Abstract
Introduction: Lowering intraocular pressure (IOP) is at present the only therapeutic approach to the treatment of glaucoma proven to be successful. The choice of therapy must take into account efficacy, tolerability, safety, quality of life, adherence and cost. Monotherapy fails to achieve a satisfactory IOP reduction in 40 – 75% of glaucoma patients after > 2 years of therapy. So far, three prostaglandin/timolol maleate 0.5% fixed combinations (FCs) are available.
Areas covered: This review provides a background on the tafluprost–timolol FC (TTFC, Santen Oy) and its individual compounds. It summarizes the data on efficacy and safety, including comparative data with prostaglandin/timolol FCs already available.
Expert opinion: Tafluprost is a preservative-free prostaglandin analog with a similar IOP efficacy when compared with other prostaglandin analogs. However, its improved adverse effect profile seems to be beneficial in patients sensitive to preservatives. The preservative-free TTFC has no market authorization yet. Only one Phase III trial was published so far, but results are promising in terms of efficacy, tolerability and safety. It is likely that the TTFC will play a role in the treatment of open-angle glaucoma and ocular hypertension.
1. Introduction
Glaucoma is a progressive optic neuropathy caused by the degeneration and death of retinal ganglion cells and their axons that form the optic nerve, causing loss of visual function up to blindness Citation[1]. Primary open-angle glaucoma is the most common form of all glaucoma and the second leading cause of blindness worldwide Citation[2]. It has been estimated that in the year 2010 there were ∼ 60 million glaucoma patients worldwide, and that this figure is expected to rise to 80 million by 2020 owing to aging Citation[3]. At present, intraocular pressure (IOP) is the only modifiable risk factor of progression. Sufficiently high IOP reduction reduces the risk of progression of visual field loss Citation[4-6]. The goal of glaucoma treatment is to preserve vision. To reach this goal, it is essential to sufficiently reduce IOP and at the same time to provide good long-term tolerance to therapy Citation[7]. For many years, β-adrenoceptor antagonists (β-blockers) have been a first choice in medical treatment of ocular hypertension and primary open-angle glaucoma. Because of their efficacy, convenience (once-daily application) and minimal systemic side effects, prostaglandin analogs launched in the last decade have nowadays become the first line of treatment Citation[8]. Many clinical trials and meta-analyses have found that prostaglandin analogs have a greater ability to reduce IOP than the other prescribed therapeutic classes, including β-blockers Citation[9-11]. A meta-analysis has shown that the three available prostaglandin analogs can provide an IOP reduction of −31 to −33% at peak and from −28 to −29% at trough Citation[9]. Prostaglandin analogs are recommended as first-line therapy by medical society guidelines (European Glaucoma Society guideline Citation[12], American Academy of Ophthalmology preferred practice Citation[13]). Despite the efficacy of prostaglandin analogs, a significant proportion of patients require more than one medication to reach an IOP at which optic nerve damage will not progress the so-called ‘target IOP.’ The Ocular Hypertension Study found that almost 40% of patients require two or more medications to reach a given target IOP Citation[14]. Prostaglandin analogs are frequently combined with other classes; several unfixed combinations are available. However, unfixed combinations increase regimen complexity and can cause decreased adherence and persistence. Moreover, they require a waiting time between two instillations in order to avoid drug washout Citation[15-18]. Fixed combinations (FCs) simplify the dosing regimen and may thus improve compliance and persistence. This is important as glaucoma is a chronic asymptomatic disease and affected patients are more prone to nonadherence than patients with a symptomatic disease Citation[19]. FCs also decrease the daily exposure to preservatives such as benzalkonium chloride, which is known to have toxic effects on the ocular surface and to increase treatment side effects Citation[20]. Ocular surface disease is very common in glaucoma and ocular hypertensive patients chronically treated with preserved topical IOP-lowering medications. The prevalence rates varied between 45 and 60% in different studies Citation[21-23]. Another benefit of FCs is elimination of the washout effect, which occurs when patients instill medications in succession so that the first-administered medication is washed out by application of the second medication. They therefore often provide better IOP control than unfixed combinations in real life Citation[24]. Finally, the cost of a FC is frequently less than the combined cost of the two ingredients prescribed separately Citation[8].
