenalidomide and pomalidomide, as well as bortezomib and carfilzomib, have revolutionized MM therapeutics and significantly improved patient outcomes, there remains a critical need for new agents in novel drug classes. Daratumumab reflects just this, both as a mAb and with a unique target expressed both on the tumor cell as well as the tumor microenvironment.
After review of Phase I data obtained to date, the US FDA recently designated DARA a ‘breakthrough therapy', a designation that reflects the agent's potential to improve patient outcome in an area of unmet medical need. As part of the ongoing Phase I study, doses of 8 and 16 mg/kg are currently being explored further with long-term dosing over a 96-week treatment period for responding patients. An additional single-arm Phase II study will evaluate the activity of DARA monotherapy in ‘double refractory' patients who are resistant to both lenalidomide and bortezomib.
7. Conclusion
The anti-CD38 mAb DARA possesses a broad spectrum of anti-MM activity including CDC, ADCC and ADCP. The initial clinical experience with DARA has been promising in terms of the agent's toxicity profile and activity in relapsed and refractory MM. PK analysis indicates that target-mediated drug clearance is blunted at doses of 4 mg/kg or higher, resulting in higher trough concentrations and more sustained exposure to the drug. Of note, among patients treated at doses of 4 mg/kg or higher, the rate of MR or better was 67% and rate of PR or better was 42%.
8. Expert opinion
In spite of the remarkable progress in MM therapeutics in recent years and corresponding improvement in patient outcomes, the disease remains incurable and there remains a critical need for effective new agents. As a cell-surface receptor that is uniformly expressed by plasma cells and that impacts fundamental cellular capabilities such as homing, adhesion and migration, CD38 is an attractive therapeutic target in MM. A critical challenge faced in the development of DARA, however, is potential toxicity stemming from the expression of CD38 on a variety of other cell types, including lymphoid, myeloid, epithelial, muscle and nerve cells. The first-in-human Phase I protocol thus incorporated an appropriately cautious dose-escalation design in which the initial dosing level was 0.005 mg/kg. Toxicity data obtained, to date, has been reassuring to a large extent, for although there have been a limited number of dose-limiting toxicities, an MTD was not reached.
The level of antitumor response observed in the Phase I setting is unprecedented for a mAb in MM. By comparison, the anti-CS1 mAb elotuzumab exhibited minimal single-agent activity, without objective responses in a Phase I dose-escalation study Citation[50] and has proven to be most promising in combinations with lenalidomide Citation[51] and bortezomib Citation[52]. Indeed, the degree of response observed with DARA at doses of 4 mg/kg and higher is reminiscent of the antitumor activity noted in early studies of single-agent thalidomide (32% with at least a 25% reduction in serum or urine M-protein) Citation[53], lenalidomide (rate of MR or better, 26%) Citation[54] and bortezomib (rate of PR or better, 38%) Citation[55].
With ‘breakthrough' status now conferred upon DARA by the FDA, critical steps in the agents' development lie ahead, with efforts to characterize the optimal dose, schedule of administration, duration of therapy, as well as short-and long-term toxicity profile of the drug.
While the single-agent antitumor activity of DARA observed in RR MM is notable, the impact of this agent may be most significant in regimens involving other agents with distinct and complementary anti-MM mechanisms of action. Daratumumab plus lenalidomide and dexamethasone is currently being evaluated in a Phase I study with very encouraging tolerability and response data to date, which in turn will provide critical insights regarding the safety and efficacy of combination therapy incorporating DARA as the study continues Citation[56]. To date, 8 of 11 patients treated have achieved at least a PR, and 3 have achieved a complete response.
In addition, it is quite likely DARA will be incorporated into regimens for frontline therapy in MM, where it has potential to improve depth and duration of treatment response.
Much work remains to be done in the development of DARA, yet current results provide reason for cautious optimism that another drug class may eventually be added to the growing armamentarium of anti-MM therapies and so improve outcome for patients with this otherwise incurable and challenging hematologic malignancy.
Declaration of interest
Y-T Tai is a consultant for Onyx Pharmaceuticals, Inc., while PG Richardson is on the advisory committees of Johnson & Johnson, Genmab A/S, Millennium Pharmaceuticals, the Celgene Corporation and Bristol-Myers Squibb. KC Anderson is on the advisory committees of Johnson & Johnson, Sanofi S.A., Millennium Pharmaceuticals, Celgene and Bristol-Myers Squibb and has stocks in Acetylon Pharmaceuticals and Oncopep, Inc. JP Laubach has nothing to declare. This work was in part supported by the Rick Corman Multiple Myeloma Research Fund.
Acknowledgements
The authors gratefully acknowledge the administrative support of M Maglio and L Langdon-Embry in the preparation of this manuscript.