2. Overview of the market
So far, three FCs containing prostaglandin analogs are available on the market. They include bimatoprost and timolol maleate FC (available with and without benzalkonium chloride), latanoprost and timolol maleate FC (containing benzalkonium chloride) and travoprost and timolol maleate (containing polyquaternium-1) Citation[25,26]. A meta-analysis demonstrated that all three available prostaglandin/timolol FCs provide greater IOP reduction than the three respective prostaglandin monotherapies Citation[27]. This meta-analysis, together with a more recent meta-analysis, has also observed that prostaglandin/timolol FCs can significantly reduce conjunctival hyperemia Citation[28]. In various studies, the latanoprost/timolol FC has shown a mean daytime IOP-lowering efficacy of 30 – 39% from untreated baseline Citation[28,29]. In patients inadequately controlled by prostaglandin analog monotherapy, evening-dosed bimatoprost/timolol FC provided significantly greater mean diurnal IOP reduction as compared to latanoprost/timolol FC dosed in the evening (2.8 ± 0.9 mmHg vs 2.1 ± 0.6 mmHg; p = 0.021) at the end of a 4-week trial Citation[30]. Martinez and Sanchez compared the efficacy of bimatoprost/timolol FC with latanoprost/timolol FC in patients with primary open-angle glaucoma and pseudoexfoliative glaucoma and found a small but statistically significant difference in IOP reduction after a period of 12 weeks (mean 12-h IOP difference: 0.8 mmHg) Citation[31]. In a prospective, investigator-masked multicenter study with 82 glaucoma patients inadequately controlled on prostaglandin monotherapy, latanoprost/timolol FC provided an additional 13.7% IOP lowering effect after 12 weeks, whereas the bimatoprost/timolol FC provided an additional 21.4% ocular hypertensive effect Citation[32]. A meta-analysis compared the mean differences in IOP lowering of the three FCs versus their corresponding prostaglandin monotherapy alone: depending on measuring time (9 am, noon, 4 pm or mean diurnal curve), mean differences were 1.31 – 2.01 mmHg for latanoprost versus FC (p < 0.01), 2.02 – 2.59 mmHg for travoprost versus FC (p < 0.001) and 0.00 – 1.14 mmHg for bimatoprost versus FC (p > 0.1 to p < 0.001) Citation[27]. So far, only one FC ophthalmic solution without preservatives is available. Previous clinical trials suggested the important benefit associated with the use of benzalkonium chloride-free formulations of prostaglandin analogs, as they significantly diminished the incidence of conjunctival irritation Citation[33-35]. Clearly, there is a need to offer more prostaglandin analogs FCs containing no preservatives.
3. Introduction of the compound
Tafluprost–timolol FC (TTFC) ophthalmic solution is a fixed-dose combination of tafluprost 15 µg/ml and timolol 5 mg/ml without preservatives (). This preservative-free FC will be labeled as a once-daily agent for glaucoma patients whose IOP is inadequately controlled with a monotherapy. It has no marketing authorization yet. Tafluprost ophthalmic solution 0.0015% is the most recently introduced prostaglandin analog Citation[34,36,37]. Tafluprost was the first prostaglandin analog produced in a preservative-free formulation. It has been shown that IOP-lowering efficacy of tafluprost, in both preserved and preservative-free formulations, is similar to that of latanoprost Citation[36-38]. Tafluprost was first introduced in Japan in 2008 as a benzalkonium chloride containing multidose formulation and in Germany in 2008 with approval for both a preserved and an unpreserved tafluprost formulation Citation[39]. Currently, however, throughout the rest of the world, only unpreserved tafluprost is marketed Citation[39]. Both formulations seem to be equally effective. Hamacher et al. found an overall efficacy difference of only 0.01 mmHg (95% CI −0.46 – 0.49; p = 0.96) at 4 weeks Citation[40]. Several open-label studies have examined the efficacy and tolerability of unpreserved tafluprost in naïve Citation[41] or previously treated patients with open-angle glaucoma or ocular hypertension, who were either poorly controlled or had tolerability issues with other medications Citation[34,35,42-44]. These studies have reported a mean diurnal IOP reduction of 22.9 – 32.1% from untreated baseline Citation[41,42,44]. Although these studies were uncontrolled, they indicate that preservative-free tafluprost has almost comparable efficacy to latanoprost and will likely benefit patients facing tolerability problems with other medications Citation[38]. Reduction of IOP starts ∼ 2 – 4 h after the first administration, with the peak effect within ∼ 8 – 12 h. Evening administration of prostaglandins is generally preferable because of a better circadian IOP profile Citation[45].
Box 1. Drug summary.
Timolol has been topically used for the treatment of glaucoma and increased IOP for over 30 years Citation[46]. β-blockers and prostaglandin analogs are used as first-line therapy in glaucoma. Of the glaucoma and ocular hypertension patients, about 70% are timolol responders Citation[47]. Timolol has been reported to decrease the aqueous humor production by up to 48% in the normal human eye, the mean suppression being 34% Citation[48]. The European Glaucoma Society quotes a mean IOP reduction of 20 – 25% Citation[12]. After the initial fall in IOP, there may be some loss in effect Citation[46]. However, it has been reported that a persistent effect is present even after years of use Citation[49]. It is usually given twice daily Citation[12].
Past clinical randomized studies suggested that both FCs of latanoprost/timolol and travoprost/timolol provided greater IOP reduction at evening dosing than at morning dosing Citation[50,51]. The TTFC is tested at 08:00 am in clinical trials Citation[52].
4. Chemistry
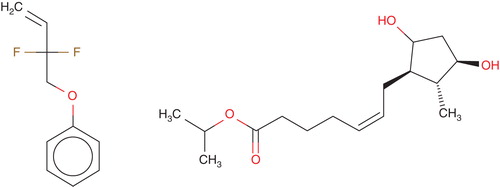
The preservative-free TTFC is a combination of two well-known ophthalmic drugs, namely tafluprost (15 µg/ml) and timolol maleate (5 mg/ml) Citation[52]. Tafluprost is a fluorinated analog of prostaglandin F2α. The chemical name for tafluprost is 1methylethyl (5Z)-7-{(1R, 2R, 3R, 5S)-2-[(1E)-3,3-difluoro-4-phenoxy-1-butenyl}-3,5-dihydroxycyclopentyl]5-heptenoate. The molecular formula of tafluprost is C25H34F2O5 and its molecular weight is 452.53. Its structural formula is shown in .
Tafluprost is a colorless to light yellow viscous liquid that is practically insoluble in water. Tafluprost ophthalmic solution 0.0015% without preservatives is supplied as a sterile solution of tafluprost with a pH range 5.5 – 6.7 and an osmolality range 260 – 300 mOsmol/kg. It contains active: tafluprost 0.015 mg/ml; inactives: glycerol, sodium dihydrogen phosphate dihydrate, disodium edetate, polysorbate 80, hydrochloric acid and/or sodium hydroxide (to adjust pH) and water for Injection.
Timolol maleate ophthalmic solution is a noncardioselective, competitive β-adrenergic receptor blocking agent. Its chemical name is (-)-l-(tert-butylamino)-3- [(4-morpholino-l, 2, 5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The optical rotation of timolol maleate is:
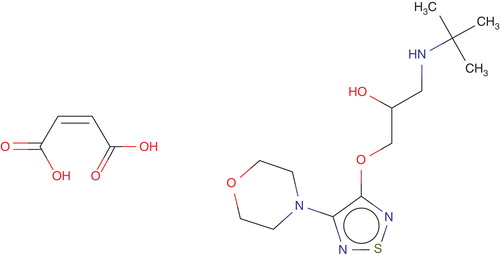
Its molecular formula is C13H24N4O3S•C4H4O4 and its structural formula is shown in .
Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder, which is soluble in water, methanol and alcohol. It is stable at room temperature and is supplied as a sterile, isotonic, buffered, aqueous solution of timolol maleate in two dosage strengths: Each ml of timolol maleate 0.5% contains 5 mg of timolol (6.8 mg of timolol maleate). The pH of the solution is ∼ 7, and the osmolarity is 274 – 328 mOsm. Inactive ingredients: monobasic and dibasic sodium phosphate and water for injection. It contains no preservatives.