Notes
Bibliography
- Malavasi F, Funaro A, Roggero S, et al. Human CD38: a glycoprotein in search of a function. Immunol Today 1994;15(3):95-7
- Deaglio S, Mehta K, Malavasi F. Human CD38: a (r)evolutionary story of enzymes and receptors. Leuk Res 2001;25(1):1-12
- Hara-Yokoyama M, Kukimoto-Niino M, Terasawa K, et al. Tetrameric interaction of the ectoenzyme CD38 on the cell surface enables its catalytic and raft-association activities. Structure 2012;20(9):1585-95
- Zhao YJ, Lam CM, Lee HC. The membrane-bound enzyme CD38 exists in two opposing orientations. Sci Signal 2012;5(241):ra67
- Malavasi F, Deaglio S, Funaro A, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 2008;88(3):841-86
- Malavasi F, Deaglio S, Damle R, et al. CD38 and chronic lymphocytic leukemia: a decade later. Blood 2011;118(13):3470-8
- Buggins AG, Pepper C, Patten PE, et al. Interaction with vascular endothelium enhances survival in primary chronic lymphocytic leukemia cells via NF-kappaB activation and de novo gene transcription. Cancer Res 2010;70(19):7523-33
- Vaisitti T, Aydin S, Rossi D, et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia 2010;24(5):958-69
- Funaro A, Spagnoli GC, Ausiello CM, et al. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol 1990;145(8):2390-6
- Ausiello CM, Urbani F, la Sala A, et al. CD38 ligation induces discrete cytokine mRNA expression in human cultured lymphocytes. Eur J Immunol 1995;25(5):1477-80
- Deaglio S, Dianzani U, Horenstein AL, et al. Human CD38 ligand. A 120-KDA protein predominantly expressed on endothelial cells. J Immunol 1996;156(2):727-34
- Horenstein AL, Stockinger H, Imhof BA, Malavasi F. CD38 binding to human myeloid cells is mediated by mouse and human CD31. Biochem J 1998;330(Pt 3):1129-35
- Howard M, Grimaldi JC, Bazan JF, et al. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 1993;262(5136):1056-9
- Aarhus R, Graeff RM, Dickey DM, et al. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J Biol Chem 1995;270(51):30327-33
- Lee HC. Structure and enzymatic functions of human CD38. Mol Med 2006;12(11-12):317-23
- Hubert S, Rissiek B, Klages K, et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med 2010;207(12):2561-8
- Chiarugi A, Dolle C, Felici R, Ziegler M. The NAD metabolome–a key determinant of cancer cell biology. Nat Rev Cancer 2012;12(11):741-52
- Deaglio S, Vaisitti T, Bergui L, et al. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood 2005;105(8):3042-50
- Deaglio S, Vaisitti T, Aydin S, et al. In-tandem insight from basic science combined with clinical research: CD38 as both marker and key component of the pathogenetic network underlying chronic lymphocytic leukemia. Blood 2006;108(4):1135-44
- Ferretti E, Bertolotto M, Deaglio S, et al. A novel role of the CX3CR1/CX3CL1 system in the cross-talk between chronic lymphocytic leukemia cells and tumor microenvironment. Leukemia 2011;25(8):1268-77
- Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol 2004;121(4):482-8
- Kim D, Park CY, Medeiros BC, Weissman IL. CD19-CD45 low/- CD38 high/CD138+ plasma cells enrich for human tumorigenic myeloma cells. Leukemia 2012;26(12):2530-7
- Hosen N. Multiple myeloma-initiating cells. Int J Hematol 2013;97(3):306-12
- Pittner BT, Shanafelt TD, Kay NE, Jelinek DF. CD38 expression levels in chronic lymphocytic leukemia B cells are associated with activation marker expression and differential responses to interferon stimulation. Leukemia 2005;19(12):2264-72
- Granziero L, Circosta P, Scielzo C, et al. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood 2003;101(5):1962-9
- Mihara K, Bhattacharyya J, Kitanaka A, et al. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia 2012;26(2):365-7
- Mihara K, Yanagihara K, Takigahira M, et al. Activated T-cell-mediated immunotherapy with a chimeric receptor against CD38 in B-cell non-Hodgkin lymphoma. J Immunother 2009;32(7):737-43
- Stevenson FK, Bell AJ, Cusack R, et al. Preliminary studies for an immunotherapeutic approach to the treatment of human myeloma using chimeric anti-CD38 antibody. Blood 1991;77(5):1071-9
- Goldmacher VS, Bourret LA, Levine BA, et al. Anti-CD38-blocked ricin: an immunotoxin for the treatment of multiple myeloma. Blood 1994;84(9):3017-25
- Ellis JH, Barber KA, Tutt A, et al. Engineered anti-CD38 monoclonal antibodies for immunotherapy of multiple myeloma. J Immunol 1995;155(2):925-37