5. Pharmacodynamics
The TTFC contains two active substances. No published evidence regarding pharmacodynamics of the TTFC is available so far. Therefore, pharmacodynamics of both active substances used as monotherapy will be described.
Tafluprost acid, a prostaglandin analog is a selective FP prostanoid receptor agonist, which is believed to reduce IOP by increasing uveoscleral outflow. The exact mechanism of action is unknown at this time Citation[53]. Reduction of the IOP starts ∼ 2 – 4 h after the first administration with the maximum effect reached after 12 h Citation[53]. In clinical studies up to 24 months in duration, patients with open-angle glaucoma or ocular hypertension and baseline pressure of 23 – 26 mmHg who were treated with tafluprost dosed once daily in the evening demonstrated reductions in IOP at 3 and 6 months of 6 – 8 and 5 – 8 mmHg, respectively Citation[53]. The reduction in IOP achieved by preservative-free tafluprost is equivalent to that obtained with the preserved formulation Citation[40].
The β-adrenergic receptors (β1 and β2) are widely distributed throughout the body, including the eye. In the eye, β-receptors are found on the ocular surface, in the ocular blood vessels, trabecular meshwork, lens epithelium, ciliary body and retina. In the ciliary processes, 75 – 90% of the β-receptors are β2-receptors Citation[54]. Timolol is a nonselective β-adrenergic blocker that blocks the activity of both β-1 and β-2 receptors Citation[46]. Timolol has been shown to lower IOP by reducing aqueous humor production by the nonpigmented ciliary epithelium Citation[55,56]. The IOP-lowering effect of timolol begins 20 min post-dosing, reaches its maximal effect 2 h after instillation and continues in a waning manner for up to 24 h Citation[56,57].
6. Pharmacokinetics and metabolism
No data is yet available on the pharmacokinetics and metabolism of TTFC. Therefore, the individual compounds are described. Tafluprost is an ester prodrug that is hydrolyzed into the biologically active metabolite, tafluprost acid, in the eye Citation[53]. The acid compound is further metabolized via β-oxidation and Phase II conjugation. Plasma concentrations of tafluprost acid peak about 10 min after instillation of one drop of eye drop solution. Plasma tafluprost acid concentrations are below detectable levels (10 pg/ml) 30 min following topical administration Citation[53]. The IOP decrease of timolol maleate 0.5% begins 30 – 60 min after administration and lasts for at least 24 h Citation[58]. Timolol is absorbed systemically, mainly from the nasal mucosa. Peak plasma concentration after a single instillation varies between 2.5 and 5 ng/ml in humans Citation[59,60]. Timolol is distributed in various tissues like conjunctiva, cornea, sclera, iris, aqueous humor, liver, kidney and lung. Its half-life is 2.5 – 5.0 h Citation[61]. Timolol and its metabolites are mainly excreted by the kidneys Citation[61].
7. Clinical efficacy
Only one publication on the TTFC ophthalmic solution is available so far Citation[52]. Three clinical trials testing this FC including the published trial are listed in clinicaltrials.gov (ClinicalTrials.gov Identifiers: NCT01434888, NCT01306461 Citation[52], NCT01292460). All of them were sponsored by Santen Oy, Finland. A Phase I study in healthy volunteers was completed in 2011 – 2012 to evaluate the pharmacokinetics, safety and tolerability of the preservative-free FC. Fifteen healthy volunteers were included and received tafluprost 0.0015%, timolol 0.5% and fixed-dose combination of both (all preservative-free) in a crossover assignment. The primary outcome measure was pharmacokinetics (plasma concentrations) after single and repeated administration of tafluprost and timolol. Safety and tolerability served as secondary outcome measures. No results were published so far. One Phase III study was performed to prove superiority of the TTFC against monotherapies in ocular hypertension and open-angle glaucoma. Six hundred patients were included in nine countries. After washout, prior timolol users received either timolol or TTFC, prior tafluprost users received either tafluprost or TTFC (all preservative-free) for a duration of 6 months. Primary outcome measure was the change from baseline in the average diurnal IOP at 3 months. Secondary outcome measures included change from baseline in average diurnal IOP at 2 weeks, 6 weeks and 6 months and change from baseline in timewise IOP, measured at 08:00, 10:00, 16:00 and 20:00. The study was finished in March 2013. No results are published so far. Another Phase III study compared the TTFC against concomitant administrations in a noninferiority design. This 6-month, prospective, randomized, double-masked, active-controlled, parallel group, multicenter Phase III study was performed in patients with ocular hypertension and open-angle glaucoma with untreated IOP ≥ 23 and ≤ 36 mmHg at baseline Citation[52]. The purpose was to compare efficacy, safety and tolerability of the preservative-free FC and non-FC (NFC) of tafluprost 0.0015% and timolol 0.5% in patients with open-angle glaucoma or ocular hypertension. Four hundred patients washed out from IOP-lowering medication (if not treatment-naïve) were randomized, 201 received the FC and 199 received the NFC. Mean IOP decreases from baseline ranged from −7.3 to −9.1 mmHg (29.6 – 34.6%) in the FC and from −7.5 to −9.4 mmHg (30.7 – 36.0%) in the NFC arm (per-protocol [PP] dataset, P <0.0001 compared with baseline for both groups). At month 6, the estimated overall treatment difference (FC–NFC) was 0.308 mmHg (PP dataset, 95% CI from −0.194 to 0.810 mmHg). An IOP decrease ≥ 30% was achieved in 58.3 and 66.9% of the patients in the FC and NFC groups, respectively (PP dataset; p = 0.105); an IOP decrease ≥ 35% was achieved in 36.6 and 43.1% of patients in the FC and NFC groups, respectively (PP dataset; p = 0.297). All measures of IOP reduction for FC of preservative-free tafluprost/timolol were statistically and clinically significant and noninferior to those of the NFC, throughout the 6-month study period.
8. Safety and tolerability
Only one Phase III trial describing the safety and tolerability of the TTFC was published so far Citation[52]. Patients with ocular adverse events were evenly distributed in both groups. A total of 169 adverse events (89 ocular, 80 nonocular) were reported by 84 patients (41.8%) in the FC treatment group, and 175 adverse events (98 ocular, 77 nonocular) were reported by 88 patients (44.2%) in the NFC treatment group (p = 0.624). Nonocular treatment-related adverse events were observed in a few subjects. In total, seven patients in the FC group and four patients in the NFC group discontinued the study due to adverse events (ocular discomfort, conjunctival/ocular hyperemia, eye irritation, eye discharge, dry eye symptoms, erythema of the eyelid, allergic conjunctivitis, foreign body sensation, eye pain and photophobia; mostly mild severity). The most common side effect, conjunctival/ocular hyperemia was found in 8 and 5% of patients in the FC and NFC arms, respectively. The severity of hyperemia was generally mild to moderate and comparable in both treatment groups. For both treatment groups, blood pressure and heart rate decreased slightly from the 08:00 to the 10:00 am measurement. No clear trends (from baseline to month 6) could be seen either in the blood pressure or heart rate in either group. However, the heart rate for the NFC group decreased slightly (−1.9 beats per minute [bpm] for FC and −4.9 bpm for NFC at 10:00; p < 0.001). Both treatment regimens were generally well tolerated with ∼ 75 – 80% of patients experiencing no drop discomfort on instillation during the study. The results are in line with the safety and tolerability described for tafluprost and timolol used as monotherapy. Timolol is topically well tolerated by most patients Citation[46]. Approximately 80% of a topically administered eye drop is reported to drain through the nasolacrimal duct and is systemically absorbed Citation[62]. This can cause severe cardiac and respiratory adverse events such as bradycardia, hypotension and syncope, bronchospasm, respiratory failure and increased cough Citation[63,64]. Cardiac and respiratory adverse effects should always be anticipated whenever timolol or other β-blockers are used, especially in susceptible patients with underlying pertinent systemic disease Citation[65].