- Flavell DJ, Boehm DA, Emery L, et al. Therapy of human B-cell lymphoma bearing SCID mice is more effective with anti-CD19- and anti-CD38-saporin immunotoxins used in combination than with either immunotoxin used alone. Int J Cancer J Int Cancer 1995;62(3):337-44
- Bolognesi A, Polito L, Farini V, et al. CD38 as a target of IB4 mAb carrying saporin-S6: design of an immunotoxin for ex vivo depletion of hematological CD38+ neoplasia. J Biol Regul Homeost Agents 2005;19(3-4):145-52
- Lokhorst H, Plesner T, Gimsing P, et al. Daratumumab, A CD38 monoclonal antibody study in advanced multiple myeloma – an open-label, dose escalation followed by open-label extension in a single-arm phase I/II study. Haematologica 2013;98(s1):241
- Lokhorst HM, Plesner T, Gimsing P, et al. Phase I/II dose-escalation study of daratumumab in patients with relapsed or refractory multiple myeloma. J Clin 2013;31(Suppl):8512
- de Weers M, Tai YT, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 2011;186(3):1840-8
- Matas-Céspedes A, Vidal-Crespo A, Rodriguez V, et al. Daratumumab, a novel human anti-CD38 monoclonal antibody for the treatment of chronic lymphocytic leukemia and B-cell non–hodgkin lymphoma. ASH Annu Meeting Abstr 2012;120(21):3935
- Groen RW, Noort WA, Raymakers RA, et al. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood 2012;120(3):e9-e16
- Noort WA, Groen RW, Raymakers R, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of refractory patient-derived multiple myeloma cells, growing in a novel humanized mouse MM model. ASH Annu Meeting Abstr 2012;120(21):940
- Noort WA, Groen RW, Raymakers R, et al. Daratumumab, a novel human CD38 monoclonal antibody for treatment of multiple myeloma, prevents intra-medullary spreading of patient derived multiple myeloma cells growing in a humanized mouse model. ASH Annu Meeting Abstr 2012;120(21):1834
- Jansen JH, Boross P, Overdijk MB, et al. Daratumumab, a human CD38 antibody induces apoptosis of myeloma tumor cells via Fc receptor-mediated crosslinking. ASH Annu Meeting Abstr 2012;120(21):2974
- Kong SY, Li XF, Nahar S, et al. Daratumumab directly induces human multiple myeloma cell death and acts synergistically with conventional and novel anti-myeloma drugs. ASH Annu Meeting Abstr 2010;116(21):3013
- van der Veer MS, de Weers M, van Kessel B, et al. The therapeutic human CD38 antibody daratumumab improves the anti-myeloma effect of newly emerging multi-drug therapies. Blood Cancer J 2011;1(10):e41
- Overdijk MB, Verploegen S, Marijn B, et al. Phagocytosis is a mechanism of action for daratumumab. ASH Annu Meeting Abstr 2012;120(21):4054
- Zheng Y, Cai Z, Wang S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 2009;114(17):3625-8
- van der Veer MS, de Weers M, van Kessel B, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica 2011;96(2):284-90
- Plesner T, Lokhorst H, Gimsing P, et al. Daratumumab, a CD38 monoclonal antibody in patients with multiple myeloma - data from a dose-escalation phase I/II study. ASH Annu Meeting Abstr 2012;120(21):73
- Plesner T, Lokhorst HM, Gimsing P, et al. Daratumumab, a CD38 mab, for the treatment of relapsed/refractory multiple myeloma patients: preliminary efficacy data from a multicenter phase I/II study. ASCO Meeting Abstr 2012;30(15_Suppl):8019
- Lokhorst HM, Plesner T, Gimsing P, et al. Phase I/II dose-escalation study of daratumumab in patients with relapsed or refractory multiple myeloma. ASCO Meeting Abstr 2013;31(15_Suppl):8512
- Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011;117(18):4691-5
- Zonder JA, Mohrbacher AF, Singhal S, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012;120(3):552-9
- Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol 2012;30(16):1953-9
- Jakubowiak AJ, Benson DM, Bensinger W, et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol 2012;30(16):1960-5
- Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999;341(21):1565-71
- Richardson P, Jagannath S, Hussein M, et al. Safety and efficacy of single-agent lenalidomide in patients with relapsed and refractory multiple myeloma. Blood 2009;114(4):772-8
- Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005;352(24):2487-98
- Plesner T, Arkenau T, Lokhorst H, et al. Preliminary safety and efficacy data of daratumumab in combination with lenalidomide and dexamethasone in relapsed or refractory multiple myeloma. ASH Annual Meeting Abstr 2013;122:1986