9. Regulatory affairs
So far, Phase I and Phase III studies were conducted, one Phase III trial was published (ClinicalTrials.gov Identifiers: NCT01434888, NCT01306461 Citation[52], NCT01292460). The preservative-free tafluprost/timolol maleate FC has no market authorization yet.
10. Conclusion
So far, only one publication on the TTFC is available. This 6-month, prospective, randomized, double-masked, active-controlled, parallel group, multicenter Phase III study was performed in patients with ocular hypertension and open-angle glaucoma and compared the FC versus both ingredients as NFC. All measures of IOP reduction for FC of preservative-free tafluprost/timolol were statistically and clinically significant and noninferior to those of the NFC, throughout the 6-month study period. Both treatment arms showed a good and comparable safety and tolerability profile.
11. Expert opinion
The choice of therapy in glaucoma must take into account not just IOP lowering, but also tolerability, cost and compliance. FCs have been increasingly used in the last 10 years. They have several advantages such as improved adherence, fewer side effects and lower costs. Tafluprost is a preservative-free prostaglandin analog, which appears to have a similar therapeutic efficacy when compared to other prostaglandin analogs. However, its improved adverse effect profile seems to be beneficial in patients with sensitivities to preservatives. The preservative-free TTFC has no market authorization yet. Only one Phase III trial was published so far, but results are promising in terms of efficacy, tolerability and safety. Though at present there is only little information and no long-term clinical experience available, the ingredients in tafluprost have been extensively used since 2008. For timolol, clinical data for > 40 years are available. This strongly indicates that the TTFC will have a role in the treatment of open-angle glaucoma and ocular hypertension.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363(9422):1711-20
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996;80(5):389-93
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in. 2010 and 2020. Br J Ophthalmol 2006;90(3):262-7
- Mao LK, Stewart WC, Shields MB. Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. Am J Ophthalmol 1991;111(1):51-5
- Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114(11):1965-72
- Investigators A. The Advanced Glaucoma Intervention Study (AGIS): 9. Comparison of glaucoma outcomes in black and white patients within treatment groups. Am J Ophthalmol 2001;132(3):311-20
- Hollo G, Bozkurt B, Irkec M. Brinzolamide/timolol fixed combination: a new ocular suspension for the treatment of open-angle glaucoma and ocular hypertension. Expert Opin Pharmacother 2009;10(12):2015-24
- Aptel F, Chiquet C, Romanet JP. Intraocular pressure-lowering combination therapies with prostaglandin analogues. Drugs 2012;72(10):1355-71
- van der Valk R, Webers CA, Schouten JS, et al. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology 2005;112(7):1177-85
- Stewart WC, Konstas AG, Nelson LA, et al. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology 2008;115(7):1117-22. e1
- van der Valk R, Webers CA, Lumley T, et al. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J Clin Epidemiol 2009;62(12):1279-83
- European Glaucoma Society. Terminology and guidelines for glaucoma. 4th edition. Editrice Dogma, Savona, Italy; 2014
- American Academy of Ophthalmology Glaucoma Panel. Preferred practice pattern guidelines. Primary open-angle glaucoma. American Academy of Ophthalmology, San Francisco, CA, USA; 2010
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):701-13. discussion 829-30
- Stewart WC, Konstas AG, Pfeiffer N. Patient and ophthalmologist attitudes concerning compliance and dosing in glaucoma treatment. J Ocul Pharmacol Ther 2004;20(6):461-9
- Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol 2008;53(Suppl 1):S57-68
- Robin AL, Novack GD, Covert DW, et al. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol 2007;144(4):533-40
- Djafari F, Lesk MR, Harasymowycz PJ, et al. Determinants of adherence to glaucoma medical therapy in a long-term patient population. J Glaucoma 2009;18(3):238-43
- DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med care 2002;40(9):794-811
- Baudouin C, Labbe A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res 2010;29(4):312-34
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma 2008;17(5):350-5
- Erb C, Gast U, Schremmer D. German register for glaucoma patients with dry eye. I. Basic outcome with respect to dry eye. Graefes Arch Clin Exp Ophthalmol 2008;246(11):1593-601
- Garcia-Feijoo J, Sampaolesi JR. A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol 2012;6:441-6
- Francis BA, Du LT, Berke S, et al. Comparing the fixed combination dorzolamide-timolol (Cosopt) to concomitant administration of 2% dorzolamide (Trusopt) and 0.5% timolol – a randomized controlled trial and a replacement study. J Clin Pharm Ther 2004;29(4):375-80
- Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol 2004;15(2):132-5
- Konstas AG, Haidich AB, Rossetti L, et al. Prostaglandin-timolol fixed combinations efficacy: myth or reality? Eur J Ophthalmol 2012;22(1):1-4
- Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin-timolol fixed combinations: a meta-analysis of randomized clinical trials. Eur J Ophthalmol 2012;22(1):5-18
- Quaranta L, Biagioli E, Riva I, et al. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysis. J Ocul Pharmacol Ther 2013;29(4):382-9
- Webers CA, Beckers HJ, Zeegers MP. The intraocular pressure-lowering effect of prostaglandin analogs combined with topical beta-blocker therapy: a systematic review and meta-analysis. Ophthalmology 2010;117(11):2067-74. e1-6
- Martinez A, Sanchez M. A comparison of the safety and intraocular pressure lowering of bimatoprost/timolol fixed combination versus latanoprost/timolol fixed combination in patients with open-angle glaucoma. Curr Med Res Opin 2007;23(5):1025-32
- Martinez A, Sanchez M. Bimatoprost/timolol fixed combination vs latanoprost/timolol fixed combination in open-angle glaucoma patients. Eye 2009;23(4):810-18
- Centofanti M, Oddone F, Vetrugno M, et al. Efficacy of the fixed combinations of bimatoprost or latanoprost plus timolol in patients uncontrolled with prostaglandin monotherapy: a multicenter, randomized, investigator-masked, clinical study. Eur J Ophthalmol 2009;19(1):66-71
- Henry JC, Peace JH, Stewart JA, et al. Efficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapy. Clin Ophthalmol 2008;2(3):613-21
- Uusitalo H, Chen E, Pfeiffer N, et al. Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol 2010;88(3):329-36
- Hommer A, Kimmich F. Switching patients from preserved prostaglandin-analog monotherapy to preservative-free tafluprost. Clin Ophthalmol 2011;5:623-31
- Traverso CE, Ropo A, Papadia M, et al. A phase II study on the duration and stability of the intraocular pressure-lowering effect and tolerability of Tafluprost compared with latanoprost. J Ocul Pharmacol Ther 2010;26(1):97-104
- Uusitalo H, Pillunat LE, Ropo A, et al. Efficacy and safety of tafluprost 0.0015% versus latanoprost 0.005% eye drops in open-angle glaucoma and ocular hypertension: 24-month results of a randomized, double-masked phase III study. Acta Ophthalmol 2010;88(1):12-19
- Konstas AG, Quaranta L, Katsanos A, et al. Twenty-four hour efficacy with preservative free tafluprost compared with latanoprost in patients with primary open angle glaucoma or ocular hypertension. Br J Ophthalmol 2013;97(12):1510-15
- Pantcheva MB, Seibold LK, Awadallah NS, et al. Tafluprost: a novel prostaglandin analog for treatment of glaucoma. Adv Ther 2011;28(9):707-15
- Hamacher T, Airaksinen J, Saarela V, et al. Efficacy and safety levels of preserved and preservative-free tafluprost are equivalent in patients with glaucoma or ocular hypertension: results from a pharmacodynamics analysis. Acta Ophthalmol Suppl 2008;242:14-19
- Rossi GC, Pasinetti GM, Raimondi M, et al. Efficacy and ocular surface tolerability of preservative-free tafluprost 0.0015%: a 6-month, single-blind, observational study on naive ocular hypertension or glaucoma patients. Expert Opin Drug Saf 2012;11(4):519-25
- Hommer A, Mohammed Ramez O, Burchert M, et al. IOP-lowering efficacy and tolerability of preservative-free tafluprost 0.0015% among patients with ocular hypertension or glaucoma. Curr Med Res Opin 2010;26(8):1905-13
- Erb C, Lanzl I, Seidova SF, et al. Preservative-free tafluprost 0.0015% in the treatment of patients with glaucoma and ocular hypertension. Adv Ther 2011;28(7):575-85
- Milla E, Stirbu O, Rey A, et al. Spanish multicenter tafluprost tolerability study. Br J Ophthalmol 2012;96(6):826-31
- Alm A, Stjernschantz J. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian Latanoprost Study Group. Ophthalmology 1995;102(12):1743-52
- Brooks AM, Gillies WE. Ocular beta-blockers in glaucoma management. Clinical pharmacological aspects. Drugs Aging 1992;2(3):208-21
- Camras CB, Hedman K; US Latanoprost Study Group. Rate of response to latanoprost or timolol in patients with ocular hypertension or glaucoma. J Glaucoma 2003;12(6):466-9
- Coakes RL, Brubaker RF. The mechanism of timolol in lowering intraocular pressure. In the normal eye. Arch Ophthalmol 1978;96(11):2045-8
- Volotinen M, Hakkola J, Pelkonen O, et al. Metabolism of ophthalmic timolol: new aspects of an old drug. Basic Clin Pharmacol Toxicol 2011;108(5):297-303
- Konstas AG, Tsironi S, Vakalis AN, et al. Intraocular pressure control over 24 hours using travoprost and timolol fixed combination administered in the morning or evening in primary open-angle and exfoliative glaucoma. Acta Ophthalmol (Copenh) 2009;87(1):71-6
- Takmaz T, Asik S, Kurkcuoglu P. Comparison of intraocular pressure lowering effect of once daily morning vs evening dosing of latanoprost/timolol maleate combination. Eur J Ophthalmol 2008;18(1):60-5
- Hollo G, Hommer A, Anton Lopez A, et al. Efficacy, safety, and tolerability of preservative-free fixed combination of tafluprost 0.0015%/timolol 0.5% versus concomitant use of the ingredients. J Ocul Pharmacol Ther 2014;30(6):468-75
- Prescribing information. Zioptan (tafluprost), Merck&Co, Whitehouse Station, NJ; 2013
- Wax MB, Molinoff PB. Distribution and properties of beta-adrenergic receptors in human iris-ciliary body. Invest Ophthalmol Vis Sci 1987;28(3):420-30
- Bartels SP, Roth HO, Jumblatt MM, et al. Pharmacological effects of topical timolol in the rabbit eye. Invest Ophthalmol Vis Sci 1980;19(10):1189-97
- Feldman RM. An evaluation of the fixed-combination of latanoprost and timolol for use in open-angle glaucoma and ocular hypertension. Expert Opin Pharmacother 2004;5(4):909-21
- Amyot M, Blondeau P. [Timolol maleate. Pharmacology and review of the literature]. Can J Ophthalmol 1979;14(3):208-14
- Zimmerman TJ, Kaufman HE. Timolol, dose response and duration of action. Arch Ophthalmol 1977;95(4):605-7
- Passo MS, Palmer EA, Van Buskirk EM. Plasma timolol in glaucoma patients. Ophthalmology 1984;91(11):1361-3
- Alvan G, Calissendorff B, Seideman P, et al. Absorption of ocular timolol. Clin Pharmacokinet 1980;5(1):95-100
- Tocco DJ, Duncan AE, Delauna FA, et al. Physiological disposition and metabolism of timolol in man and laboratory animals. Drug Metab Dispos 1975;3(5):361-70
- Shell JW. Pharmacokinetics of topically applied ophthalmic drugs. Surv Ophthalmol 1982;26(4):207-18
- Zimmerman TJ, Baumann JD, Hetherington J Jr. Side effects of timolol. Surv Ophthalmol 1983;28(Suppl):243-51
- Nelson WL, Fraunfelder FT, Sills JM, et al. Adverse respiratory and cardiovascular events attributed to timolol ophthalmic solution. 1978Am J Ophthalmol 1986;102(5):606-11
- Taniguchi T, Kitazawa Y. The potential systemic effect of topically applied beta-blockers in glaucoma therapy. Curr Opin Ophthalmol 1997;8(2):55-8